Abstract
AIM: To study the effect of CXC chemokine receptor-4 (CXCR4) expression on disease progression and prognosis in esophageal cancer.
METHODS: CXCR4 expression was evaluated in 37 patients with histologically confirmed esophageal squamous carcinomas (ESCC) undergoing preoperative chemoradiotherapy (CRT) by immunohistochemical staining.
RESULTS: Eleven out of 37 ESCC patients showed a pathological complete response (CR) after CRT. CXCR4 protein expression was observed in cell cytoplasms of 13 tumors, and null expression was seen in 13 tumors. Distant recurrence was significantly more common in patients with positive CXCR4 expression (P = 0.0318). After a median follow-up time of 31.6 mo, 19 patients progressed (12 of 19 expressed positive CXCR4) and 11 died (10 of 11 expressed positive CXCR4). Overall survival was significantly correlated with lymph node metastasis (952.1 ± 53.8 d in negative group vs 475.1 ± 56.2 d in positive group, P = 0.023), distant metastasis (874.0 ± 60.4 d in negative group vs 434.9 ± 75.2 d in positive group, P = 0.014) and CRT (811.5 ± 51.2 d in responder group vs 459.6 ± 94.0 d in non-responder group, P = 0.00038) and further with an absence of CXCR4 expression or no residual tumor (959.8 ± 51.0 d in null expression or no tumor group vs 412.0 ± 57.1 d in positive expression group, P = 0.0001).
CONCLUSION: Persistent positive CXCR4 expression is implicated in tumor aggressiveness and poor prognosis in ESCC after CRT, and preoperative CRT may improve the prognosis of ESCC via CXCL12-CXCR4 signaling pathway.
Keywords: CXC chemokine receptor-4, Metastasis, Chemoradiotherapy, Esophageal cancer
INTRODUCTION
The migration of tumor cells to a secondary site from their primary location is a crucial issue in cancer metastasis. Recently, a novel “homing” signaling mechanism has been proposed, in which target organs produce and release specific chemokines that attract cancer cells bearing corresponding receptors[1,2]. This mechanism was originally characterized for organogenesis, hematopoiesis and inflammation, and draws on the principles of the “seed and soil” hypothesis advocated by Paget more than one century ago[3]. Signaling results in directional, site-specific cancer cell migration leading to implantation in the favorable “soil” of organs. A large number of studies support this “homing” mechanism by demonstrating that malignant cells can target specific organs or tissues using select chemokine receptors, mainly through the CXCL12-CXC chemokine receptor-4 (CXCR4) pathway[4-8].
Chemokines are signaling molecules that function in myriad cell trafficking events. They have been classified into four subgroups (C, CC, CXC, and CX3C) based on the positioning of their cysteine residues[9]. CXCL12, also known as stromal cell-derived factor-1 (SDF-1), belongs to the CXC chemokine family and CXCR4 is the only known physiological receptor for SDF-1[10]. Chemokine receptor activation can lead to growth, adhesion and, most importantly, directional migration[11]. In hematopoiesis and development, stem cells and progenitor immune cells migrate to and from various organs and tissues under the directional guidance of chemokines[12]. Chemokine migratory activation also plays a role in integrating T-cell migration during immune and inflammatory responses[13]. The discovery that chemokine receptors are expressed on nonhemopoietic cell types, such as endothelial and epithelial cells, will inevitably lead to the receptors being implicated in other biological and disease processes such as angiogenesis, organ development, metastasis and tumorigenesis. Indeed, clinical studies have already revealed that CXCR4 expression is associated with increased metastasis and decreased survival of some cancer patients[14-18].
Esophageal cancer is one of the most aggressive forms of cancer with rapid growth. Common to other cancers, the presence of lymph node metastasis and vascular invasion indicate a highly malignant potential[19]. Surgery is the treatment of choice for patients with locoregionally confined esophageal cancer, but the five-year survival rate is only 10%-30%, even after curative surgery[20]. The reason for this is that esophageal cancer shows extensive local invasion or frequent regional lymph node metastasis, often at the time of initial diagnosis. Since 1996, we have introduced preoperative chemoradiotherapy (CRT) combined with radical surgery for the treatment of esophageal cancers, and have reported that adjuvant preoperative CRT increased resectability, reduced the incidence of both local recurrence and distant metastasis, and achieved better prognosis for CRT responders[21]. However, no data are currently available on the role of CXCR4 expression in esophageal cancer progression, and the prognosis of patients undergoing CRT has not as yet been reliably estimated. In this study, therefore, we retrospectively investigated the expression of CXCR4 protein in human esophageal squamous cell carcinoma (ESCC) tissues and evaluated the clinical implications of these patients who underwent preoperative CRT and radical surgery.
MATERIALS AND METHODS
Patients and therapy
Thirty-seven patients, seven women and 30 men with a mean age of 60.32 (range 44-78) years and surgically excised ESCC were studied at the Hyogo College of Medicine, Hyogo, Japan, between April 1996 and June 2003. Preoperative CRT was performed as follows (schedule shown in Figure 1): 5-flurouracil (5-FU) (500 mg/m2 per day) was administered as a 120-h continuous intravenous infusion starting on d 1, and cisplatin (CDDP) (15 mg/m2 per day) as a 2-h iv infusion on d 1-5. Radiation therapy was performed on d 1-5, after CDDP infusion, using a linear accelerator (Mevatron KD2, Siemens, Germany). The radiation method has been previously reported[16]. Chemotherapy was combined with radiation therapy during the first week, and then radiation therapy alone was repeated for the next 3 wk (d 8-12, 15-19, and 22-26). A single dose was 2 Gy/d, with a total dose of 40 Gy.
Figure 1.
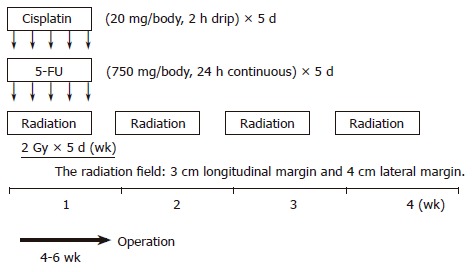
Schedule of preoperative chemoradiotherapy (CRT).
Surgery was usually performed 4 to 6 wk after the completion of CRT. Resected specimens were cut open longitudinally and fixed with formalin. Follow-up information was obtained from office charts, hospital records, and telephone interviews.
Immunohistochemistry
ESCC tissue specimens were processed using conventional procedures for paraffin embedding, cut into 4-µm sections, and mounted onto poly-L-lysine-coated slides. Sections were dewaxed in xylene, rehydrated in descending alcohols, and heated twice by microwave oven for 5 min each time in 10 mmol/L sodium citrate (pH 6.0) for antigen retrieval. They were then blocked for endogenous peroxidase with 30 mL/L H2O2 in methanol, and blocked again for non-specific antibody binding with normal rabbit serum. After then they were incubated for 2 h at room temperature with mouse mAb against human CXCR4 (R&D Systems, Minneapolis, MN, USA), followed by a standard avidin-biotin-peroxidase complex method. The slides were developed with 3, 3’-diaminobenzidine tetrahydrochloride solution containing 1 mL/L H2O2 and were lightly counterstained with hematoxylin. Normal mouse IgG was substituted for the primary antibody as a negative control. The sections were finally counterstained with Lillie-Mayer’s hematoxylin and mounted.
The sections were examined microscopically by three of the authors (R.Y., T.T., and Y.F.) without knowledge of clinicopathologic features. CXCR4 expression was categorized into four grades according to staining intensity in comparison with interstitial infiltrates[18]: score 3 (strong), staining intensity greater than interstitial infiltrates; score 2 (moderate), staining intensity equal to interstitial infiltrates; score 1 (mild), staining intensity less than interstitial infiltrates; and score 0 (negative), no staining. Additional CXCR4 expression scores were also assigned: score 3, CXCR4 high; scores 0-2, CXCR4 null or low.
Statistical analysis
Overall survival (OS) was defined as the time from the date of initial diagnosis to patient death or the date of the last available information on vital status. In univariate analysis, the difference between the cumulative survival rates of patient groups was calculated by the log-rank test for comparison using Kaplan-Meier survival curves. Statistical significance was considered at P < 0.05. Statistical analyses were carried out using Statistica statistical software, version 06J (Statistica, Tulsa, OK, USA).
RESULTS
Patient and tumor characteristics
Patient and tumor characteristics are summarized in Table 1. The patient gender bias was male (M:F, 30:7). Histology of all tumors was shown to be ESCC by histological examination. Thirty-six tumors originated in the thorax. According to the TNM system of the American Joint Committee on Cancer, stage II tumors were seen in 17 patients (46%), stage III in 13 (35.1%), and stage IV in seven (18.9%). Thirteen (35.1%) patients had lymph node metastasis (N1 in six, M1a in three, and M1b in four patients) at the time of diagnosis. All lesions before CRT presented with a T3 or T4 extent of invasion. Three-quarters of patients had tumors between 6 and 8 cm in diameter. M+ classification was described in seven tumors. Two patients had distant metastasis of the liver. All patients experienced a disease-free period. During the follow-up period, four (10.8%) patients developed local recurrence or residual tumors, six (16.2%) developed neck or celiac lymph node recurrence, and seven (18.9%) developed distant metastasis. Fifteen (40.5%) patients died during follow-up: 13 (35.1%) died from their tumors and the remaining two (5.4%) were tumor free and died of intercurrent diseases.
Table 1.
Patient characteristics
| Characteristics | n |
| Sex (M/F) | 37 (30/7) |
| Mean age (yr) | 60.32 |
| Location of tumor | |
| Cervical | 1 |
| Upper thoracic | 6 |
| Middle thoracic | 19 |
| Lower thoracic | 11 |
| T-classification | |
| T3 | 14 |
| T4 | 15 |
| N-classification | |
| N0 | 24 |
| N1 | 13 |
| M-classification | |
| M0 | 28 |
| M1 | 9 |
| Disease stage (UICC TNM stage) | |
| IIa | 17 |
| III | 13 |
| IVa | 2 |
| IVb | 5 |
Prognostic value of persistent CXCR4 expression
Eleven tumors were totally eradicated by CRT, resulting in an absence of visible tumor cells: a pathological complete response (CR). CXCR4 expression was absent from 13 tumors (35.1%). Another 13 tumors (35.1%) were positive for CXCR4 expression: 11 scored 1, one scored 2, and the last scored 3. The patient who scored 3 died of multiple lung metastasis two months after surgery. Staining was observed predominantly in the cytoplasm of tumor cells (Figure 2). In the positive CXCR4 expression group (n = 13), recurrences were found in 10 patients: two locally and eight distant in the liver, bone, thyroid gland, lung and neck lymph nodes. By contrast, in the null CXCR4 expression group (n = 11), recurrences were found in only two patients, in the liver and bone. Statistical analysis showed that distant recurrence was more common in those patients with positive CXCR4 expression (P = 0.0318).
Figure 2.
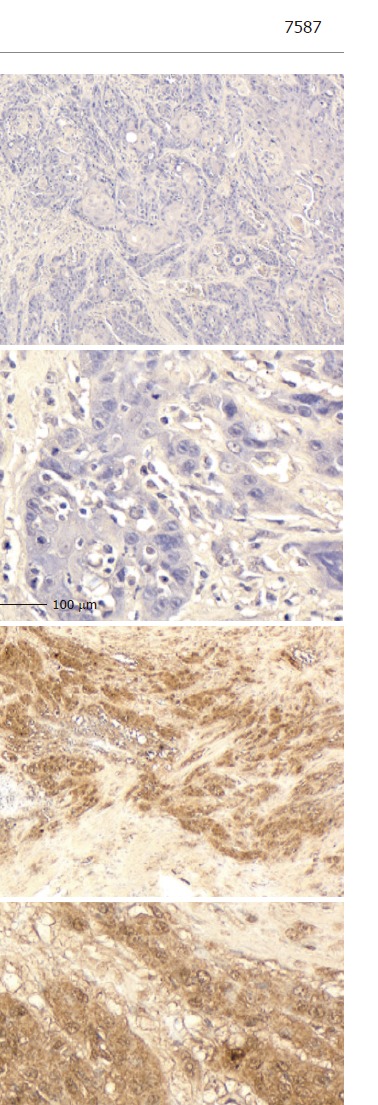
Immunohistochemical detection of CXCR4 in ESCC specimens. A: Null expression (A1: × 40; A2: × 200); B: Strong expression (B1: × 40; B2: × 200).
After follow-up, disease progression had occurred in 12 of the 13 patients with positive CXCR4 expression, and 11 of them died. Univariate analysis of prognostic factors for OS is summarized in Table 2. Lymph node metastasis and distant metastasis had a significant prognostic value (952.1 ± 53.8 d in lymph node metastasis negative group vs 475.1 ± 56.2 d in positive group, P = 0.023, and 874.0 ± 60.4 d in distant metastasis negative group vs 434.9 ± 75.2 d in positive group, P = 0.014, respectively). Furthermore, there was a very significant effect of CRT on OS prognosis (811.5 ± 51.2 d in responder group vs 459.6 ± 94.0 d in non-responder group, P = 0.00038). Kaplan-Meier analyses (Figures 3 and 4) suggest that prognosis was particularly unfavorable for patients with persistent positive CXCR4 expression in their primary tumors compared with CXCR4 null expression patients or those with no residual tumor (959.8 ± 51.0 d in CXCR4 null expression or no tumor group vs 412.0 ± 57.1 d in positive expression group, P = 0.0001). In addition, CXCR4 null expression patients showed an improved OS compared with positive expression patients (P = 0.0005).
Table 2.
Univariate analysis of prognostic factors for overall survival
| Covariate | n | P | |
| Age (yr) | < 70 | 31 | |
| ≥ 70 | 6 | NS | |
| Gender | Male | 30 | |
| Female | 7 | NS | |
| CRT | Effective | 28 | |
| Not effective | 9 | 0.00038b | |
| Lymph node metastasis | Positive | 13 | |
| Negative | 24 | 0.023a | |
| Distant metastasis | Positive | 14 | |
| Negative | 23 | 0.014a | |
| Depth of tumor invasion | T3 | 23 | |
| T4 | 14 | NS | |
| Tumor location1 | Upper | 14 | |
| Lower | 23 | NS | |
| CXCR4 expression | Positive | 13 | |
| Negative | 24 | 0.0001b |
Upper or lower: Above or below the tracheal bifurcation. NS: Not significant.
P < 0.05,
P < 0.01 comparison between two corresponding groups.
Figure 3.
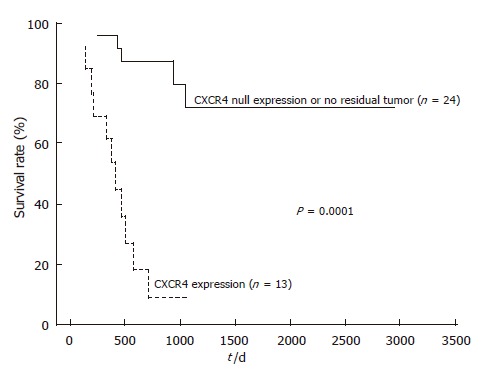
Overall survival in ESCC patients with no residual tumor or null CXCR4 expression and those with positive CXCR4 expression.
Figure 4.
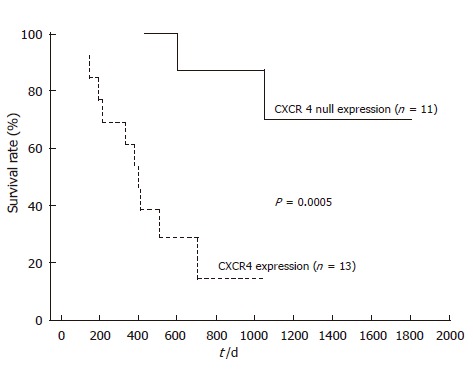
Overall survival in ESCC patients with null or positive CXCR4 expression.
DISCUSSION
The understanding of the molecular basis of tumor development has progressed dramatically in the last few decades. It is well known that esophageal cancer shows poor prognosis because of its aggressiveness; therefore, it is important to understand the role of molecular factors in the acquisition of malignant potential. The presence and number of metastases in ESCC is important both for staging and prognosis[22]. The metastatic potential of primary ESCC is considerably higher than other primary solid tumors when the size of the primary lesion is compared. Notably, in the present study, univariate analyses demonstrated that persistent CXCR4 expression was the most influential factor for OS in operable ESCC patients who underwent CRT. Patients with advanced ESCC, even in T4 stage, demonstrated improved prognosis when preoperative CRT was successful. Persistent CXCR4 expression after CRT may therefore be a useful biomarker for screening and management of high-risk patients with poor prognosis. To date, this is the first report demonstrating the prognostic role of CXCR4 expression in ESCC patients undergoing CRT.
It is likely that several mechanisms are involved in cancer metastasis including those of the “homing” signaling process described earlier. In ESCC, the signaling mechanism together with a mechanical drainage pattern might facilitate specific metastasis to lymph nodes, liver and lung. After distant metastatic cells have passed through vascular channels and implanted, a favorable “soil” might be responsible for further growth and proliferation. Frequently, these esophageal metastases occur in multiple foci, but little is known about these patterns. Furthermore, the concept that cancer implantation enacts an immune response, thus accentuating chemoattraction of CXCR4-positive tumor and immune cells, cannot be discounted[23]. It has recently been reported that CXCR4 is highly expressed in malignant but not normal breast tissue, and that CXCL12 is expressed in those organs where breast cancer metastasis is frequently found such as bone marrow, lymph nodes, lung, and liver[1]. Moreover, neutralizing the interactions of CXCL12-CXCR4 by administration of an anti-CXCR4 antibody significantly impairs the metastasis of breast cancer cells to the lung and regional lymph nodes in mice[1]. Taken together with our results, these findings indicate that the CXCL12-CXCR4 interaction is important for the metastasis of solid tumors that fail to respond to CRT. Persistent CXCR4 expression even after CRT appears to have a prognostic value, although the alteration of CXCL12-CXCR4 signaling by CRT was not directly analyzed in our study. CXCR4 antagonists have already been studied in phase I clinical trials in multiple myeloma and non-Hodgkin’s lymphoma[24-26]. These preliminary data regarding hematopoiesis suggested that AMD3100, a CXCR4 antagonist, is safe and effective in reversibly inhibiting CXCL12-CXCR4 binding and in mobilizing WBCs, CD34+ cells and hematopoietic progenitor cells (HPCs) into the circulation. Utilization of CXCL12-CXCR4 pathway may be a promising therapeutic approach in the prevention and treatment of metastasis.
In conclusion, we have demonstrated that positive CXCR4 expression after CRT correlates with distant recurrence and provides an independent prognostic factor for ESCC survival. These findings strongly suggest that CXCR4 plays an important role in ESCC progression and could provide a novel molecular target for the treatment of ESCC. Large cohort studies in a multicenter setting will be necessary to validate our findings and explore the potential use of CXCR4 antagonists in the treatment of ESCC patients with a high risk of early relapse following CRT.
COMMENTS
Background
The chemokine CXCL12, also known as stromal cell-derived factor-1 (SDF-1), and its receptor CXCR4 have been implicated in organ-specific metastases of several malignancies. Recently, we have reported that adjuvant preoperative chemoradiotherapy (CRT) reduced the incidence of both local recurrence and distant metastasis. However, the impact of CXCR4 expression on disease progression and prognosis in esophageal cancer patients undergoing preoperative CRT remains unknown.
Research frontiers
It has recently been reported that CXCR4 is highly expressed in malignant but not normal breast tissue, and that CXCL12 is expressed in those organs where breast cancer metastasis is frequently found such as bone marrow, lymph node, lungs, and the liver. Moreover, neutralizing the interactions of CXCL12-CXCR4 by administration of an anti-CXCR4 antibody significantly impairs the metastasis of breast cancer cells to the lungs and regional lymph nodes in mice.
Innovations and breakthroughs
We have demonstrated that positive CXCR4 expression after CRT correlates with distant recurrence and provides an independent prognostic factor for ESCC survival. These findings strongly suggest that CXCR4 plays an important role in ESCC progression and could provide a novel molecular target for the treatment of ESCC. To date, this is the first report demonstrating the prognostic role of CXCR4 expression in ESCC patients undergoing CRT.
Applications
Persistent CXCR4 expression after CRT may be a useful biomarker for the screening and management of high risk patients with poor prognosis. CXCR4 antagonists have already been studied in phase I clinical trials in multiple myeloma and non-Hodgkin’s lymphoma. Potential use of CXCR4 antagonists should be explored in the treatment of ESCC patients with a high risk of early relapse following CRT.
Terminology
Chemokines are signaling molecules that function in myriad cell trafficking events. They have been classified into four subgroups (C, CC, CXC, and CX3C) based on the positioning of their cysteine residues. CXCL12, also known as stromal cell-derived factor-1 (SDF-1), belongs to the CXC chemokine family and CXC chemokine receptor-4 (CXCR4) is the only known physiological receptor for SDF-1. Chemokine receptor activation can lead to growth, adhesion and, most importantly, directional migration.
Peer review
The paper is scientific and innovative contents as well as readability can reflect the advanced levels of the clinical research in gastroenterology both at home and abroad.
Footnotes
Supported by grants from the Medical Research Fund of Hyogo Medical Association
S- Editor Wang GP L- Editor Zhu LH E- Editor Liu WF
References
- 1.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 3.Paget S. The distribution of secondary growths in cancer of the breast. The Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 4.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203–7206. [PubMed] [Google Scholar]
- 5.Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62:6304–6311. [PubMed] [Google Scholar]
- 6.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 7.Bertolini F, Dell'Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, Gobbi A, Pruneri G, Martinelli G. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma. Cancer Res. 2002;62:3106–3112. [PubMed] [Google Scholar]
- 8.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 9.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 10.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 11.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 12.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 13.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- 14.Schimanski CC, Bahre R, Gockel I, Müller A, Frerichs K, Hörner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–217. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, Heinecke A, Pantel K, Izbicki JR. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst. 2005;97:1840–1847. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 17.Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 18.Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, Möhle R, Meister B. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol. 2001;115:545–553. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- 19.Sugimachi K, Matsuoka H, Ohno S, Mori M, Kuwano H. Multivariate approach for assessing the prognosis of clinical oesophageal carcinoma. Br J Surg. 1988;75:1115–1118. doi: 10.1002/bjs.1800751122. [DOI] [PubMed] [Google Scholar]
- 20.Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology. 1991;48:411–420. doi: 10.1159/000226971. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara Y, Kamikonya N, Inoue T, Koishi K, Yoshikawa R, Nakao K, Yagyu R, Nishiwaki M, Fujiwara M, Kojima S, et al. Chemoradiotherapy for T3 and T4 squamous cell carcinoma of the esophagus using low-dose FP and radiation: a preliminary report. Oncol Rep. 2005;14:1177–1182. [PubMed] [Google Scholar]
- 22.Wang LS, Chow KC, Chi KH, Liu CC, Li WY, Chiu JH, Huang MH. Prognosis of esophageal squamous cell carcinoma: analysis of clinicopathological and biological factors. Am J Gastroenterol. 1999;94:1933–1940. doi: 10.1111/j.1572-0241.1999.01233.x. [DOI] [PubMed] [Google Scholar]
- 23.Prehn RT. Stimulatory effects of immune reactions upon the growths of untransplanted tumors. Cancer Res. 1994;54:908–914. [PubMed] [Google Scholar]
- 24.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, Calandra G, DiPersio JF. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 26.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]


