Abstract
The number of patients with gastric cancer has more than doubled since 1985 in developing countries. Thus, the questions of whether it can be predicted from gastritis morphology, who is at risk and who has a lower risk of developing gastric carcinoma are raised. H pylori-infection leads to erosions, ulcerations, carcinoma, mucosa associated lymphoid tissue (MALT)-lymphoma and extragastric diseases only in some individuals. The frequency of ulcerations among H pylori-infected individuals is estimated to be 13%, gastric cancer about 1% and MALT lymphoma around 0.1%. In the literature a multistep model from chronic active H pylori-infection through multifocal atrophy, intestinal metaplasia, dysplasia (intraepithelial neoplasia) and carcinoma has been described. But this model cannot be applied to all routine cases. Since risk factors such as metaplasia and atrophy are paracancerous rather than precancerous conditions, this raises the question whether there is a better morphological marker. Differences in topography, grade and activity of Helicobacter gastritis in the antrum and corpus might be good markers for identifying those who are at risk of developing gastric cancer. It is known that the so-called corpus dominant
H pylori gastritis is found more frequently among individuals with early and advanced gastric cancer and within high risk populations. This is valid both for first-degree relatives of gastric cancer patients and for patients with gastric adenoma and hyperplastic polyps. In conclusion, corpus-dominant H pylori gastritis is significantly more common in patients with advanced and early gastric cancer, first-degree relatives of patients with gastric cancer, patients with gastric adenoma and gastric hyperplastic polyps. Therefore, all these patients are at risk of developing gastric cancer. Next, the question of who is at risk of developing corpus-dominant gastritis is raised. It appears that patients with a low acid output more frequently develop gastric cancer. Eradication therapy is never performed too early but probably sometimes too late after the patients pass a “point of no return”. Large prospective long term studies are necessary to prove this and identify new reliable markers for gastric cancer development.
Keywords: H pylori, Corpus-dominant helicobacter gastritis, Low acid output, Gastric adenocarcinoma, Histomorphology
INTRODUCTION
H pylori is recognized as the major pathogenetic factor in chronic active gastritis. Chronic active gastritis not only leads to gastric inflammation in various degrees but also can be the cause of gastric and duodenal ulcer disease and might give rise to gastric mucosa associated lymphoid tissue (MALT) lymphoma (marginal B-cell lymphoma). Interestingly, low grade MALT lymphoma regresses after antibiotic treatment for H pylori eradication in a high percentage of cases.
HISTORICAL BACKGROUND
Prior to the morphological identification of H pylori as the causative agent, gastric inflammation had already been considered a risk factor for gastric carcinoma. Correa postulated in 1988[1] that chronic gastritis may lead via a multistep process to intestinal metaplasia and atrophy as an additional morphological risk factor for the development of carcinoma since these are frequently found to be closely related to the intestinal type of gastric cancer. In addition, today it has become clear that at least 50% of all cases of autoimmune atrophic gastritis which is considered to some varying degree as a precancerous condition, are probably induced by H pylori[2].
EPIDEMIOLOGICAL EVIDENCE
Mortality of gastric cancer is a significant burden not only on patients but also on the whole health system. There are regional differences depending on the geographical location (Table 1). For example, about 360 000 deaths occur due to gastric cancer each year in China with the world largest population[3]. This means that every 2 min one patient dies of gastric cancer in China.
Table 1.
Gastric cancer mortality in different populations (Modified from Winawer et al[3])
| Countries | Yr | Yr |
| 2000 | 2020 | |
| Developed | 334.000 | 440.000 |
| Developing | 543.000 | 983.000 |
The knowledge on a pathogenetic association between gastric carcinoma and H pylori infection is mainly based on retrospective studies by observing resected stomachs. H pylori gastritis is usually present in gastric mucosa adjacent to cancerous lesions. Furthermore, a statistically significant correlation between the rate of infection and the incidence of gastric cancer has been observed in a number of populations, although in some populations, especially in Africa, such a correlation is not found[4]. Thirdly, case-control studies demonstrated that the risk of developing gastric carcinoma is significantly higher in the presence of H pylori. Such an association is only described for the distal stomach but not for carcinomas in the proximal stomach, that may arise due to an increasing number of Barrett’s esophagus[5,6]. Many of these studies have methodological problems and weakness of data strength. The situation has improved nowadays. At least some prospective studies are available showing that if certain morphological features are present the patients are at risk of developing gastric carcinoma.
In Germany with 80 million inhabitants, more than 26 000 patients suffer from gastric carcinoma[7]. Approximately 50% of them will not survive for 5 years, which underlines the importance of this disease for the health system.
WHO has classified H pylori as a class 1 carcinogen for the development of gastric carcinoma, but only a minority of individuals infected with H pylori develop gastric carcinoma. It is not clear up till now how the complex interaction occurs between host development of neoplasia in some patients but not in the majority of all others. It has long been known that nutritional factors, high salt intake, smoked meat, few vitamins might increase the risk of gastric carcinoma[8]. From our routine practice we know that cancer develops very rarely in normal gastric mucosa. It is of particular interest to identify risk markers capable of predicting the risk of developing gastric carcinoma. Healing of such high risk gastritis can then decrease the risk of developing gastric adenocarcinoma. Before H pylori was re-introduced into medicine as the causative agent for gastritis, it has been known that the presence of multifocal atrophic gastritis with intestinal metaplasia is a risk factor for gastric carcinoma[1]. Interestingly, the diffuse (signet ring cell) type of carcinomas is not covered by such a hypothesis that gastric cancer might develop even in normal gastric mucosa, especially in very young adults, indicating a genetic background in those patients.
MORPHOLOGICAL FEATURES OF GASTRITIS WITH HIGHER RISK OF DEVELOPMENT OF ADENOCARCINOMA
Initial investigations on the topographic aspects of H pylori showed that H pylori colonization is less frequent and activity is less marked in the gastric corpus compared to the gastric antrum[9-12]. Recent studies including the gastric cardia also suggest that H pylori colonization is denser in the gastric cardia than in the gastric corpus, in turn leading to more pronounced gastritis in this region[13]. Nowadays it is well accepted that H pylori colonizes in the whole gastric mucosa. The infection induces chronic active inflammation anywhere in the gastric mucosa. An important task is therefore, to search for bacteria- and host-related factors that favor the development of gastric carcinoma. The results of studies carried out by our group at the end of the 1980s[14] have prompted us to carry further studies in this field.
Comparison of gastritis scores in antrum and corpus biopsies
In a matched control study, our group has compared the gastritis score[15] of individuals with NUD, duodenal ulcer, gastric ulcer, MALT-lymphoma and gastric carcinoma. These studies show that patients with chronic active H pylori gastritis, gastric ulcer and duodenal ulcer have significantly lower scores in the corpus than in the antrum, compared to cases of gastric cancer and MALT-lymphoma (Figure 1)[15], but the scores are significantly lower in MALT lymphoma than in gastric cancer.
Figure 1.
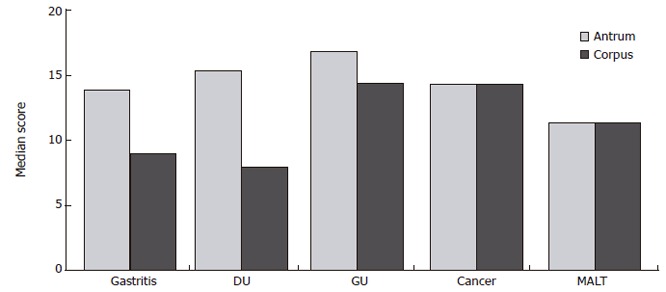
Median gastritis scores in antrum and corpus mucosa of patients with chronic active H pylori gastritis alone, duodenal ulcer (DU), gastric ulcer (GU), gastric cancer or MALT lymphoma (n = 50) (Modified from Meining et al[16]).
Comparison of antral and corpus gastritis in patients with gastric carcinoma or duodenal ulcer
In a next step, our group compared the grade and activity of H pylori gastritis in the antrum of 215 patients with gastric carcinoma or patients suffering from duodenal ulcer, showing that there are no differences in the grade and activity of antrum mucosa between the two patient groups, but significant differences in the corpus mucosa. The activity and grade of gastritis in the gastric corpus are significantly higher than those in the antrum of patients with gastric carcinoma, but only in a few patients with duodenal ulcer (Figure 2)[16]. An additional study showed that intestinal metaplasia occurs significantly more often both in antrum and in corpus of patients with gastric carcinoma[17]. According to Hattori et al[18], intestinal metaplasia might only be a paracancerous condition and just an expression of prior or ongoing severe inflammation, but does not cause cancer.
Figure 2.
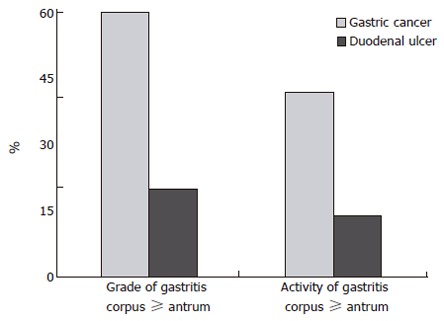
Topographic distribution of grade and activity of H pylori gastritis in individuals with early gastric cancer or duodenal ulcer (Modified from Meining et al[16]).
Gastric carcinoma risk index
In 1998, our group proposed a gastric carcinoma risk index based on previously described results. This index score consists of the presence of intestinal metaplasia (1 point) and the grade and activity of gastritis in the corpus. The maximum point is 3 when all features are present (Table 2). The positive predictive value for the presence of gastric carcinoma is 79% for score 2 and 94% for score 3[16]. Leodolter et al[19] found that there is a strong relationship between age and the prevalence of so-called high risk gastritis with a score of 3 (Table 3).
Table 2.
Gastric cancer risk index in patients with active H pylori gastritis
| Grade of gastritis (infiltration by lymphocytes and plasma cells) is more severe in the corpus compared to the antrum or at least equally distributed |
| Activity of gastritis (infiltration by neutrophilic granulocytes) is more severe in the corpus compared to the antrum or at least equally distributed |
| Intestinal metaplasia present in the antrum and/or in the corpus |
Each feature is scored with one point. Values between 0 and 3 and a total sum of 3 are possible (Adapted from Meining et al[16]).
Table 3.
Prevalence of H pylori-associated high risk gastritis (3-index-points) in relation to age (n = 845) (Modified from Leodolter et al[19])
| Age | n | 3-index-points |
| < 35 yr | 114 | 0.9% |
| 35-54 yr | 116 | 2.7% |
| 55-64 yr | 186 | 6.5% |
| 65-74 yr | 145 | 12.7% |
| > 74 yr | 83 | 12.6% |
Comparison of grade and activity in first degree relatives of gastric carcinoma patients
Due to the lack of large prospective studies, it can be argued that analyses of the topographic grading of H pylori- infected gastric mucosa in patients with gastric carcinoma do not allow transfer of the findings to individuals without gastric cancer since it might influence the status of gastric mucosa. Therefore, our group has analyzed a set of 237 first degree relatives of gastric cancer patients and 237 controls with H pylori infection alone[20]. The updated Sydney System for histological classification of gastritis[21] consists of a four-scale semiquantitative grading system with values being not present, slight, moderate and marked.
When the risk profile is analyzed by grading the grade and activity of gastritis according to the updated Sydney System, it becomes clear that the first degree relatives of gastric cancer patients do have more severe grade and activity of gastritis (Figure 3), supporting the familial background of gastric cancer.
Figure 3.
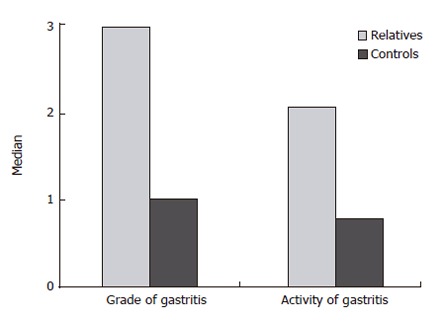
Median grade and activity of H pylori gastritis in the corpus in relatives of gastric cancer patients (n = 237) and matched control patients (n = 237) (Modified from Meining et al[20]).
Gastritis status in patients with gastric adenomas and hyperplastic polyps
Adenomas of the stomach are unequivocal intraepithelial neoplasms that are limited to the gastric epithelium and can be subdivided into low-grade (adenoma) and high grade intraepithelial neoplasia (formerly dysplasia). From our routine practice, we know that the diagnosis of high grade intraepithelial neoplasia is very rare, and that most reports on follow-up studies of such lesions show that most of these “high grade lesions” are invasive cancers within a few months[22], which probably contribute more to diagnostic uncertainty in biopsy specimens than real neoplastic progression. Adenomas can show a polypoid or a flat growth within the gastric epithelium. For gastric adenomas, the adenoma-carcinoma-sequence[23] can also be used to represent a precancerous lesion that should be removed completely to avoid progression to cancer[24].
In a series of 118 patients with gastric adenomas, our group showed[25] that adenomas occur more frequently in patients with autoimmune gastritis but also in patients with H pylori gastritis (Figure 4), and a subsequent analysis showed that patients with gastric adenoma have the same distribution of corpus dominant H pylori gastritis like patients with early gastric cancer. In a further study, our group analyzed the gastritis status of gastric pyloric gland adenomas which is also a precancerous condition (approximately 30% show invasive carcinoma at the time of first diagnosis), and found that the proportion of atrophic autoimmune gastritis and corpus dominant H pylori gastritis is high in elderly women[26] (data not shown).
Figure 4.
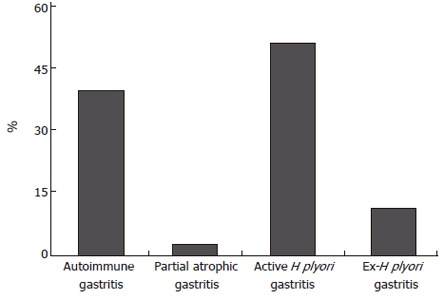
Prevalence of different gastritis in 118 individuals with gastric adenomas (Adapted from Meining et al[25]).
Hyperplastic polyps are also considered a precancerous lesion. A few case series in the literature and our own data[27] indicate that hyperplastic polyps bear a risk of malignant transformation between 0.6% and 6.6%. Therefore, these polyps belong to the group of precancerous lesions and should be removed completely. Follow-up studies showed that gastric carcinoma may develop in 8.6% of the patients at other gastric sites[28]. As adenomas, hyperplastic polyps arise quite often in autoimmune gastritis. H pylori infection is present in 37% of the cases of hyperplastic polyps. Most of these cases also show corpus-dominant H pylori gastritis (data not shown) (Figure 5).
Figure 5.
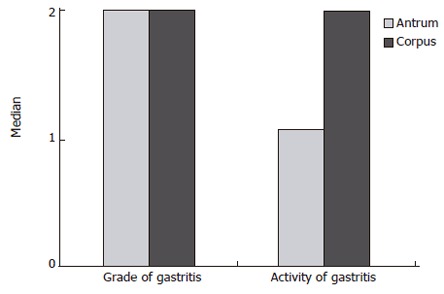
Grading of gastritis according to the updated Sydney System for classification of gastritis in the antrum and corpus of 91 patients with hyperplastic polyps (Adapted from Oberhuber[27]).
Gastritis status in populations with different risks of developing gastric carcinoma
A high incidence of gastric carcinoma is present in Fuzhou in China and in Japan, compared to Beijing in China, Thailand and Vietnam. Corpus dominant gastritis is present in Japan and China (Fuzhou), especially in the elderly whereas antrum predominant gastritis is present in Beijing (China), Thailand and Vietnam. These results correlate with the low incidence of gastric adenocarcinoma in Thai, Vietnamese and certain Chinese populations[29] (Figure 6). Interestingly, the activity of corpus gastritis increases with age in Japanese individuals while antrum predominant H pylori gastritis does not shift to more severe corpus gastritis in Vietnamese, Thai and certain Chinese populations. This is followed by a more severe colonization of H pylori in cases of corpus dominant H pylori gastritis. In contrast to the literature, we could not confirm the finding of improved antrum gastritis after proton-pump inhibitor (PPI) therapy. We have found aggraved corpus gastritis as described in the literature but decreased colonization in the antrum and corpus[30]. This might explain why increased incidence of gastric carcinoma is not reported among the millions of individuals who use acid-suppressive therapy, although corpus dominant H pylori gastritis is treated with PPI. Future prospective studies especially in China with its large background of individual genetic differences and environmental conditions are necessary to investigate the topography of gastritis in different Chinese populations searching for explanations for different incidences of gastric cancer in China as reported in the literature[31]. It has been speculated that differences in environmental factors might provide a crucial clue to all these questions rather than genetic backgrounds[32].
Figure 6.
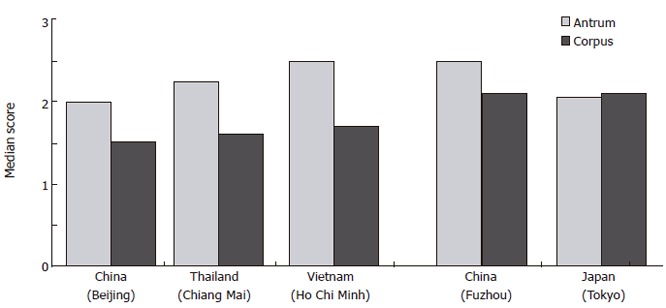
Mean scores of active gastritis (neutrophilic granulocytes) in the antrum and corpus in different Asian populations (Modified from Matsuhisa et al[29]).
DISCUSSION
Corpus dominant H pylori gastritis is found more frequently in patients with advanced gastric carcinomas, or early gastric carcinomas and even in first degree relatives of patients with gastric cancer than in patients with duodenal ulcer or functional dyspepsia. While more than 90% of patients with gastric carcinoma are H pylori positive. Combined autoimmune gastritis and gastric cancer is very rarely found. On the other hand, 30% of patients with hyperplastic polyps and gastric adenoma of the intestinal or gastric type (pyloric gland adenoma) suffer from autoimmune gastritis[16,26]. Corpus dominant H pylori gastritis is mainly diagnosed in cases infected with H pylori. All the above studies have confirmed that corpus dominant H pylori gastritis bears a higher risk developing gastric neoplasms rather than other forms of H pylori gastritis.
It is difficult to explain the different incidences of gastric cancer in patients with corpus dominant H pylori gastritis and autoimmune gastritis, because at least 50% of autoimmune gastritis are probably induced by H pylori[2], indicating that more than one risk exists in patients with precancerous lesions and patients with corpus dominant H pylori gastritis have a higher risk than those with autoimmune gastritis. Corpus dominant H pylori gastritis is a risk factor for gastric carcinoma in individuals with precancerous conditions and in the first degree relatives of gastric carcinoma patients[20,25]. The predictive value of corpus dominant H pylori gastritis needs to be investigated in future prospective studies.
Lee et al[33] are the first to consider the possibility of differences in acid secreting capacity in answering the underlying causes of corpus dominant H pylori gastritis. According to their hypothesis that low acid output leads to more severe gastritis, which has been confirmed laters[34,35]. Moreover, it is important to emphasize that the proposed gastritis-atrophy-intestinal metaplasia- dysplasia-carcinoma sequence by Correa[1] is an ideal model only and fails to be proven in most cases[18]. But intestinal metaplasia (as a paracancerous phenomenon) is frequently picked up in biopsy specimens from individuals with corpus dominant H pylori gastritis, indicating a close relationship.
Whether H pylori eradication is capable of preventing gastric cancer is to clarified in prospective studies such as the German PRISMA study[36]. Eradication stops at least the progression of intestinal metaplasia and atrophy[37] and improves gastritis[38]. However, it has been indicated that “a point of no return” might have been reached before H pylori eradication cures the infection. This possibility is demonstrated by the development of 3 early gastric carcinomas in 92 patients 4 and 5 years after H pylori eradication and complete remission of MALT lymphomas in our German MALT lymphoma study[39] and 3 additional cases having a similar clinical history (Malfertheiner’s personal communication). Further proof is given by the study of Wong et al[40] who showed that the incidence of gastric carcinoma is similar in individuals receiving eradication therapy or placebo during a 7.5-year follow- up period. It was reported that if no precancerous lesions (atrophy/dysplasia/intestinal metaplasia) are present, eradication of H pylori leads to no gastric cancer[40-42]. Another factor that should not be disregarded is a genetic background shown by the analysis of first-degree relatives of individuals with gastric carcinoma[43-46]. Whether metaplasia and atrophy are precancerous or paracancerous lesions[18], they may not have a great clinical impact since these lesions occur especially in individuals with an elevated risk of gastric cancer[47]. Large prospective studies are needed to further analyze the risk factors for gastric cancer and to clarify whether and when eradication therapy is capable of preventing gastric carcinoma.
Footnotes
S- Editor Wang GP L- Editor Wang XL E- Editor Bi L
References
- 1.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 2.Müller H, Rappel S, Wündisch T, Bayerdörffer E, Stolte M. Healing of active, non-atrophic autoimmune gastritis by H. pylori eradication. Digestion. 2001;64:30–39. doi: 10.1159/000048836. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Classen M, Rozen P. Report of the International Digestive Disease Alliance, New Orleans 2004--in collaboration with the OMED Colorectal Cancer Screening Committee. Eur J Cancer Prev. 2004;13:457–459. doi: 10.1097/00008469-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tatsuta M, Iishi H, Okuda S, Taniguchi H, Yokota Y. The association of Helicobacter pylori with differentiated-type early gastric cancer. Cancer. 1993;72:1841–1845. doi: 10.1002/1097-0142(19930915)72:6<1841::aid-cncr2820720608>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Fiocca R, Villani L, Luinetti O, Gianatti A, Perego M, Alvisi C, Turpini F, Solcia E. Helicobacter colonization and histopathological profile of chronic gastritis in patients with or without dyspepsia, mucosal erosion and peptic ulcer: a morphological approach to the study of ulcerogenesis in man. Virchows Arch A Pathol Anat Histopathol. 1992;420:489–498. doi: 10.1007/BF01600253. [DOI] [PubMed] [Google Scholar]
- 7.Arbeitsgemeinschaft bevölkerungsbezogener Krebsregister in Deutschland (ed) Krebs in Deutschland-Häufigkeiten und Trends. 3rd ed. Berlin: Robert Koch Institut; 2003. Available from: http://www.rki.de/Krebs. [Google Scholar]
- 8.Boeing H, Jedrychowski W, Wahrendorf J, Popiela T, Tobiasz-Adamczyk B, Kulig A. Dietary risk factors in intestinal and diffuse types of stomach cancer: a multicenter case-control study in Poland. Cancer Causes Control. 1991;2:227–233. doi: 10.1007/BF00052138. [DOI] [PubMed] [Google Scholar]
- 9.Bayerdörffer E, Oertel H, Lehn N, Kasper G, Mannes GA, Sauerbruch T, Stolte M. Topographic association between active gastritis and Campylobacter pylori colonisation. J Clin Pathol. 1989;42:834–839. doi: 10.1136/jcp.42.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackelsberger A, Günther T, Schultze V, Labenz J, Roessner A, Malfertheiner P. Prevalence and pattern of Helicobacter pylori gastritis in the gastric cardia. Am J Gastroenterol. 1997;92:2220–2224. [PubMed] [Google Scholar]
- 11.Eidt S, Stolte M. Helicobacter pylori and gastric malignancy. Zentralbl Bakteriol. 1993;280:137–143. doi: 10.1016/s0934-8840(11)80949-7. [DOI] [PubMed] [Google Scholar]
- 12.Stolte M, Stadelmann O, Bethke B, Burkard G. Relationships between the degree of Helicobacter pylori colonisation and the degree and activity of gastritis, surface epithelial degeneration and mucus secretion. Z Gastroenterol. 1995;33:89–93. [PubMed] [Google Scholar]
- 13.Genta RM, Graham DY. Helicobacter pylori: the new bug on the (paraffin) block. Virchows Arch. 1994;425:339–347. doi: 10.1007/BF00189571. [DOI] [PubMed] [Google Scholar]
- 14.Stolte M, Eidt S, Ohnsmann A. Differences in Helicobacter pylori associated gastritis in the antrum and body of the stomach. Z Gastroenterol. 1990;28:229–233. [PubMed] [Google Scholar]
- 15.Meining A, Stolte M, Hatz R, Lehn N, Miehlke S, Morgner A, Bayerdörffer E. Differing degree and distribution of gastritis in Helicobacter pylori-associated diseases. Virchows Arch. 1997;431:11–15. doi: 10.1007/s004280050063. [DOI] [PubMed] [Google Scholar]
- 16.Meining A, Bayerdörffer E, Müller P, Miehlke S, Lehn N, Hölzel D, Hatz R, Stolte M. Gastric carcinoma risk index in patients infected with Helicobacter pylori. Virchows Arch. 1998;432:311–314. doi: 10.1007/s004280050171. [DOI] [PubMed] [Google Scholar]
- 17.Miehlke S, Hackelsberger A, Meining A, Hatz R, Lehn N, Malfertheiner P, Stolte M, Bayerdörffer E. Severe expression of corpus gastritis is characteristic in gastric cancer patients infected with Helicobacter pylori. Br J Cancer. 1998;78:263–266. doi: 10.1038/bjc.1998.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori T. Development of adenocarcinomas in the stomach. Cancer. 1986;57:1528–1534. doi: 10.1002/1097-0142(19860415)57:8<1528::aid-cncr2820570815>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Leodolter A, Guenther T, Ebert M, Kahl S, Glasbrenner B, Malfertheiner P. Prevalence of H. pylori associated high-risk gastritis in patients with normal endoscopic findings. Gut. 2000;47 Suppl 3:358. [Google Scholar]
- 20.Meining AG, Bayerdörffer E, Stolte M. Helicobacter pylori gastritis of the gastric cancer phenotype in relatives of gastric carcinoma patients. Eur J Gastroenterol Hepatol. 1999;11:717–720. doi: 10.1097/00042737-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Vieth M, Stolte M. Pathology of early upper GI cancers. Best Pract Res Clin Gastroenterol. 2005;19:857–869. doi: 10.1016/j.bpg.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;70:1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.BERG JW. Histological aspects of the relation between gastric adenomatous polyps and gastric cancer. Cancer. 1958;11:1149–1155. doi: 10.1002/1097-0142(195811/12)11:6<1149::aid-cncr2820110610>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer. J Clin Pathol. 2002;55:770–773. doi: 10.1136/jcp.55.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieth M, Kushima R, Borchard F, Stolte M. Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Arch. 2003;442:317–321. doi: 10.1007/s00428-002-0750-6. [DOI] [PubMed] [Google Scholar]
- 27.Oberhuber G, Stolte M. Gastric polyps: an update of their pathology and biological significance. Virchows Arch. 2000;437:581–590. doi: 10.1007/s004280000330. [DOI] [PubMed] [Google Scholar]
- 28.Seifert E, Gail K, Weismüller J. Gastric polypectomy. Long-term results (survey of 23 centres in Germany) Endoscopy. 1983;15:8–11. doi: 10.1055/s-2007-1018596. [DOI] [PubMed] [Google Scholar]
- 29.Matsuhisa T, Matsukura N, Yamada N. Topography of chronic active gastritis in Helicobacter pylori-positive Asian populations: age-, gender-, and endoscopic diagnosis-matched study. J Gastroenterol. 2004;39:324–328. doi: 10.1007/s00535-003-1329-y. [DOI] [PubMed] [Google Scholar]
- 30.Logan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995;36:12–16. doi: 10.1136/gut.36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong BC, Ching CK, Lam SK, Li ZL, Chen BW, Li YN, Liu HJ, Liu JB, Wang BE, Yuan SZ, et al. Differential north to south gastric cancer-duodenal ulcer gradient in China. China Ulcer Study Group. J Gastroenterol Hepatol. 1998;13:1050–1057. doi: 10.1111/j.1440-1746.1998.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsuhisa TM, Yamada NY, Kato SK, Matsukura NM. Helicobacter pylori infection, mucosal atrophy and intestinal metaplasia in Asian populations: a comparative study in age-, gender- and endoscopic diagnosis-matched subjects. Helicobacter. 2003;8:29–35. doi: 10.1046/j.1523-5378.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee A, Dixon MF, Danon SJ, Kuipers E, Mégraud F, Larsson H, Mellgård B. Local acid production and Helicobacter pylori: a unifying hypothesis of gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995;7:461–465. [PubMed] [Google Scholar]
- 34.Miyaji M, Ogoshi K, Tajima T, Mitomi T. Association between serum gastrin levels, gastric acid secretion and age in early gastric cancer. Tumour Biol. 1997;18:311–320. doi: 10.1159/000218044. [DOI] [PubMed] [Google Scholar]
- 35.Meining A, Kiel G, Stolte M. Changes in Helicobacter pylori-induced gastritis in the antrum and corpus during and after 12 months of treatment with ranitidine and lansoprazole in patients with duodenal ulcer disease. Aliment Pharmacol Ther. 1998;12:735–740. doi: 10.1046/j.1365-2036.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- 36.Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, Dite P, Läuter J, Labenz J, Leodolter A, et al. Helicobacter pylori and gastric cancer: current status of the Austrain Czech German gastric cancer prevention trial (PRISMA Study) World J Gastroenterol. 2001;7:243–247. doi: 10.3748/wjg.v7.i2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Comparison of Helicobacter pylori infection and gastric mucosal histological features of gastric ulcer patients with chronic gastritis patients. World J Gastroenterol. 2005;11:976–981. doi: 10.3748/wjg.v11.i7.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solcia E, Villani L, Fiocca R, Luinetti O, Boldorini R, Trespi E, Perego M, Alvisi C, Lazzaroni M, Bianchi Porro G. Effects of eradication of Helicobacter pylori on gastritis in duodenal ulcer patients. Scand J Gastroenterol Suppl. 1994;201:28–34. [PubMed] [Google Scholar]
- 39.Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248–253. doi: 10.3748/wjg.v7.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 41.Fichman S, Niv Y. Histological changes in the gastric mucosa after Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2004;16:1183–1188. doi: 10.1097/00042737-200411000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Uemura N, Okamoto S. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer in Japan. Gastroenterol Clin North Am. 2000;29:819–827. doi: 10.1016/s0889-8553(05)70149-7. [DOI] [PubMed] [Google Scholar]
- 43.La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70:50–55. doi: 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Zanghieri G, Di Gregorio C, Sacchetti C, Fante R, Sassatelli R, Cannizzo G, Carriero A, Ponz de Leon M. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer. 1990;66:2047–2051. doi: 10.1002/1097-0142(19901101)66:9<2047::aid-cncr2820660934>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 45.Miehlke S, Yu J, Ebert M, Szokodi D, Vieth M, Kuhlisch E, Buchcik R, Schimmin W, Wehrmann U, Malfertheiner P, et al. Expression of G1 phase cyclins in human gastric cancer and gastric mucosa of first-degree relatives. Dig Dis Sci. 2002;47:1248–1256. doi: 10.1023/a:1015358127751. [DOI] [PubMed] [Google Scholar]
- 46.Yu J, Ebert MP, Miehlke S, Rost H, Lendeckel U, Leodolter A, Stolte M, Bayerdörffer E, Malfertheiner P. alpha-catenin expression is decreased in human gastric cancers and in the gastric mucosa of first degree relatives. Gut. 2000;46:639–644. doi: 10.1136/gut.46.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol. 2005;11:791–796. doi: 10.3748/wjg.v11.i6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]


