Abstract
Transgenic Bacillus thuringiensis (Bt) crops play an increasing role in pest control, and resistance management is a major issue in large-scale cultivation of Bt crops. The fitness cost of resistance in targeted pests is considered to be one of the main factors delaying resistance when using the refuge strategy. By comparing 10 resistant Helicoverpa armigera (Hubner) strains, showing various resistance levels to Bt toxin (Cry1Ac), to a susceptible strain, we showed an increasing fitness cost corresponding with increasing levels of resistance. The relationship between overall fitness cost C and the resistance ratio Rr could be described by C = 24.47/(1 + exp([1.57 - Log10Rr]/0.2)). This model predicted that the maximum overall fitness cost would be ~24% (±5.22) in the strains with the highest resistance level. The overall fitness cost was closely linked to egg hatching rate, fecundity, emergence rate, larval survival rate, and developmental duration of adults. Among fitness components measured, fecundity was the most sensitive trait linked to the resistance selection. To integrate the results into simulation models would be valuable in evaluating how variation in fitness cost may influence the development of resistance in pest populations, thus helping to develop enhanced refuge strategies.
Transgenic plants producing the Bacillus thuringiensis toxins (Bt), which primarily target lepidopteran pests, have been planted over widespread areas for almost two decades. Bt corn and Bt cotton, for instance, covered 69 million hectares globally in 20121,2. Bt crops have led to a drastic drop in insecticide use as well as steadily increasing biocontrol services provided by generalist predators3,4,5. Widespread adoption of Bt crops has also benefited neighboring non-Bt crops through suppression of major insect pest populations and conservation of natural enemies3,4,5. Nevertheless, sustainability of Bt crops may be compromised due to the development of resistance by pests, e.g. field-evolved resistance has occurred in populations of several major targeted pests 7–8 years after Bt crops were first implemented6,7. Various theoretical mathematical models have suggested that the refuge strategy8, if widely adopted, would be effective in delaying insect resistance to Bt crops6,7. Analyses of more than a decade of global resistant monitoring data matched with these predictions as most targeted pests remained susceptible to Bt crops when the refuge strategy was used6,7.
The basic theory underlying the refuge strategy is to reduce resistance heritability by providing numerous susceptible moths in refuges to mate with the resistant moths from Bt crops6,9. To delay pest resistance, the main method is to promote survival of susceptible pests by planting refuges of non-Bt host plants near Bt crops6,7,8. Fitness costs associated with Bt resistance, i.e. lower fitness in resistant insects than in susceptible ones in the absence of Bt toxins, can further delay the development of resistance by selecting against Bt-resistant genotypes in refuges, and this strategy can even reverse resistance development10.
Resistance to Bt is controlled by multiple genes, and multiple resistance alleles with different types of mutations have been found on the same locus10,11. If each mutation may cause a cost, and the costs may accumulate with more genes or alleles involved in conferring a higher resistance level. Thus, fitness costs are expected to increase steadily with the development of increased resistance. Although fitness costs have been identified in various targeted pest populations, with the higher costs found linked to higher resistance levels to Bt10,12, successive changes in fitness cost due to development of resistance to Bt have not been fully demonstrated.
Fitness costs associated with Bt resistance are often detected by (i) comparing one fitness component, e.g. survival, development rate or weight, between Bt-resistant and Bt-susceptible strains in the absence of Bt toxins, or by (ii) evaluating the stability of resistance to Bt toxin(s) in heterogeneous laboratory strains reared on non-Bt plants10. The former method provides insight into which fitness components are affected by resistance, and the latter method measures the cumulative effect of all fitness components over many generations10. However, these two methods do not enable clear identification of actual fitness component(s) that may respond to selection events for resistance to Bt toxins. Moreover, it leaves unknown how to compare the various costs detected with the two different methods.
In the present study, ten resistant strains of H. armigera representing different stages of resistance development to Cry1Ac were used to compare fitness cost with a susceptible strain to (1) depict how the fitness cost changes through resistance development and (2) identify sensitive fitness components related to overall fitness cost changes and the increase of resistance level through resistance development in H. armiger to Cry1Ac.
Results
Eleven H. armigera strains were established by screening field-collected populations with different dose of Cry1Ac toxins under laboratory conditions (Fig. 1). When compared with the susceptible strain 96S, the other ten strains showed different resistance levels against Cry1Ac toxin with the BtR showing the highest resistance level (3536.5-fold) and the other strains showing intermediated resistance levels (Table S1). The intrinsic rate of population increase, rm, estimated on non-Bt diet, was the highest in the susceptible strain 96S, and dropped in the other resistant strains showing various overall fitness costs (Table S1). This enabled the assessment of a possible relationship between overall fitness cost and level of resistance to Cry1Ac toxin in H. armigera.
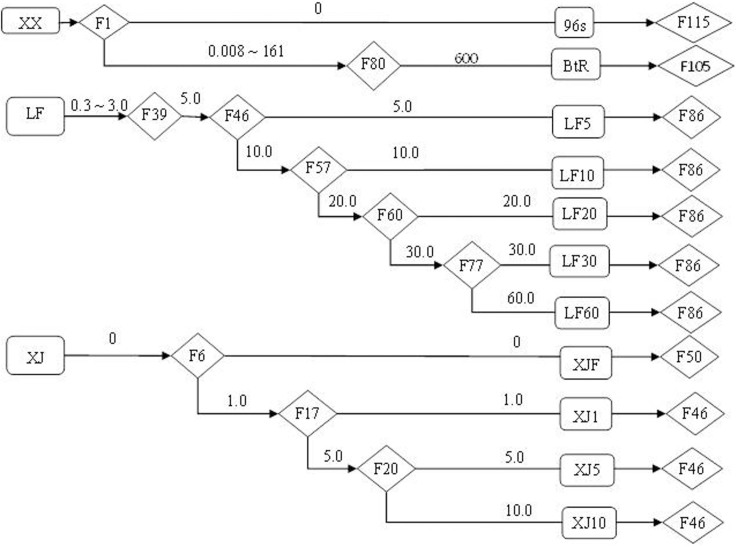
Figure 1. Flowchart of selection of 11 strains of H. armigera from three field collected populations in the laboratory.
The names of the strains were shown in rectangle frames, numbers of generations were shown in the diamond frames, doses of Bt toxins (mg Cry1Ac/L artificial diet) imposed on H. armigera larvae were shown on the arrows. The last generation of each strains were tested for fitness cost in the present study.
With linear and non-linear regression analysis, a three-parameter logistic model C = 24.47/(1 + exp([1.57 - Log10Rr]/0.2)), with the lowest AIC (Akaike's Information Criterion) value of 81.77, was established to describe the relationship between overall fitness cost and resistance level (Fig. 2). This logistic model predicted that the overall fitness cost would increase to a limit of 24.47% (±5.22) in the highest Cry1Ac-resistant H. armigera strains.
Figure 2. The increase of fitness cost with development of resistance in H. armigera to Cry1Ac.
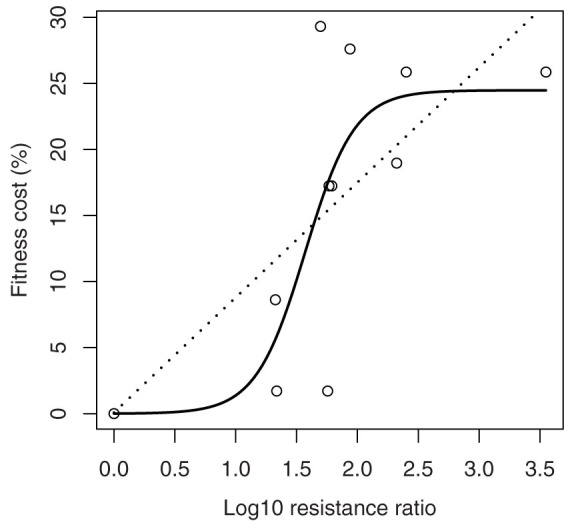
The dotted line indicates the linear tendency C = 0.09 + 8.71 Log10Rr (p = 0.023, AIC = 82.50), while the solid line indicates the logistical tendency C = 24.47/(1 + exp([1.57 - Log10Rr]/0.2)) (AIC = 81.77).
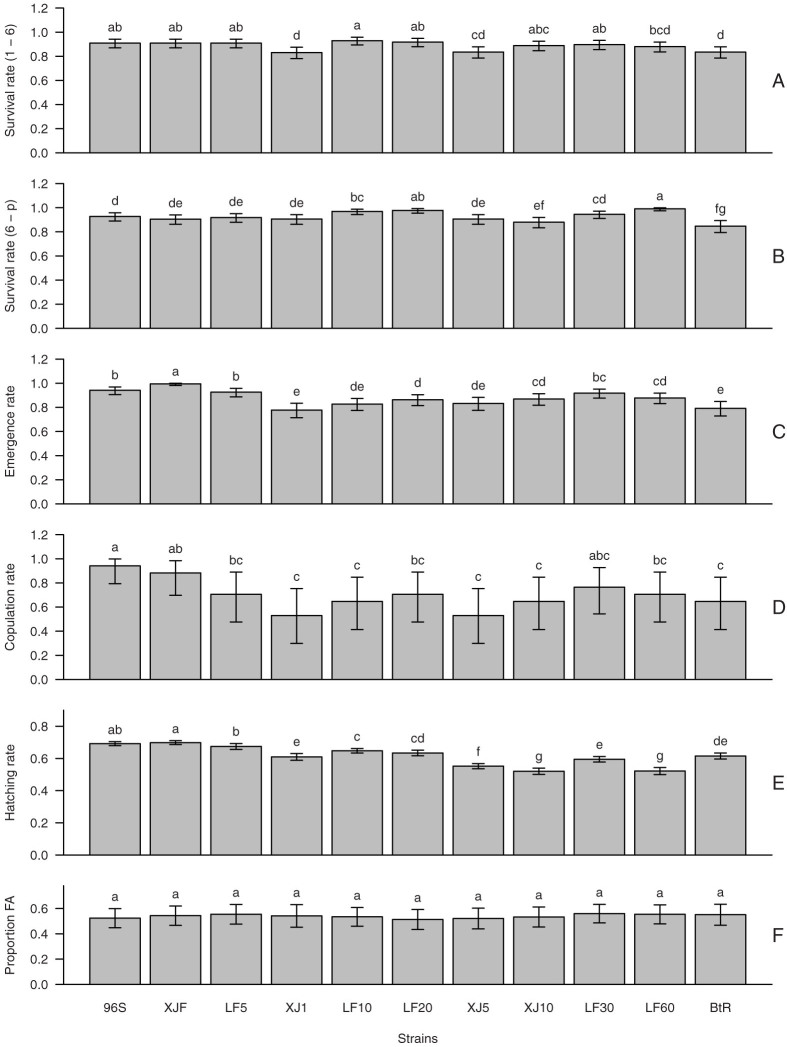
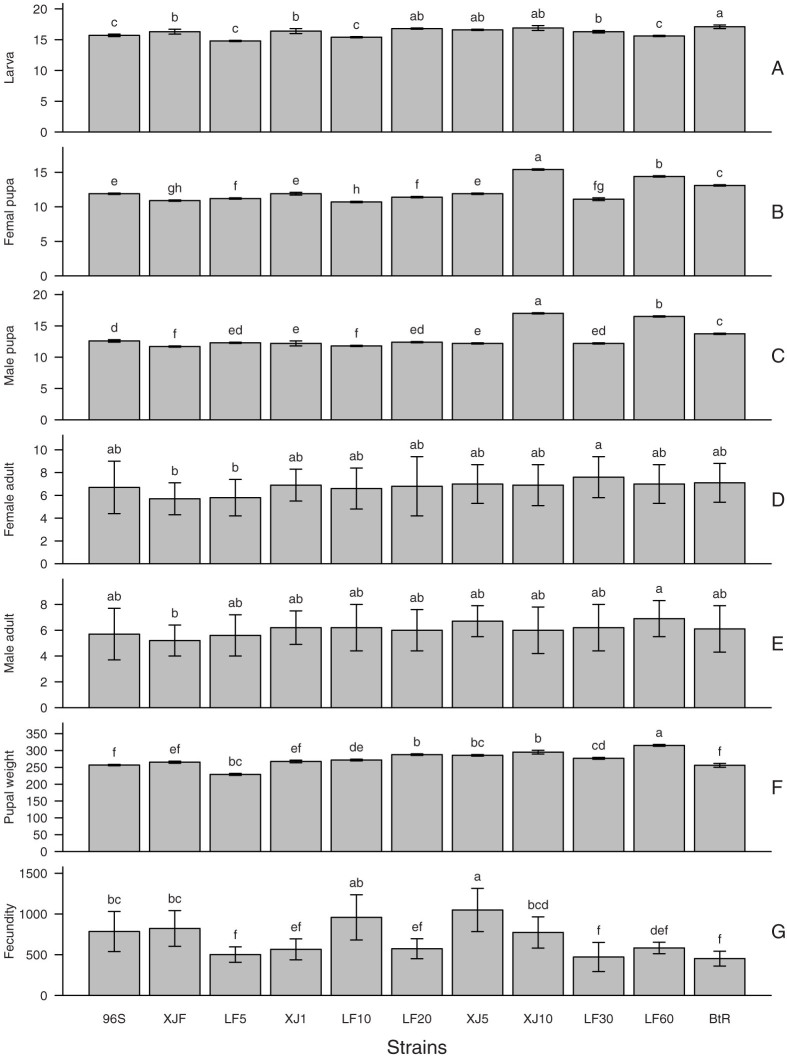
Thirteen fitness components were estimated for each strains with life tables. Comparing with the susceptible strain 96S, fitness component costs were often observed in resistant strains, i.e. they had lower survival rates, lower copulation rate, lower fecundity and longer developmental durations (Fig. 3 and 4), with exceptions for sex ratio and developmental duration of male and female adults, where no significant differences were found among the 11 strains on non-Bt diet (Fig. 3F and 4D & E). However, the variation of 10 fitness components showed various complicated trends with the increase of resistance to Cry1Ac (Fig. 3A–E and 4A–C & F–G, Fig. S1KA-KJ, Fig. S2KA-KJ & LA-LJ) or the overall fitness cost (Fig. S1LA-LJ) and thus were analyzed with multiple regressions to find the most influential fitness components.
Figure 3. Survival rate from neonates to the 6th instar larvae (A) survival from 6th instar larvae to pupa (B), emergence rate (C), copulation rate (D), hatching rate (E) and proportion of female adults (F) for each strains.
The resistance level increased through the strains 96S to BtR. Bars indicate the 95% Credible Interval. Histograms bearing different letters are significantly different at P < 0.05. The statistical analysis is based on Bayesian methods and Monte Carlo approximations (see Table S2, S3–S8 for detailed statistical results).
Figure 4. Developmental duration (days) of larva (A), female pupa (B), male pupa (C), female adult (D), male adult (E), pupal weight (F) and fecundity (G).
Bars indicated the standard error (SE). Different letters on the bars indicate significantly differences among the 11 strains (ANOVA followed by Tukey's HSD posthoc test, P < 0.05 level). Data and statistics to produce this figure were listed in Table S9.
A multiple regression model C = −1.591 + 0.03CRh2 + 0.258CFec + 0.618CRe + 0.618CRl + 0.662CDa (F5,5 = 46.7, p = 0.000338), with lowest AIC value of 54.64, was the best one to describe the contribution of fitness components to the overall fitness cost. In this model, CRh, CFec, CRe, CRl and CDa are five fitness component costs estimated using egg hatching rate, fecundity, emergence rate, larval survival rate and developmental duration of adult, and their importance decreased according to the significance of their coefficients (Table 1). We reported the scatter plots of predicted overall fitness cost against observed data in Fig. S3.
Table 1. Major influential fitness component costs to overall fitness cost and regression coefficients.
| Fitness component cost | Coefficient | Estimated coefficient | Std. Error | t value | Pr(>|t|) | Significance |
|---|---|---|---|---|---|---|
| (Intercept) | α | −1.590681 | 1.376196 | −1.156 | 0.299968 | |
| CRh2 | β1 | 0.030426 | 0.003947 | 7.708 | 0.000587 | *** |
| CFec | β2 | 0.258139 | 0.044570 | 5.792 | 0.002161 | ** |
| CRe | β3 | 0.617304 | 0.116802 | 5.285 | 0.003232 | ** |
| CRl | β4 | 0.617594 | 0.132943 | 4.646 | 0.005605 | ** |
| CDa | β5 | 0.662258 | 0.185108 | 3.578 | 0.015914 | * |
*indicates significant at significant level of 0.05.
**indicates significant at significant level of 0.01.
***indicates significant at significant level of 0.001.
A multiple linear regression model Log10Rr = 0.502 + 0.032CFec + 0.026CRc − 0.063CWp + 0.099CR6p (F4,6 = 10.54, p = 0.007) was established to describe the relationship between fitness component costs and development of resistance. The significance of coefficients and the scatter plot of predicted resistance against observed data are reported in Table 2 and Fig. S4, respectively. The variables used to predict resistance levels were similar to those predicting overall fitness cost (described above). Among these four explanatory variables, the cost in fecundity CFec, was the most important, followed by the cost in copulation rate, CRc, in pupal weight, CWp and in survival rate from the 6th instar larvae to pupae, CR6p.
Table 2. Major influential fitness components costs to resistance ratio and coefficients.
| Fitness component cost | Coefficient | Estimated coefficient | Std. Error | t value | Pr(>|t|) | Significance |
|---|---|---|---|---|---|---|
| (Intercept) | α | 0.502961 | 0.264468 | 1.902 | 0.10591 | |
| CR6p | β1 | 0.098970 | 0.047561 | 2.081 | 0.08263 | . |
| CWp | β2 | −0.062893 | 0.025234 | −2.492 | 0.04701 | * |
| CFec | β3 | 0.031711 | 0.007606 | 4.169 | 0.00589 | ** |
| CRc | β4 | 0.026279 | 0.008480 | 3.099 | 0.02115 | * |
Discussion
In the present study, the comparison of fitness in 10 resistant H. armigera strains with that in a susceptible strain indicated a logistic increase in overall fitness costs associated with the log transformed resistance level to the Bt toxin Cry1Ac. Using multiple regressions, we established models that predict overall fitness cost and resistance level with fitness costs detected in key life history traits. These models would enable researchers and extension specialists to estimate overall fitness cost or resistance level in the field populations using several key life historical traits. Our results will help modelers optimizing models that simulate selection of resistance to Cry1Ac in H. armigera; this would ultimately help in assessing efficacy of resistance management strategies (e.g. for refuge strategies, which are important for sustainable use of Bt cotton).
In the well-known simulation models, on which the current widely adopted resistance management refuge strategy is rooted, the fitness cost was often treated as a fixed one10. Although the fitness cost had been found to vary in different environments and higher costs were linked with higher resistance levels, the relationship between fitness cost and resistance level has not yet been well characterized. In the present study, the overall fitness cost seemed to increase in a non-linear manner with the Log10Rr, which described the relationship better than any linear models. The logistic model suggested that the increase in fitness cost of the resistance to Cry1Ac may asymptotically reach a maximum of 24.47% (±5.22). This is actually quite similar to the standard value of 25% suggested for modelers in 2009 by Gassmann et al.10. The logistic model was not only statistically significant but also biologically meaningful.
To explain the logistic increase of the overall fitness cost with the Log10Rr, we hypothesized that development of resistance to Cry1Ac might occur throughout three distinct phases in H. armigera. In the first phase, we assumed that only physiological adaptations (a common phenomenon in arthropods to any hazard that causes no obvious fitness costs13) were involved and the fitness cost increased very slowly. In a second phase, we assumed that selection of genetic mutations conferring high level of resistance was involved. The regulation of resistance genes, such as over-expression of detoxification enzymes14,15,16,17,18,19,20,21, change in hormones induced by detoxification enzymes, and structural modification of targeted genes14,17,22,23, has been considered the most important factor producing fitness cost. In this phase, fitness cost increased quickly with an increasing number of mutated genes because of the pleiotropic effects of these genes. In a third phase, the fitness cost reached a plateau. This might result from new mutations providing higher resistance level but fewer pleiotropic effects, or from compensatory mutations (other mutations) that might have been selected. Alternatively, new mutations conferring higher resistance levels but lower fitness cost could have been selected and have taken over resistance alleles that caused high fitness costs.
The logistic increase of overall fitness cost with the Log10Rr was observed for H. armigera strains selected on artificial diet with added toxin in laboratory, rather than on cotton plants in the field, or collected directly in the field. Thus the logistic relationship and the above explanations might have some limitations and should be cautious to apply to the field evolved cases. In the laboratory, the mutations conferring higher resistance were always selected no matter how high the accompanied fitness cost was. However, if the mutations conferring no or lower fitness cost exist, they would have always been selected firstly in the field, because the fitness cost may counterbalance the benefit conferred by resistant mutation on refuge crops. This has been demonstrated in the field-evolved cost free resistant populations of the fall armyworm Spodoptera frugiperda24, the western corn rootworm, Diabrotica virgifera virgifera25 and the sugarcane borer Diatraea saccharalis26 on Bt maize. The D. virgifera virgifera have no fitness cost even for laboratory selected strains27. These three species evolved resistance in the field might be lack of mutations with fitness costs. This reflected some intrinsic diference between D. virgifera virgifera and H. armigera or difference in complicated interactions between pest and host exhibited by corn pests and cotton pests. For those species with fitness cost being widely identified in laboratory, fitness cost must have been playing an importance role in delaying resistance, such as in H. armigera.
In our study, we have considered the overall fitness cost with the actual time elapsed since strains were established in the laboratory (i.e. number of generations kept on a constant dose of Cry1Ac toxin since resistant strains were established) to detect potential compensatory effects. However, in our study, the elapsed time since establishment was significantly related to resistance level. Therefore we could not rule out the influence of development of resistance in the observed fitness cost increase when testing for compensatory mutation effects. It would be valuable to collect fitness cost data chronologically while the resistance strains are kept on a constant dose of toxin, with the ideal situation of keeping resistance level constant. Such a study would also help clarify whether a cost-free resistance to Bt toxin Cry1Ac may evolve. In terms of practical applications, such possibility of cost-free resistance would prompt re-thinking of the refuge method to maintain satisfactory management of resistance in targeted pests (H. armigera in Bt cotton in the present case).
The logistic model was established using strains showing moderate resistance levels (LF and XJ series) and one showing high resistance levels (XX series). Therefore, our findings would need to be confirmed through additional studies, notably using strains that would show higher resistance levels in the LF and XJ series. In addition, using pooled data from three series of resistant strains might not have been optimal conditions for assessing how fitness cost can vary with resistance level, notably because the three series of strains might have showed differences in genetic background (owing to field populations collected from three different locations). However, it has been demonstrated that there were four distinct H. armigera geotypes (geographical genotypes) in China28,29, and the three H. armigera strain series used for our study all came from the same genotype. Therefore, the three strain series did share similar genetic backgrounds, and pooling data was valid in our study. Genetic deviations could exist among different populations; however, such differences could not have been enough to cause the important differences in life history traits that we observed among the populations. This conclusion is also consistent with the lack of significant difference in linear regression slopes between LF and XJ series. Therefore, the costs detected in the various strains can be attributed confidently to the development of resistance to Cry1Ac.
A meta-analysis suggested that fitness costs were more frequently detected in selection experiments than in fitness component comparisons10. The present study provided an explanation by analyzing the correlation between overall fitness cost and each fitness component cost. With multiple regression analyses, we demonstrated that the egg hatching rate (squared), fecundity, emergence rate, larval survival rate, and developmental duration of adults (ranked in decreasing importance) affected the overall fitness cost. Although costs in developmental duration of larvae and pupae were highly correlated with the overall fitness cost (pairwise correlation coefficients, Fig. S2CL and EL), both were discarded through multiple regressions. This might be because (1) the developmental duration of larvae varied among strains in LF series, whereas it did not in XJ series (Table S9) and (2) the developmental duration of pupae did not change much in strains showing low resistance levels, but it did increase in strains showing the highest resistance levels in each series (Table S9). The strains with the highest resistance level in XJ and LF series also had the shortest elapsed time for establishment (Fig. 1). This implied that the cost in developmental duration of pupae might have been compensated in those strains with lower resistance levels; therefore, this trait should be considered in future studies aiming to understand the compensation of fitness costs with development of resistance to toxins in arthropods, or at least in the case of H. armigera.
Overall, a low probability of detecting fitness cost when comparing life history traits may be due to (i) an absence of cost related to Cry1Ac resistance in various life history traits in H. armigera (e.g. sex ratio), (ii) a quick compensation of the cost in some traits (e.g. developmental duration of pupa), and to (iii) random variations in traits evaluated.
We identified the life history traits linked to the development of resistance to Cry1Ac in H. armigera. Both fecundity and larval survival were identified as important traits influencing overall fitness cost related to the resistance to Cry1Ac. The survival from 6th instar larva to pupal stage was actually more sensitive than survival of the whole larval stage when assessing development of resistance to the Bt toxin. Pupa is the life stage undergoing important physiological and morphological transformation; it can therefore be the crucial stage to reveal disadvantages linked to the selection of mutations conferring resistance to Bt toxin. Because Cry1Ac toxin primarily targets the midgut of the larval stage of Lepidoptera, and reduction or loss of binding of Bt toxin to midgut membrane receptors is the most common general mechanism of resistance30,31,32, it has been hypothesized that mutations conferring resistance to Bt toxins might cause fitness costs by reduction of nutrient uptake in guts of the Lepidopteran pests (e.g. by increasing permeability of the gut membrane to toxic phytochemicals or pathogens, or by impairing protein digestion)10. Our findings suggest that such mutations may be particularly detrimental when the larvae pupate.
Methods
Insect strains
Eleven H. armigera strains were reared under laboratory conditions (27 ± 2°C, 75 ± 10% RH, 14L:10D). According to the origin of the strains, they were grouped into 3 series: (i) the XX series included the 96S and BtR strains (ii) the LF series included the LF5, LF10, LF20, LF30 and LF60 strains, and (iii) the XJ series included the XJF, XJ1, XJ5 and XJ10 strains (Fig. 1). The original populations of the three series of strains were all collected from Yellow River Region and were subject to various screening programs to produce strains with different resistance levels (Fig. 1 and supplementary text). All bioassays described below were carried out in similar conditions.
Bioassay of resistance to Cry1Ac toxin
The H. armigera strains were reared and assessed simultaneously for susceptibility to Cry1Ac using the methods reported by Liang et al.12,33. In brief, the susceptibility was evaluated by feeding H. armigera larvae with artificial diets containing various concentrations of Cry1Ac toxin. Four-day-old larvae were placed individually on Cry1Ac-contaminated artificial diet in plastic wells (depth: 1.5 cm, vol. 3 ml) in 24-well plates. The plates were covered with a plastic lid to prevent escape and 96 larvae were tested per Cry1Ac concentration. After seven days, we recorded the mortality rate and measured body mass of individuals still alive. Both dead larvae and those with a body mass of less than 5 mg were recorded as dead34. For each strain, the Lethal Concentration 50 (LC50) value was determined using a probit analysis35 and the resistance level to Cry1Ac indicated by the resistance ratio was calculated with LC50 of the tested strains divided by the LC50 of the susceptible strain.
Assessment of fitness cost
Thirteen fitness components (life history parameters) were obtained with life table techniques (Supplementar text). The components considered were (i) survival rate from the 1st to 6th instar larvae, (ii) survival rate from the 6th instar larvae to pupae, (iii) duration of larval development, (iv) pupal weight, (v) developmental duration of female pupa, (vi) developmental duration of male pupa, (vii) emergence rate, (viii) proportion of females (or sex ratio), (ix) copulation rate, (x) developmental duration of female adult and (xi) of male adult, (xii) fecundity, and (xiii) hatching rate.
Each fitness component cost, Ci, for each strain was calculated as:
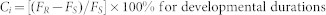 |
or
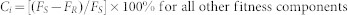 |
where FS and FR are the mean values of fitness components for susceptible and resistant strains, respectively10.
For each strain, the intrinsic rate of population increase, rm, was calculated basing on the life table data with the methods described by Bird and Akhurst36:
 |
where T is the developmental time from egg to adult eclosion.
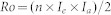 |
where n is the mean number of eggs per female, Ie is the proportion of fertile eggs, Ia is the proportion of reproductive adults produced from the 1st instar larvae, and 2 is the sex ratio coefficient for H. armigera.
The overall fitness cost C was then calculated as:
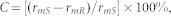 |
where rmS and rmR are the intrinsic rate of population increase for susceptible and resistant strains, respectively.
The Ci and C were calculated with reference to either the susceptible strain 96S or the relative susceptible strains in each series (i.e., LF5 and XJF for LF and XJ series, respectively), and calculated negative values (if any) were converted to 0.
Statistical analysis
The survival rates were calculated using the Bayesian method and compared among strains with Monte Carlo approximations37 (Supplementary text). The means of developmental duration, weight and fecundity were compared among strains using an ANOVA. The resistance ratio was log10 transformed to normalize the data distribution before regression analysis.
To assess how the fitness cost varied with the development of resistance, a series of linear and nonlinear regressions were performed for each series of strains separately and for pooled data (Supplementary text). Multiple linear regressions were used to analyze how each fitness component cost influenced the overall fitness cost and resistance level (Supplementary text).
The above analysis was carried out for overall fitness costs calculated with reference to either the susceptible strain 96S or the relative susceptible strains in each series (i.e., LF5 and XJF for LF and XJ series, respectively). Only the former produced satisfied results (i.e., statistically significant results) and reported here. All statistics were performed with R38.
Author Contributions
G.C. and K.W. designed the study. G.C. and F.G. performed all the experiments. G.C., H.F. and K.W. analyzed data and wrote the manuscript. G.L. provided the insect and Cry1Ac. G.C., X.L., K.W. and N.D. shared in the scoping and writing responsibilities. All authors have read and approved the manuscript for publication.
Supplementary Material
Supplementary information
Acknowledgments
This research was supported by the Key Project for Breeding Genetically Modified Organisms (2014ZX08012-004) and China Postdoctoral Science Foundation (20080440053).
References
- James C. ISAAA Brief No. 44: Global Status of Commercialized Biotech/GM Crops: 2012. Ithaca NY: ISAAA (2012). [Google Scholar]
- Sanahuja G., Banakar R., Twyman R., Capell T. & Christou P. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotech. J. 9, 283–300 (2011). [DOI] [PubMed] [Google Scholar]
- Wu K. M., Lu Y. H., Feng H. Q., Jiang Y. Y. & Zhao J. Z. Suppression of cotton bollworm in multiple crops in china in areas with Bt toxin–containing cotton. Science 321, 1676–1678 (2008). [DOI] [PubMed] [Google Scholar]
- Hutchison W. D. et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 330, 222–225 (2010). [DOI] [PubMed] [Google Scholar]
- Lu Y. H., Wu K. M., Jiang Y. Y., Guo Y. Y. & Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–365 (2012). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E., Brévault T. & Carrière Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31, 510–521 (2013). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E., Brévault T. & Carrière Y. Insect resistance to genetically engineered crops: successes and failures. ISB news report. (2014).
- Lu Z. Z., Zalucki M. P., Perkins L. E., Wang D. Y. & Wu L. L. Towards a resistance management strategy for Helicoverpa armigera in Bt-cotton in northwestern China: an assessment of potential refuge crops. J. Pest. Sci. 86, 695–703 (2013). [Google Scholar]
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 43, 701–726 (1998). [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Carrière Y. & Tabashnik B. E. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 54, 147–163 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang H. N. et al. Diverse genetic basis of field evolved resistance to Bt cotton in cotton bollworm from China. Proc. Natl. Acad. Sci. USA 109, 10275–10280 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G. M. et al. Changes of inheritance mode and fitness in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin. J. Invertebr. Pathol. 97, 142–149 (2008). [DOI] [PubMed] [Google Scholar]
- Liu N. N., Zhu F., Xu Q., Pridgeon J. W. & Gao X. W. Behavioral change, physiological modification, and metabolic detoxification: mechanisms of insecticide resistance. Acta. Entom. Sin. 49, 671–679 (2006). [Google Scholar]
- Chevillon C., Raymond M., Guillemaud T., Lenormand T. & Pasteur N. Population genetics of insecticide resistance in the mosquito Culex pipiens. Biol. J. Linn. Soc. Lond. 68, 147–157 (1999). [Google Scholar]
- Gunning R. V., Dang H. T., Kemp F. C., Nicholson I. C. & Moores G. D. New resistance mechanism in Helicoverpa armigera threatens transgenic crops expressing Bacillus thuringiensis Cry1Ac toxin. Appl. Environ. Microbiol. 71, 2558–2563 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. H., Han L. Z. & Zhang C. Y. Influence of the intrinsic rate of natural increase for different malathion-resistant genotypes on the evolution of resistance in culex pipiens pallens. Acta. Entom. Sin. 33, 385–392 (1990). [Google Scholar]
- Bourguet D. et al. Independent duplications of the acetylcholinesterase gene conferring insecticide resistance in the mosquito Culex pipiens. Genetics 147, 1225–1234 (1997). [DOI] [PubMed] [Google Scholar]
- Boyera S., Parisc M., Jego S., Lempérière G. & Ravanel P. Influence of insecticide Bacillus thuringiensis subsp. israelensis treatments on resistance and enzyme activities in Aedes rusticus larvae (Diptera: Culicidae). Biol. Control. 62, 75–81 (2012). [Google Scholar]
- Roush R. T. & Plapp R. W. Jr. Effects of insecticide resistance on biotic potential of the house fly (Diptera: Muscidae). J. Econ. Entomol. 75, 708–713 (1982). [DOI] [PubMed] [Google Scholar]
- McKenzie J. A., Whitten M. J. & Adena M. A. The effect of genetic background on the fitness of diazinon resistance genotypes of the Australian sheep blowfly, Lucilia cuprina. Heredity 49, 1–9 (1982). [Google Scholar]
- Teesea M. G. et al. Gene identification and proteomic analysis of the esterases of the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 40, 1–16 (2010). [DOI] [PubMed] [Google Scholar]
- Caccia S. et al. Association of Cry1Ac toxin resistance in Helicoverpa zea (Boddie) with increased alkaline phosphatase levels in the midgut lumen. Appl. Environ. Microbiol. 78, 5690 –5698 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. C., Schuler M. A. & Berenbaum M. R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52, 231–253 (2007). [DOI] [PubMed] [Google Scholar]
- Jakka S. R. K., Knight V. R. & Jurat-fuentes J. L. Fitness costs associated with field-evolved resistance to Bt maize in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 107, 342–351 (2014). [DOI] [PubMed] [Google Scholar]
- Hoffmann A. M., French B. W., Jaronski S. T. & Gassmann A. A. J. Effects of entomopathogens on mortality of western corn rootworm (Coleoptera: Chrysomelidae) and fitness costs of resistance to Cry3Bb1 maize. J. Econ. Entomol. 107, 352–360 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Fitness costs and stability of Cry1Ab resistance in sugarcane borer, Diatraea saccharalis (F.). J. Invertebr. Pathol. 117, 26–32 (2014). [DOI] [PubMed] [Google Scholar]
- Petzold-Maxwell J. L., Cibils-Stewart X., French B. W., & Gassmann A. J. Adaptation by western corn rootworm (Coleoptera: Chrysomelidae) to Bt maize: Inheritance, fitness Costs, and feeding preference. J. Econ. Entomol. 105, 1407–1418 (2012). [DOI] [PubMed] [Google Scholar]
- Wu K. M. & Guo Y. Y. The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 50, 31–52 (2005). [DOI] [PubMed] [Google Scholar]
- Wu K. M. & Guo Y. Y. Geotype differentiation and regional migratory regularity of Helicoverpa armigera in China. Plant Protec. 33, 6–11 (2007). [Google Scholar]
- Morin S. et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. USA 100, 5004–5009 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. J., Yu L. Y. & Wu Y. D. Disruption of a cadherin gene associated with resistance to Cry1Ac {delta}-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 71, 948–954 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J., Chen H. Y., Wu Y. D., Yang Y. H. & Wu S. W. Mutated cadherin alleles from a field population of Helicoverpa armigera confer resistance to Bacillus thuringiensis toxin Cry1Ac. Appl. Environ. Microbiol. 73, 6939–6944 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G. M., Tan W. J. & Guo Y. Y. Studies on the resistance screening and cross-resistance of cotton bollworm to Bacillus thuringiensis (Berliner). Sci. Agric. Sci. 33, 46–53 (2000). [Google Scholar]
- Yang Y. H. et al. Introgression of a disrupted cadherin gene enables susceptible Helicoverpa armigera to obtain resistance to Bacillus thuringiensis toxin Cry1Ac. Bull. Entomol. Res. 99, 175–181 (2009). [DOI] [PubMed] [Google Scholar]
- Finney D. J. Probit Analysis. (Cambridge University Press, Cambridge 1971).
- Bird L. J. & Akhurst R. J. Relative fitness of Cry1A-resistant and -susceptible Helicoverpa armigera (Lepidoptera: Noctuidae) on conventional and transgenic cotton. J. Econ. Entomol. 97, 1699–1709 (2004). [DOI] [PubMed] [Google Scholar]
- Hoff P. D. A First Course in Bayesian Statistical Methods. (Springer, New York 2009). [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. (2013) Date of access: 19/05/2013. URL http://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information