Abstract
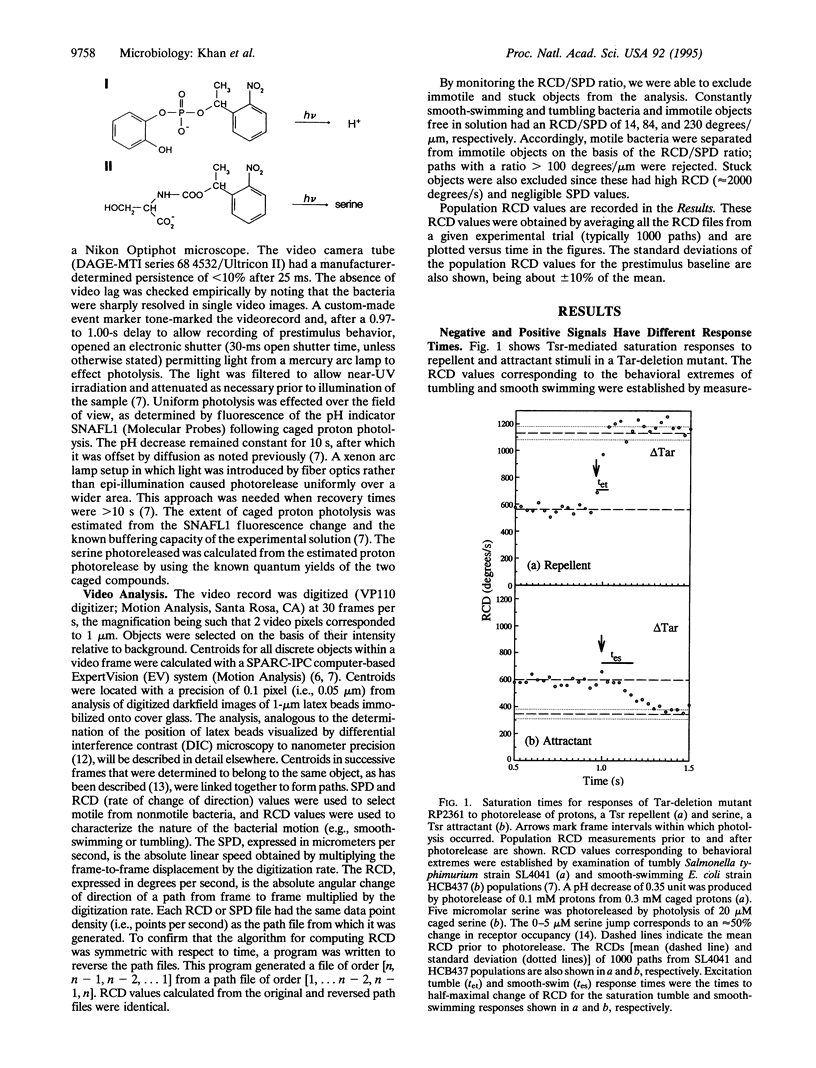
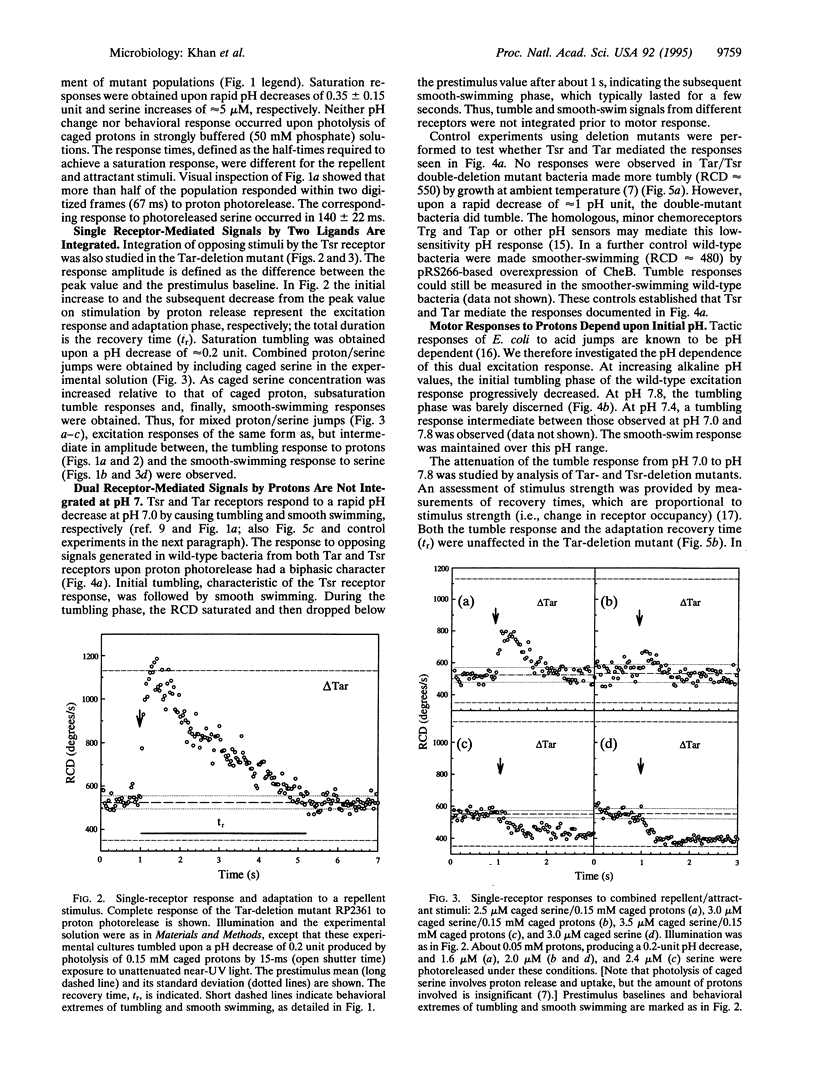
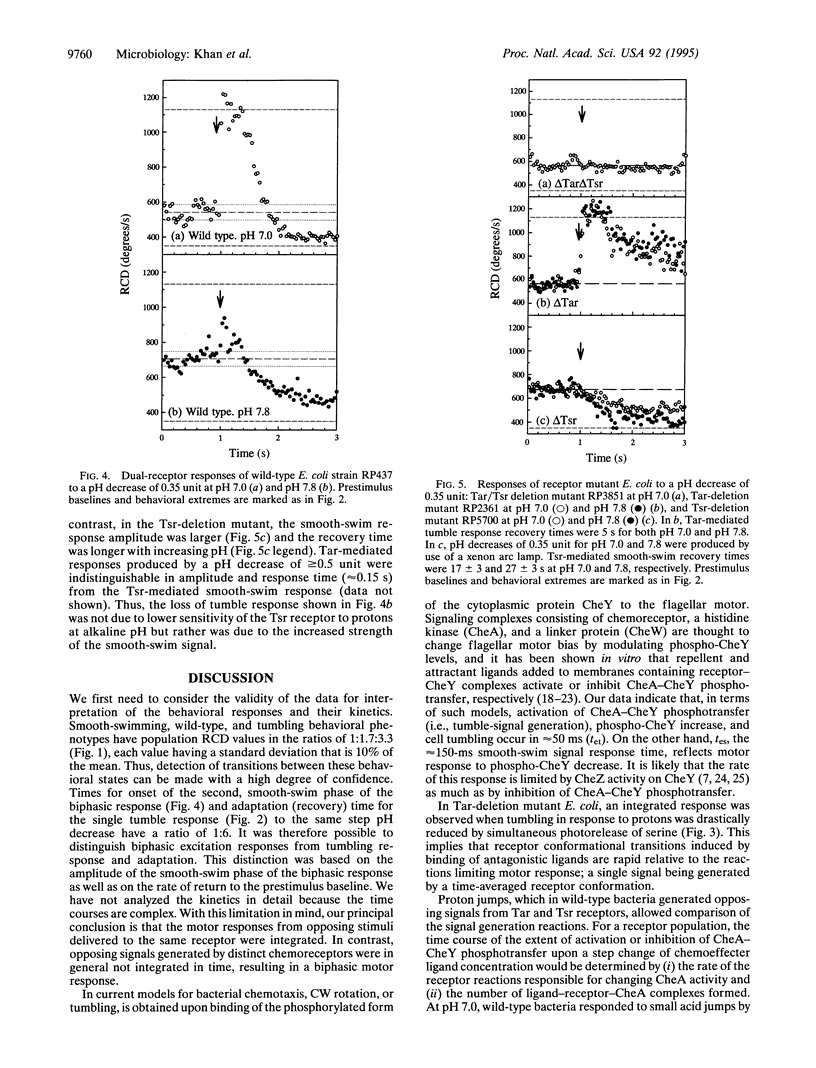
Chemotactic signaling in Escherichia coli involves transmission of both negative and positive signals. In order to examine mechanisms of signal processing, behavioral responses to dual inputs have been measured by using photoactivable "caged" compounds, computer video analysis, and chemoreceptor deletion mutants. Signaling from Tar and Tsr, two receptors that sense amino acids and pH, was studied. In a Tar deletion mutant the photoactivated release of protons, a Tsr repellent, and of serine, a Tsr attractant, in separate experiments at pH 7.0 resulted in tumbling (negative) or smooth-swimming (positive) responses in ca. 50 and 140 ms, respectively. Simultaneous photorelease of protons and serine resulted in a single tumbling or smooth-swimming response, depending on the relative amounts of the two effectors. In contrast, in wild-type E. coli, proton release at pH 7.0 resulted in a biphasic response that was attributed to Tsr-mediated tumbling followed by Tar-mediated smooth-swimming. In wild-type E. coli at more alkaline pH values the Tar-mediated signal was stronger than the Tsr signal, resulting in a strong smooth-swimming response preceded by a diminished tumbling response. These observations imply that (i) a single receptor time-averages the binding of different chemotactic ligands generating a single response; (ii) ligand binding to different receptors can result in a nonintegrated response with the tumbling response preceding the smooth-swimming response; (iii) however, chemotactic signals of different intensities derived from different receptors can also result in an apparently integrated response; and (iv) the different chemotactic responses to protons at neutral and alkaline pH may contribute to E. coli migration toward neutrality.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Tso W. W. "Decision"-making in bacteria: chemotactic response of Escherichia coli to conflicting stimuli. Science. 1974 Jun 21;184(4143):1292–1294. doi: 10.1126/science.184.4143.1292. [DOI] [PubMed] [Google Scholar]
- Armitage J. P. Behavioral responses in bacteria. Annu Rev Physiol. 1992;54:683–714. doi: 10.1146/annurev.ph.54.030192.003343. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Anderson R. A. Bacteria swim by rotating their flagellar filaments. Nature. 1973 Oct 19;245(5425):380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Tedesco P. M. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. M., Segall J. E., Berg H. C. Impulse responses in bacterial chemotaxis. Cell. 1982 Nov;31(1):215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Simon M. I. The dynamics of protein phosphorylation in bacterial chemotaxis. Cell. 1990 Dec 21;63(6):1339–1348. doi: 10.1016/0092-8674(90)90429-i. [DOI] [PubMed] [Google Scholar]
- Bourret R. B., Borkovich K. A., Simon M. I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- Bray D., Bourret R. B., Simon M. I. Computer simulation of the phosphorylation cascade controlling bacterial chemotaxis. Mol Biol Cell. 1993 May;4(5):469–482. doi: 10.1091/mbc.4.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Koshland D. E., Jr Membrane receptors for aspartate and serine in bacterial chemotaxis. J Biol Chem. 1979 Oct 10;254(19):9695–9702. [PubMed] [Google Scholar]
- Gegner J. A., Graham D. R., Roth A. F., Dahlquist F. W. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992 Sep 18;70(6):975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Kehry M. R., Doak T. G., Dahlquist F. W. Sensory adaptation in bacterial chemotaxis: regulation of demethylation. J Bacteriol. 1985 Sep;163(3):983–990. doi: 10.1128/jb.163.3.983-990.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Amoyaw K., Spudich J. L., Reid G. P., Trentham D. R. Bacterial chemoreceptor signaling probed by flash photorelease of a caged serine. Biophys J. 1992 Apr;62(1):67–68. doi: 10.1016/S0006-3495(92)81781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Castellano F., Spudich J. L., McCray J. A., Goody R. S., Reid G. P., Trentham D. R. Excitatory signaling in bacterial probed by caged chemoeffectors. Biophys J. 1993 Dec;65(6):2368–2382. doi: 10.1016/S0006-3495(93)81317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Dapice M., Reese T. S. Effects of mot gene expression on the structure of the flagellar motor. J Mol Biol. 1988 Aug 5;202(3):575–584. doi: 10.1016/0022-2836(88)90287-2. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikos A., Conley M. P., Boyd A., Berg H. C., Simon M. I. Chimeric chemosensory transducers of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1326–1330. doi: 10.1073/pnas.82.5.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Gelles J., Sheetz M. P. Nanometer-scale measurements using video light microscopy. Cell Motil Cytoskeleton. 1988;10(1-2):47–53. doi: 10.1002/cm.970100109. [DOI] [PubMed] [Google Scholar]
- Schuster S. C., Swanson R. V., Alex L. A., Bourret R. B., Simon M. I. Assembly and function of a quaternary signal transduction complex monitored by surface plasmon resonance. Nature. 1993 Sep 23;365(6444):343–347. doi: 10.1038/365343a0. [DOI] [PubMed] [Google Scholar]
- Segall J. E., Ishihara A., Berg H. C. Chemotactic signaling in filamentous cells of Escherichia coli. J Bacteriol. 1985 Jan;161(1):51–59. doi: 10.1128/jb.161.1.51-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J. E., Manson M. D., Berg H. C. Signal processing times in bacterial chemotaxis. Nature. 1982 Apr 29;296(5860):855–857. doi: 10.1038/296855a0. [DOI] [PubMed] [Google Scholar]
- Slonczewski J. L., Macnab R. M., Alger J. R., Castle A. M. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli. J Bacteriol. 1982 Oct;152(1):384–399. doi: 10.1128/jb.152.1.384-399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Sensory rhodopsins of halobacteria. Annu Rev Biophys Biophys Chem. 1988;17:193–215. doi: 10.1146/annurev.bb.17.060188.001205. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Koshland D. E., Jr Quantitation of the sensory response in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Feb;72(2):710–713. doi: 10.1073/pnas.72.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R. C. Activating and inhibitory mutations in the regulatory domain of CheB, the methylesterase in bacterial chemotaxis. J Biol Chem. 1993 Jan 25;268(3):1921–1930. [PubMed] [Google Scholar]
- Sundberg S. A., Alam M., Spudich J. L. Excitation signal processing times in Halobacterium halobium phototaxis. Biophys J. 1986 Nov;50(5):895–900. doi: 10.1016/S0006-3495(86)83530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso W. W., Adler J. Negative chemotaxis in Escherichia coli. J Bacteriol. 1974 May;118(2):560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Macnab R. M., Imae Y. Repellent response functions of the Trg and Tap chemoreceptors of Escherichia coli. J Bacteriol. 1990 Jan;172(1):383–388. doi: 10.1128/jb.172.1.383-388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



