Abstract
AIM: To analyze the biological role of the surface antigen of Toxoplasma gondii (T gondii) in development of vaccine.
METHODS: The surface antigen of T gondii (SAG1) was expressed in vitro. The immune response of the host to the antigen was investigated by detection of specific antibody reaction to SAG1 and production of cytokines. Mice were immunized with recombinant SAG1 and challenged with lethal strain of T gondii RH. The monoclonal antibody to r-SAG1 was prepared and used to study the effects of SAG1 on T gondii tachyzoites under electromicroscope.
RESULTS: The mice immunized with recombinant SAG1 delayed death for 60 h compared to the control group. The recombinant SAG1 induced specific high titer of IgG and IgM antibodies as well as IFN-γ, IL-2 and IL-4 cytokines in mice. In contrast, IL-12, IL-6 and TNF-α were undetectable. When T gondii tachyzoites were treated with the monoclonal antibody to r-SAG1, the parasites were gathered together, destroyed, deformed, swollen, and holes and gaps formed on the surface.
CONCLUSION: SAG1 may be an excellent vaccine candidate against T gondii. The immune protection induced by SAG1 against T gondii may be regulated by both hormone- and cell-mediated immune response.
Keywords: Toxoplasma gondii, Recombinant SAG1, Monoclonal antibody, Cytokines, Morphology change
INTRODUCTION
Toxoplasma gondii (T gondii) is an intracellular coccidian parasite and causes the most common parasitic disease of animals and human beings[1]. The definitive hosts for the parasite are members of the Felidae family. The clinical manifestations associated with feline toxoplasmosis are anorexia, weight loss, lethargy, dyspnoea, ocular signs, pyrexia, vomiting and diarrhea, jaundice, myositis and abortion. Humans become infected when they ingest the Toxoplasma at infective stages (oocysts and tissue cysts) found in some cat feces and in raw meats. People weak in immune function may develop severe diseases such as encephalitis, pneumonia or other life-threatening conditions. Infants born with congenital toxoplasmosis may develop permanent diseases such as mental retardation or eye, liver and brain diseases. In cirrhotic patients, Toxoplasma IgG and IgM antibody positivity is as high as 68.5%[2]. In patients with AIDS, T gondii colitis can occur[3]. In veterinary medicine, T gondii infection may influence economics due to neonatal loss in sheep and goats[4], or as a source of transmission to humans[5]. Thus, it is of great value to develop an effective vaccine against T gondii.
The characterization of the molecules which play the role in the pathogenesis and immune protection is the important step in vaccine development. The surface of T gondii is the first component to contact with the host cells and the surface antigen of the parasite is recognized as the major study target. It was reported that there are 5 proteins in the superfamily of the surface antigens (SAG) of T gondii, including SAG1, SAG2 (22 Ku), p23, p35, and SAG3 (43 Ku). SAG1 is a 30 Ku glycoprotein and is therefore designated as SAG1/P30[6] and can be detected in the tachyzoite and sporozoite stages[7,8]. It was reported that SAG1 can elicit a lethal inflammatory process in mouse model of pathogen-driven ileitis[9]. However, the biological role of this surface protein remains unclear. In the current study, the biological function of SAG1 was studied through the analysis of the induction of specific antibody, elicitation of specific cytokines by the recombinant protein for SAG1 (r-SAG1 or r-P30) of T gondii. The immune protection ability of SAG1 was studied by challenging experiments. The results show that SAG1 is a very important protein and its biological function is regulated by multiple mechanisms.
MATERIALS AND METHODS
Parasites and antigens
T gondii RH tachyzoites were maintained by two weekly passages of tachyzoites to peritoneum of BALB/C mouse. Four days later parasites in the peritoneal fluid were collected and the cavity was washed with 5 mL of phosphate buffered saline (PBS). The total tachyzoite crude antigen was obtained from washed and pelleted tachyzoites, resuspended in PBS and freeze-thawed three times, then subjected to 2 cycles of ultrasound disruption (UTR200) for 10 min and incubated at 37 °C for 2 h with 1% decanoyl-N-methylglucamide (MEGA 10, Sigma). After centrifugation at 36 000 r/min for 30 min, the pellet was discarded and the supernatant was aliquoted and stored at -70°C. The protein concentration was determined by BCA assay (Pierce) using BSA as standard.
Cloning and expression of SAG1 gene in E. coli
About 5 × 107 T gondii RH strain tachyzoites were concentrated by centrifugation, washed with PBS, then lysed in 0.1 mol/L Tris-HCl (pH 8.0) containing 1% sodium dodecyl sulphate (SDS), 0.1 mol/L NaCl and 10 mmol/L EDTA and then treated with proteinase K (100 µg/mL) at 55°C for 2 h. The genomic DNA was extracted by phenol/chloroform method followed by ethanol precipitation. After centrifugation the pellet was dissolved in TE buffer (10 mmol/L Tris-HCl, pH 8.0 and 1 mmol/L EDTA) and used as a template for polymerase chain reaction (PCR) amplification, which used the primers (5’ TGGtttcactcttaagtgccctaaaacagc-3’and 5’ ctgcattaacctgcagccccggcaaactc-3’) together with PCR buffer, dNTP and Taq polymerase. The amplified SAG1 gene was inserted into the NcoI and HindIII sites of the plasmid pET-30a, and expressed as a His-tag fusion protein in E. coli BL21 (DE3) strain according to the manufacturer’s instructions. The transformed bacteria were centrifuged and lysed by a combination of detergent Triton X-100, lysozyme and ultrasonication. The suspension was centrifuged and the pellet was dissolved in 8 mol/L urea solution containing 50 mmol/L Tris-HCl (pH 8.0), 1 mmol/L dithiothreitol (DTT) and 1 mmol/L EDTA. One hour after incubation at room temperature (RT), the supernatant was contrifuged and dialyzed at 4°C followed by 2 mol/L urea solution at 4°Cfor 1 h each. Dialysis was done twice in 50 mmol/L Tris-HCl (pH 8.0) with 1 mmol/L DTT at 4°C and each lasting for 1 h. This was followed by overnight dialysis at 4°C in the same buffer. The dialyzed sample was centrifuged and the supernatant was recovered and used as antigen.
Immunization and challenge
Five to 7-wk-old female BALB/c mice (purchased from Shandong University) housed under approved conditions of the animal research facility, were used in this study. Twenty-one BALB/c mice were immunized at two locations at the base of the tail with 30 µg of T gondii recombinant SAG1 in 0.1 mL of saline, which was emulsified with an equal volume of complete Freund’s adjuvant (CFA) (Sigma, USA). Sixteen mice were injected with PBS. Booster was done 2 wk after the first injection using Freund’s incomplete adjuvant for the r-SAG1 group. Sera were collected at 3 wk post immunization for antibody test. For cytokine test, mice were killed at 6 wk post immunization. The mice inoculated with r-SAG1 of T gondii were challenged intraperitoneally with 1×105 tachyzoite forms of T gondii RH strain 9 wk after the first immunization.
ELISA
To measure antigen-specific antibodies, plates were coated overnight at 4°C with 10 µg/mL solution of antigen in 0.05 mol/L potassium phosphate buffer pH 8 (50 µL per well). Blocking was carried out with 2% BSA in PBS (pH 7.2) for 2 h at RT. After washed with PBS containing 0.05% Tween 20 (PBST20), sera were diluted in 1% BSA-PBST20 (50 µL per well) and incubated for 1 h at RT. After washed, bound antibodies were detected by incubation at RT for 1 h with horseradish peroxidase-labeled goat anti-mouse immunoglobulins at 1:2 000 dilution in 1% BSA-PBST20 (50 µL per well). Peroxidase activity was revealed by adding 50 µL per well of a solution containing 12.5% H2O2, 0.1 mol/L citrate-phosphate pH 4 and 10 mg/mL of TMB. The reaction was stopped by adding 50 µL of 2 mol/L H2SO4 and the absorbance (A) was read at 450 nm in an ELISA microplate reader (Bio-Rad, USA). A sample was considered positive when A > A mean + 3 SD (cut off), where A is that of the tested sample, and A mean and SD are the mean and the standard deviation of the A of the sample from PBS, respectively.
SDS-PAGE and immunoblotting
SDS-PAGE was performed on 12% polyacrylamide gels according to the procedure of Laemmli[10] using a total tachyzoite extract or r-SAG1. Gels were transferred to nitrocellulose membranes as previously described[11] and blocked in PBS containing 5% nonfat dry milk. Membranes were incubated with primary Abs and then with anti-species alkaline phosphatase conjugates, both were diluted in PBS containing 1% nonfat dry milk. The alkaline phosphatase activity was detected with the ProtoBlot nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate color development system (Promega, Madison, WI).
Cytokine determination
At 6 wk post immunization, mice were sacrificed and single-cell suspension was prepared as previously described[12]. A volume of 10 µg/mL of r-SAG1 of T gondii was added and the supernatants were collected from cultures after 72 h and assayed by ELISA for cytokines. The monoclonal antibody (mAb) was used for assay of IL-6 (MP5-20F3), IL-2 (JES6-1A12), IL-12 (9A5), IL-4 (BVD4-1D11), TNF-α (TN3-19.12), IFN-γ (AN-18) (BD Pharmingen).
Preparation of monoclonal antibodies
Monoclonal antibodies against recombinant SAG1 of T gondii were obtained by fusion of SP2/0 myeloma cells with the spleen cells of BALB/c mice immunized with r-SAG1 in the presence of polyethylene-glycol (Sigma) according to standard protocols. Hybrid cells were cultured in hypoxantine, aminopterin and thymidine (HAT) in DMEM/20% FBS medium. Clones growing in selected media were expanded and tested for antibody production. Positive clones were cloned twice by limiting dilution. Hybridomas were screened for specific antibody production by ELISA and immunoblot against the T gondii antigen. The identification of mAb isotype was made by ELISA using goat anti-mouse IgG subclasses (Nordic) appropriately diluted in PBS containing 0.1% Tween-20, 2% normal goat serum, followed by incubation with alkaline phosphatase conjugated rabbit anti-goat IgG (Sigma). Hybrid cells producing mAbs were injected to mouse intraperitoneally and after 2 wk ascites fluid was collected.
Electron microscopy
T gondii strains were harvested from BALB/C mice and washed twice with 0.05 mol/L pH7.2 PBS. The ascites fluid mAb against r-SAG1 was added (1:100) to the parasites. After incubation at 37°C for 1h, the parasites were washed 3 times with PBS and fixed in 4% paraformaldehyde, 0.05% glutaraldehyde in cacodylate buffer + 3% sucrose for 2 h at RT. After dehydration and embedding, the slides were observed under electron microscope (Hitachi 600, Hitachi, Japan).
RESULTS
Characterization of SAG1 recombinant protein
To study the biological function of the P30 gene encoding SAG1, the P30 gene of T gondii RH strain was amplified by PCR, the PCR product was subcloned into bacterial expression vector pET-30a and SAG1 was expressed as Histidin-fusion protein in E. coli. The gene was sequenced and the recombinant SAG1 was analyzed by Western blotting. A strong band at 30 Ku position was found after reaction with serum from mice infected with T gondii (Figure 1), indicating that the P30 gene could encode a functional protein.
Figure 1.
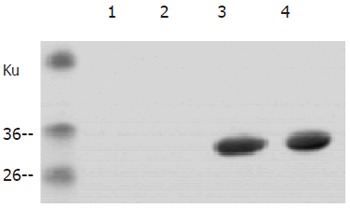
A strong band at 30 ku position after reaction with serum from mice infected with T. r-SAG1 of Toxoplasma gondii tachyzoites. The band was electrophoresed on SDS-12% polyacrylamide gel and then transferred onto a nitrocellulose membrane probed with sera (diluted at 1:100) from PBS-inoculated mice (lanes 1 and 2) or from mice infected with crude antigens of T. gondii (lanes 3 and 4). Bound antibodies were detected using anti-mouse-alkaline phosphatase conjugate. The positions of molecular masses are shown on the left.
Immunization with r-SAG1 protein could protect mice from a lethal T gondii challenge
To test whether r-SAG1 could afford protection against a lethal T gondii RH strain infection, BALB/c mice were immunized with r-SAG1 as described in the methods. Nine weeks after the first injection, the animals were intraperitoneally infected with 1×105 tachyzoites of the highly virulent RH strain. The death number of the mice was counted after challenge. It was found that after challenge with RH tachyzoites, mice started to die on day 4 in PBS-inoculated control group. On day 7, all the mice in control group were dead. However, mice immunized with r-SAG1 delayed death for 60 h compared to those in the control group (Figure 2).
Figure 2.
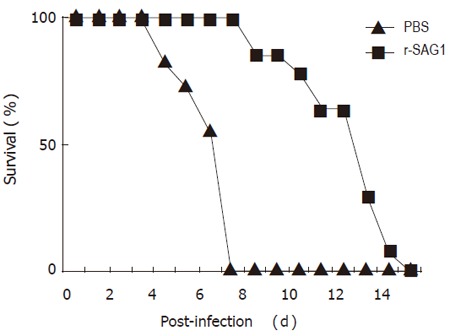
Protection of r-SAG1 against infection with tachyzoites of Toxoplasma gondii. Sixteen BALB/C mice were immunized with r-SAG1 of T. gondii and 11 mice were injected with PBS as a control. The mice were challenged with 1X105 tachyzoites of Toxoplasma gondii RH at 9 wk post immunization. The number of the dead mice was counted.
r-SAG1 induced specific antibody response
To investigate whether r-SAG1 could induce humoral immune response, the sera from mice were collected 3 wk after the last immunization. The titer and classes of immunoglobulin were tested by ELISA. It was obvious that vaccination of the mice with r-SAG1 induced a strong antibody response. The highest reaction antibody was immunoglobulin G (IgG). IgM was detected with a lower titer than that of IgG. IgA antibody response to r-SAG1 was very weak (Figure 3).
Figure 3.
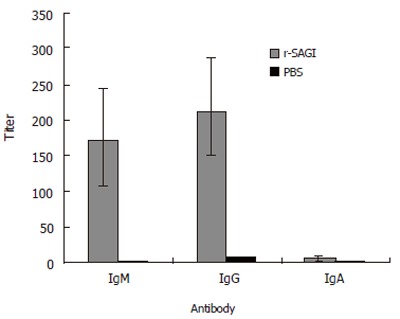
Antibody responses to r-SAG1. BALB/C mice were immunized with 30 µg r-SAG1 of T gondii or with PBS as a control. Booster was done with Freund’s incomplete adjuvant 2 wk after the first injection. Three weeks post-immunization, sera were collected from 5 mice of each group. Antibodies were tested individually by ELISA.
To test the specificity of the reaction of the antigen and antibody, the crude antigen of T gondii tachyzoites was separated on 12% SDS-PAGE and reacted with one of the mouse sera immunized with r-SAG1 by Western blotting. A strong band was present on the 30 Ku position (Figure 3), corresponding to the predicted size from amino acid sequence.
Profile of cytokines induced by r-SAG1
To investigate the possibility of cell immune response induced by r-SAG1, the spleens of the mice immunized by r-SAG1 were isolated and the cytokines in the spleen cell suspensions were detected by ELISA. Compared to the control group, the spleen cells from r-SAG1-vaccinated BALB/c mice produced significant high levels of IFN-γ, IL-2 and IL-4, while IL-12, IL-6 and TNF-α were undetectable in splenocyte supernatants from all experimental and control animals analyzed (Figure 4).
Figure 4.
Profile of cytokines induced by r-SAG1 in PBS-inoculated mice (A) or r-SAG1 of T gondii immunized mice (B). Crude antigens of Toxoplasma gondii tachyzoites were electrophoresed on SDS-12% polyacrylamide gel and then transferred onto a nitrocellulose membrane probed with sera (diluted at 1:100). Bound antibodies were detected using anti-mouse-alkaline phosphatase conjugate. The positions of molecular masses are shown on the left.
Morphology change of T. gonddi tachyzoites caused by r-SAG1 antibody
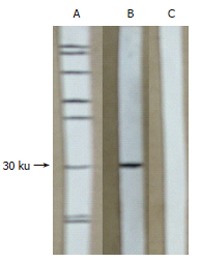
To further elucidate the biological function of r-SAG1, monoclonal antibodies against r-SAG1 were prepared. Two specific clones against r-SAG1 were obtained and designated as mAb2B3 and mAb1H6, respectively. Both clones were IgM isotype. The antibody produced a specific band at 30 Ku in Western blotting with T gondii crude antigens and r-SAG1 antigen.
To study the morphology effects of r-SAG1 antibody on T gondii, T gonddi tachyzoites were treated with specific antibody mAb2B3 and the morphology was observed under electron microscope. Compared to the PBS-treated control group, T gondii tachyzoites were gathered together and some tachyzoites were destroyed, some parasites were deformed(Figure 5). The holes and gaps on the surface of tachyzoites as well as the swollen parasites were observed (Figure 6).
Figure 5.
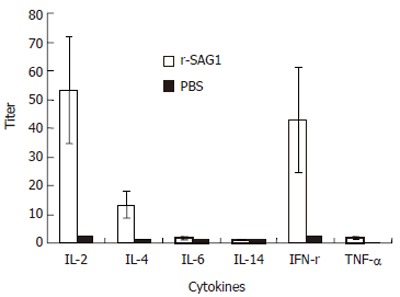
Production of cytokines induced by r-SAG1. BALB/C mice were immunized with r-SAG1 of T gondii or with PBS as a control. Six weeks after immunization, cytokines were tested individually by ELISA in 5 mice of each group.
Figure 6.

Effects of monoclonal antibody to r-SAG1 on tachyzoites of Toxoplasma gondii. A: Agglutination of the parasites, hole and gap formation on the surface, destroyed parasites and membrane thickness; B: Deformed parasite; C: Swollen parasites.Toxoplasma gondii tachyzoites were collected from mice and washed with PBS. Monoclonal antibody mAb2B3 to r-SAG1 from mouse ascites fluid was diluted and reacted with the parasites and observed under electro-microscope at 6800 x.
DISCUSSION
In the current study, the recombinant protein for SAG1 of T gondii was prepared and the biological function of SAG1 was studied through the investigation of the lethal protection of mice. The possible mechanism was studied by the observation of the induction of specific antibody, elicitation of specific cytokines and the reaction of SAG1 specific antibody with T gondii tachyzoites. The study showed that SAG1 elicited a strong immune protective reaction which might be controlled by multiple mechanisms.
This study showed that the mice immunized with r-SAG1 delayed death for 60 h when challenged with T gondii RH tachyzoites, demonstrating that SAG1 is an important antigen of T gondii. The protection of the host against organism infection may involve many factors including specific antibody. It was reported that antibodies can regulate toxoplasma tachyzoites and thereby promote their killing by normal macrophages[13]. Kang et al[14] have proved that antibodies can block infection of host cells. In addition, antibodies in the presence of complement can kill extracellular toxoplasmas[15]. In the present study, the morphology changes of the parasite, such as agglutination, deformation, swelling hole and gap formation on the surface, corresponding to SAG1 antibody treatment indicated that antibody might play an important role in the protection. The inference was verified further by the specific antibody production following the immunization of r-SAG1. After three weeks of vaccination with r-SAG1, high titer specific IgG and IgM were detected and the high titer of the antibodies was detected (data not shown). IgM antibodies could persist for at least 50 d and IgG could persist for an even longer time[16]. Therefore, when the mice were challenged at 9 wk, both IgG and IgM could contact with parasites to destroy the parasites, inhibit the infection of host cells, and thereby protect the mice from death.
In this study, the expression of cytokines such as IFN-γ, IL-4 and IL-2 was up-regulated in SAG1 immunized mice compared with control group. It was reported that IFN-γ induces inflammatory responses, thus limiting parasite proliferation[17]. In patients with alveolar echinococcosis, IFN-γ is higher, which might play a role in immunological defense against the parasite infection[18]. IL-2 (-/-) mice are unable to generate a protective IFN-γ response following infection with T gondii while IL-2 (-/-) mice have an intrinsic defect in their ability to activate and expand IFN-γ, producing T cells required for resistance to T gondii[19]. The production of IL-4 and IFN-γ allows identification of the Th0, Th1, or Th2 orientation of helper T cell clones. The production of antigen-specific cytokines suggests that SAG1 is able to elicit anti-SAG1 specific CD4 + T-cells. Cell immune response is involved in the protection of the parasites. In addition, IFN-γ producs CD8 + CTL which plays a prominent role in controlling T gondii infection[20]. It is possible that at least part of the detected IFN-γ can lead to CD8 + CTL activation. However, further experiments are required to elucidate this question.
In conclusion, SAG1 induces dominant antibody response and a strong Th1-like T cell response characterized by high titer IFN-γ production during infection. The protective role of SAG1 may be controlled cooperatively by humoral and cellular immune response. The evidence of SAG1 protection against challenge of the parasite proves that SAG1 is a good vaccine candidate for the control of toxoplasmosis.
Footnotes
Supported by China Ministry of Human Affairs and Department of Science and Technology of Shandong Province, No. 031050115
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
References
- 1.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ustun S, Aksoy U, Dagci H, Ersoz G. Incidence of toxoplasmosis in patients with cirrhosis. World J Gastroenterol. 2004;10:452–454. doi: 10.3748/wjg.v10.i3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauwels A, Meyohas MC, Eliaszewicz M, Legendre C, Mougeot G, Frottier J. Toxoplasma colitis in the acquired immunodeficiency syndrome. Am J Gastroenterol. 1992;87:518–519. [PubMed] [Google Scholar]
- 4.Dubey JP, Kirkbride CA. Toxoplasmosis and other causes of abortions in sheep from north central United States. J Am Vet Med Assoc. 1990;196:287–290. [PubMed] [Google Scholar]
- 5.Dubey JP, Thulliez P. Persistence of tissue cysts in edible tissues of cattle fed Toxoplasma gondii oocysts. Am J Vet Res. 1993;54:270–273. [PubMed] [Google Scholar]
- 6.Handman E, Goding JW, Remington JS. Detection and characterization of membrane antigens of Toxoplasma gondii. J Immunol. 1980;124:2578–2583. [PubMed] [Google Scholar]
- 7.Kasper LH, Bradley MS, Pfefferkorn ER. Identification of stage-specific sporozoite antigens of Toxoplasma gondii by monoclonal antibodies. J Immunol. 1984;132:443–449. [PubMed] [Google Scholar]
- 8.Radke JR, Gubbels MJ, Jerome ME, Radke JB, Striepen B, White MW. Identification of a sporozoite-specific member of the Toxoplasma SAG superfamily via genetic complementation. Mol Microbiol. 2004;52:93–105. doi: 10.1111/j.1365-2958.2003.03967.x. [DOI] [PubMed] [Google Scholar]
- 9.Rachinel N, Buzoni-Gatel D, Dutta C, Mennechet FJ, Luangsay S, Minns LA, Grigg ME, Tomavo S, Boothroyd JC, Kasper LH. The induction of acute ileitis by a single microbial antigen of Toxoplasma gondii. J Immunol. 2004;173:2725–2735. doi: 10.4049/jimmunol.173.4.2725. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu LX, Liu J, Ye Y, Xie YH, Kong YY, Li GD, Wang Y. A candidate DNA vaccine elicits HCV specific humoral and cellular immune responses. World J Gastroenterol. 2004;10:2488–2492. doi: 10.3748/wjg.v10.i17.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res. 1998;29:289–310. [PubMed] [Google Scholar]
- 14.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 15.Kasper LH. Isolation and characterization of a monoclonal anti-P30 antibody resistant mutant of Toxoplasma gondii. Parasite Immunol. 1987;9:433–445. doi: 10.1111/j.1365-3024.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 16.Strannegård O. Regulatory effects of antigen and antibody on the reagin response in rabbits. Clin Exp Immunol. 1971;8:963–972. [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper LH, Khan IA, Ely KH, Buelow R, Boothroyd JC. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol. 1992;148:1493–1498. [PubMed] [Google Scholar]
- 18.Shi DZ, Li FR, Bartholomot B, Vuitton DA, Craig PS. Serum sIL-2R, TNF-alpha and IFN-gamma in alveolar echinococcosis. World J Gastroenterol. 2004;10:3674–3676. doi: 10.3748/wjg.v10.i24.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villegas EN, Lieberman LA, Carding SR, Hunter CA. Susceptibility of interleukin-2-deficient mice to Toxoplasma gondii is associated with a defect in the production of gamma interferon. Infect Immun. 2002;70:4757–4761. doi: 10.1128/IAI.70.9.4757-4761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]


