Abstract
AIM: To investigate the behaviour of total plasma homocysteine (tHcy) and its most common genetic determinant defect, the methylenetetrahydrofolate reductase C677T (C677TMTHFR) polymorphism in patients with early stage colorectal carcinoma.
METHODS: tHcy was quantified by Abbott IMx immunoassay; screening for C677TMTHFR substitution was performed by PCR and restriction analysis.
RESULTS: The frequency of the C/T and T/T genotypes of the C677TMTHFR gene polymorphism did not differ between the groups. The mean tHcy was statistically higher in cancer patients than in control subjects carrying the same C/C or C/T genotype, whereas there was no difference in the T/T homozygous carriers of the two groups. tHcy was significantly higher in the T/T homozygous carriers than in C/C and C/T genotype carriers.
CONCLUSION: The statistically significant increase of tHcy observed in C/C and C/T genotype carriers among our cancer patients is related to substrate consumption dependent on the tumor cell proliferation rate, whereas the tHcy increase observed in T/T genotype carriers of both groups probably depends on the enzymatic deficit of the homocysteine conversion to methionine and/or on the folate deficiency.
Keywords: Homocysteine, Colorectal cancer, Methylenetetrahydrofolate reductase C677T polymorphism
INTRODUCTION
Homocysteine, a sulfur-containing amino acid produced by the adenylation and subsequent demethylation of dietary methionine, is an essential intermediate in folate metabolism. Two enzymes and three vitamins play a key role in the regulation of circulating homocysteine levels. Of the enzymes, cystathionine-β-synthase controls the breakdown of homocysteine to cystathionine in the transsulfuration pathway, while methylene tetrahydrofolate reductase (MTHFR) is involved in the remethylation pathway, in which homocysteine is converted back to methionine. Homocysteine is present in human plasma in four forms: 1% as free thiol, 20%-30% as disulfur with itself and others thiols, 70%-80% as disulphide bound to plasma proteins. The sum of all the forms of homocysteine existing in plasma is referred as “total plasma homocysteine” (tHcy)[1,2].
Folic acid, vitamin B6 and vitamin B12 are essential cofactors in homocysteine metabolism and a lack of them due to a deficient diet or disease can produce elevated plasma homocysteine[2,3]. Increased homocysteine concentrations are thought to directly affect carcinogenesis by diminishing DNA methylation in critical tissues through a simultaneous increase in intracellular S-adenosylhomocysteine[4]. Inclusion of homocysteine in the assessment of folate in carcinogenesis is important because we may deal with an issue of inadequate folate metabolism, which is indicated by the reduced function of enzymes involved in homocysteine metabolism, rather than merely a state of folate deficiency. However, to our knowledge, only one study has reported that there is no significant positive association between serum homocysteine and colorectal cancer[5]. Despite the substantial amount of published data related to folate and colorectal carcinogenesis, the mechanisms responsible for this effect remain unclear. Plausible mechanisms described in the literature include the role of folate in the metabolism of one-carbon units, such as methyl, methylene, and formyl groups involved in various substrates and a variety of enzymatic reactions that are intimately related to DNA and RNA synthesis and cell proliferation[4-6]. It was recently proposed that an imbalance between biological methylation and nucleotide synthesis is a key to clarifying the mechanisms responsible for the role of folate in carcinogenesis[4-7]. In addition, lower folate intakes, compared with higher supplements, have been shown to be associated with a significantly higher risk of Ki-ras mutations (prominent in colorectal neoplasia) in adenomas[8] and carcinomas[9].
Homocysteine metabolism has been shown to be dependent on genetic factors as well as acquired factors[10]. Of the gene defects, the most common is the C-to-T substitution at nucleotide 677 in the coding region of the gene for MTHFR, the so-called thermolabile variant. There are an elevated homocysteine concentration and a decreased plasma folate concentration in the homozygous mutant genotype of C677TMTHFR gene. An adequate folate intake is thus required to decrease the elevated homocysteine concentration[11,12]. The C677TMTHFR polymorphism might modulate the risk of developing neoplasia through its effects on folate metabolism. It regulates the production of thymidylate and purines for DNA synthesis and supply of methyl groups for the synthesis of methionine and DNA methylation[4]. It has also been proposed that the protective effect of folate against the risk of colon cancer is stronger among subjects with a positive family history of colorectal cancer than among those without such a history[13]. This study was to investigate the pattern of tHcy levels and the C677TMTHFR polymorphism in a group of neoplastic patients with non metastatic colorectal cancer, who were eligible for curative surgery, by determining tHcy levels in neoplastic patients and then clarifying whether this was due to a genetic enzymatic defect or was a consequence of cancer cell proliferation if the levels were increased.
MATERIALS AND METHODS
Subjects
Ninety-three consecutive colorectal carcinoma patients (51 men and 42 women, mean age 62 years, range 48-78 years), admitted to our surgery department for elective and curative surgery, were enrolled in the study. The criteria for exclusion from the study were as follows: malnutrition, serum creatinine level > 13 mg/L, assumption of vitamin supplements or other drugs which might interfere with plasma tHcy levels, pernicious anaemia, skin diseases, heavy smoking, and alcohol abuse. The subsite distribution of the 93 colorectal cancers showed 19 in right colon, 6 in transverse colon, 45 in left colon, and 23 in rectum. Diagnosis of adenocarcinoma was confirmed in all patients by histology and cytologic investigations. TNM stages of resected tumors were as follows: stage 1 = 6 cases (28%), stage 2 = 25 cases (27%) and stage 3 = 42 cases (45%). Histopatological grading of adenocarcinoma was: 2% well differentiated, 81% moderately differentiated, or 17% poorly differentiated (Table 1). The control group included 100 healthy individuals (56 men and 44 women, mean age 64 years, range 46-79 years), enrolled with the same exclusion criteria as the cancer patients, selected from the hospital staff and people attending our out-patient surgery (Table 1). The study was approved by the local ethical committee and informed consent was obtained from all participants.
Table 1.
Characteristics of control subjects and cancer patients
| Group | Sex (M/F) | Mean age ± SD (yr) (range) | TNM staging |
| Controls | 100 (56/44) | 64 ± 6 (46-79) | // |
| Cancer patients | 93 (51/42) | 62 ± 8 (48-78) | T1-3, N0-2, M0 |
Homocysteine determination
Blood samples were collected from the antecubital vein, after overnight fasting. They were drawn into vacuum tubes containing K3-EDTA, immediately put on ice and centrifuged at 2200 r/min for 20 min at 4°C. The supernatant platelet-poor plasma was stored at -80°C until assay. Total Hcy was quantified using the fluorescence polarization immunoassay (FPIA) on the IMx analyzer from Abbott Laboratories (Abbott Park, IL, USA). The Imx Hcy assay is based on reduction of the plasma samples with dithiothreitol and subsequent conversion of free Hcy to S-adenosyl homocysteine by hydrolase in the presence of added adenosine. The sample and the tracer compete in binding to the monoclonal antibody. This reaction is followed by detection of S-adenosyl homocysteine by a fluorescence polarization immunoassay. The concentration of tHcy in plasma is inversely related to the intensity of the polarized light. The within-run CV for fasting tHcy was 2.2%-1.5% and the between-run CV was 2.2%-3.0%[14].
Genotyping of C677T MTHFR mutation
Genomic DNA was isolated from peripheral blood leukocytes. Screening for the MTHFR 677C→T substitution was performed by polymerase chain reaction (PCR) of genomic DNA, followed by Hinf I digestion and agarose gel electrophoresis. The region surrounding position 677 in the MTHFR gene was amplified by PCR technique, and the alleles were identified through restriction enzyme digestion as previously described[15]. PCR reactions of 15 μL contained 30 ng template genomic DNA and 1.5 U AmpliTaqGold DNA polymerase and the following concentrations of reagents: 0.16 μmol/L of each oligonucleotide primer, 1.6 mol/L of each dNTP, 15 mmol/L Tris-HCl (pH 8.0), 50 mmol/L KCl and 1.5 mmol/L MgCl2. Verification of amplification was achieved by comparing the 173-bp products with molecular weight standards in a gel made from a combination of 3% BibcoBRL ultrapure agarose and 1.5% NuSieve GTG agarose run for 1 h at 150 V and visualized with ethidium bromide. Reaction products (5 μL) were digested for 1 h at 37°C with 2.5 U of Hinf I in buffer at pH 7.9 of 10 mmol/L Tris-HCl, 10 mmol/L MgCl2, 50 mmol/L NaCl and 1 mmol/L dithiothreitol. Genotypes were determined by analysis of restriction patterns after electrophoresis on agarose gels (as above) with T at position 677, resulting in fragments of 125 and 48 bp in length and with C at position 677, resulting in a single fragment of 173 bp. We referred to the C/C genotype as wild-type, the C/T genotype as the heterozygous variant and the TT genotype as the homozygous variant.
Statistical analysis
Normally distributed continuous variables were analysed using the Student’s t test. To assess the normal distribution we applied the Kolmogorov-Smirnov test on each sample data. Non-normally distributed variables were analysed using the Mann-Whitney test. We used the χ2 test for group comparison frequencies. Continuous variables were compared by Student’s t test or the ANOVA when multiple statistical hypotheses were assumed. All P values were two-sided. A significance level of 95% (P < 0.05) was assumed. Statistical analysis was performed by SPSS (v. 11.0).
RESULTS
In the control group there were 51 heterozygous and 19 homozygous carriers of the C677T MTHFR polymorphism (Table 2), with a prevalence of 51% of the C/T genotype and 19% of the T/T genotype that corresponded to a T allele frequency of 44.5%. Among the colorectal cancer group we found 40 heterozygous and 21 homozygous carriers of the C677T MTHFR polymorphism, with a prevalence of 45.5% of the C/T genotype and 23.8% of the T/T genotype that corresponded to a T allele frequency of 46.5%. The prevalence of both the genotypes (Table 2) like the frequency of the T allele was not statistically different between the groups.
Table 2.
tHcy plasma levels according to C677T MTHFR polymorphism [mean ± SD (μmol/L) (n)]
| Group (n) | CC genotype | CT genotype | TT genotype |
| Control subjects (100) | 9.01 ± 2.76 (30) | 9.71 ± 2.34 (51) | 17.56 ± 10.81df (19) |
| Cancer patients (93) | 11.82 ± 3.09a (32) | 12.45 ± 4.46b (40) | 16.88 ± 9.25 df (21) |
P < 0.05,
P < 0.01 vs control;
P < 0.01 TT vs CC;
P < 0.01 TT vs CT.
When patients were subdivided according to cancer stage (regional versus in situ/localized), the prevalence of T/T genotype was 21.42% (9 cases) in stage 3 and 23.5% (12 cases) in stage 1/2, while the prevalence of T/T genotype according to cancer location (colon versus rectum) was 21.42% (15 cases) in colon and 26.08% (6 cases) in rectum. The prevalence of T/T genotype was not statistically different when both stage and location were considered. The mean total Hcy level was statistically higher in cancer patients than in control subjects carrying the same C/C or C/T genotype (Table 2 and Figure 1, P < 0.04 and P < 0.01 respectively). In addition, when all the C/C and C/T carriers were considered together and the two study groups were compared, tHcy was still more significantly higher in the cancer group (12.17 ± 3.90 vs 9.45 ± 2.51, P < 0.002, Table 3, Figure 2). In contrast there was no difference in the mean total Hcy concentrations among the T/T genotype carriers of the two study groups (P = 0.6). In both groups tHcy was significantly higher in the T/T genotype than in the C/C and C/T genotypes, although there was no difference between the C/T and C/C genotype carriers (Table 2, Figure 2). Total plasma Hcy was not associated with tumor stage (regional versus in situ/localized) or location (colon versus rectum): 12.53 ± 6.43 μmol/L in colon, 13.82 ± 6.08 μmol/L in rectum, 12.62 ± 5.21 μmol/L in stage 3 and 12.97 ± 7.15 μmol/L in stages 1 and 2 .
Figure 1.
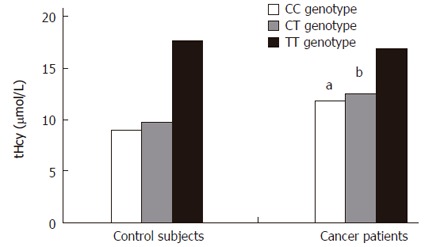
Mean tHcy plasma levels according to C677T MTHFR genotype, aP < 0.04, bP < 0.01 vs control.
Table 3.
tHcy plasma levels according to CC + CT genotype of C677T MTHFR polymorphism [mean ± SD (μmol/L) (n)]
| Group | (CC+CT) genotype |
| Control subjects | 9.45 ± 2.51 (81) |
| Cancer patients | 12.17 ± 3.90b (72) |
P < 0.002 vs control.
Figure 2.
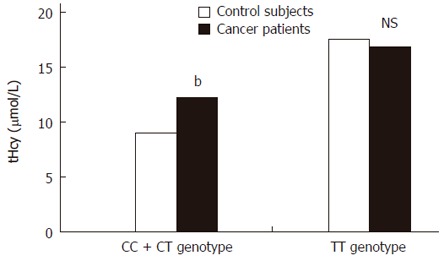
Mean tHcy plasma levels according to C677T MTHFR polymorphism, bP < 0.002 vs control; NS: Not significant.
DISCUSSION
In our group of colorectal cancer patients the T allele frequency, like the prevalence of the C/T and T/T genotypes of the C677T MTHFR polymorphism, was similar to that in the control subjects, whereas the mean tHcy concentration was significantly increased in the cancer patients carrying the C/C or C/T genotype. This difference was even more evident when the C/C plus C/T genotype carriers of each group were compared. This finding is difficult to explain because the increase of tHcy was not be simply due to the decreased MTHFR enzyme activity, but rather due to a more complex disorder of folate metabolism, probably partly related to the methionine-dependency of the proliferation rate of colorectal tumor cells. It is well known that most malignant cells, including colon cancer cell lines in primary histoculture need methionine, but endogenous methionine conversion from homocysteine does not meet the increased metabolic demand of these cells[16]. Insufficient methionine synthesis in cancer cells can result from a lack of reduced folate as a cofactor in the homocysteine 5-methyl-tetrahydrofolate methyltransferase reaction. In fact the reduced folates may be drained into other pathways that are enhanced in proliferating cells, such as the synthesis of purines and pyrimidines. As a consequence there is an increase of homocysteine in dividing cells, resulting in a greater egress of it from such cells. High tHcy levels may therefore be a phenotypic expression of malignancy, as well as a marker for cancer cell activity.
Serum concentration of homocysteine increases with folate deficiency because the metabolic disposal of homocysteine depends on a remethylation reaction in which N-5-methyltetrahydrofolate serves as a co-substrate with homocysteine[17]. In practice, an elevation of this amino acid has been found to be a more sensitive indicator of cellular folate depletion than its blood folate concentations[18]. The importance of homocysteine concentration as a biological marker is highlighted by Kim et al[19], who found that blood folate and homocysteine are correlated with colonic mucosal folate concentration.
Since we did not measure plasma folic acid, vitamin B12 and B6 concentrations, if the increase of tHcy observed in our cancer patients is a consequence of nutritional deficiency or substrate consumption induced by active neoplastic proliferation remains unknown. Assuming that our cancer patients had the same nutritional habits as our control subjects, the hypothesis is that more active consumption of substrates is more likely secondary to the metabolism of one-carbon units related to DNA and RNA synthesis[5-8].
According to previous reports regarding the Italian population[20], we found a similar and increased prevalence of T/T genotype carriers, in both our cancer and control groups (21% and 22.4% respectively). The total Hcy concentration in these subgroups was similar both in cancer patients and in control subjects, g suggesting that the T/T genotype has a stronger influence on tHcy concentration, thus overcoming the biochemical cancer effect.
It has been reported that the T/T genotype of the C677T MTHFR gene polymorphism is associated with a decreased risk of colorectal cancer[21,22]. We can argue that in our subjects, both in the control and cancer groups, there is a depletion of folate. In the folate deficiency, the high activity of the enzyme or the C/C genotype may be disadvantageous because 5, 10-methylenetetrahydrofolate is converted and the thymidylate pool is depleted. Increased risk for T/T genotype versus C/C genotype would be seen in the folate-depleted situation if aberrant DNA methylation is a primary mechanism, but no such increase has been observed[23]. We have no data about serum folate, but the increase of total Hcy observed in our T/T genotype carriers may also be an index of low folate status, a condition that confers no protection against cancer risk, as reported by Ulvik et al[24]. Moreover, because the number of individuals carrying the T/T genotype in both our groups is low, it is possible to hypothesize that a modest influence of cancer on tHcy exists, although it was not shown by our data. Of note is that a previous study investigating a large cohort of Caucasian colorectal cancer cases and matched controls, reported that C677T MTHFR polymorphism is associated with total Hcy[24]. It cannot be excluded that the difference found in our study may have been biased by the relatively small number of patients and controls with different genotypes. The relatively high SD value for T/T genotype carriers supports this possibility. The T/T genotype of the C677T MTHFR variant has less than one third of the functional activity of the normal MTHFR enzyme and is associated with reduced cellular folate and methionine levels, reduced cellular ratio of S-adenosylmethionine to S-adenosylhomocysteine, as well as elevated homocysteine levels[25]. Based on Frosst’s data, a normal C/C homozygote has 100% activity, a C/T homozygote approximately 65% activity, and a T/T homozygote approximately 30% activity[26]. Finally, it is important to emphasise that hyperhomocysteinaemia is a well-known and independent risk factor for thrombosis[27]. Moderately elevated levels of tHcy are associated with an increased risk of thrombosis, through mechanisms that are incompletely understood. In particular, there is evidence that hyperhomocysteinemia increases the risk of venous thromboembolism approximately two to four-folds[28-30], contributing to the hypercoagulable state that characterises the malignant disease[31].
In summary, the results of our study suggest that the modest but significant increase of plasma tHcy observed in the C/C and C/T genotype carriers in our cancer group, may be related to the methionine-dependent proliferation rate of colorectal cancer cells and moreover, may act as a permissive factor for thrombosis in the context of cancer thrombophilia. The tHcy increase observed in T/T genotype carriers in both groups, on the other hand, is probably dependent on the enzymatic deficit associated with the homocysteine conversion to methionine and/or the depletion of folate.
Footnotes
Supported by a research grant from the University of Siena (PAR)
S- Editor Pan BR L- Editor Wang XL E- Editor Liu WF
References
- 1.Jacobsen DW. Homocysteine and vitamins in cardiovascular disease. Clin Chem. 1998;44:1833–1843. [PubMed] [Google Scholar]
- 2.Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists' Collaboration. BMJ. 1998;316:894–898. [PMC free article] [PubMed] [Google Scholar]
- 3.Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001;73:613–621. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- 4.Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr. 2002;132:2413S–2418S. doi: 10.1093/jn/132.8.2413S. [DOI] [PubMed] [Google Scholar]
- 5.Kato I, Dnistrian AM, Schwartz M, Toniolo P, Koenig K, Shore RE, Akhmedkhanov A, Zeleniuch-Jacquotte A, Riboli E. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer. 1999;79:1917–1922. doi: 10.1038/sj.bjc.6690305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 7.Ryan BM, Weir DG. Relevance of folate metabolism in the pathogenesis of colorectal cancer. J Lab Clin Med. 2001;138:164–176. doi: 10.1067/mlc.2001.117161. [DOI] [PubMed] [Google Scholar]
- 8.Martínez ME, Maltzman T, Marshall JR, Einspahr J, Reid ME, Sampliner R, Ahnen DJ, Hamilton SR, Alberts DS. Risk factors for Ki-ras protooncogene mutation in sporadic colorectal adenomas. Cancer Res. 1999;59:5181–5185. [PubMed] [Google Scholar]
- 9.Slattery ML, Curtin K, Anderson K, Ma KN, Edwards S, Leppert M, Potter J, Schaffer D, Samowitz WS. Associations between dietary intake and Ki-ras mutations in colon tumors: a population-based study. Cancer Res. 2000;60:6935–6941. [PubMed] [Google Scholar]
- 10.Yates AA, Schlicker SA, Suitor CW. Dietary Reference Intakes: the new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J Am Diet Assoc. 1998;98:699–706. doi: 10.1016/S0002-8223(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 11.Christensen B, Frosst P, Lussier-Cacan S, Selhub J, Goyette P, Rosenblatt DS, Genest J Jr, Rozen R. Correlation of a common mutation in the methylenetetrahydrofolate reductase gene with plasma homocysteine in patients with premature coronary artery disease. Arterioscler Thromb Vasc Biol. 1997;17:569–573. doi: 10.1161/01.atv.17.3.569. [DOI] [PubMed] [Google Scholar]
- 12.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs CS, Willett WC, Colditz GA, Hunter DJ, Stampfer MJ, Speizer FE, Giovannucci EL. The influence of folate and multivitamin use on the familial risk of colon cancer in women. Cancer Epidemiol Biomarkers Prev. 2002;11:227–234. [PubMed] [Google Scholar]
- 14.Zighetti ML, Chantarangkul V, Tripodi A, Mannucci PM, Cattaneo M. Determination of total homocysteine in plasma: comparison of the Abbott IMx immunoassay with high performance liquid chromatography. Haematologica. 2002;87:89–94. [PubMed] [Google Scholar]
- 15.Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet. 1998;62:1258–1260. doi: 10.1086/301836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo HY, Herrera H, Groce A, Hoffman RM. Expression of the biochemical defect of methionine dependence in fresh patient tumors in primary histoculture. Cancer Res. 1993;53:2479–2483. [PubMed] [Google Scholar]
- 17.Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J Clin Invest. 1988;81:466–474. doi: 10.1172/JCI113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992;55:131–138. doi: 10.1093/ajcn/55.1.131. [DOI] [PubMed] [Google Scholar]
- 19.Kim YI, Fawaz K, Knox T, Lee YM, Norton R, Arora S, Paiva L, Mason JB. Colonic mucosal concentrations of folate correlate well with blood measurements of folate status in persons with colorectal polyps. Am J Clin Nutr. 1998;68:866–872. doi: 10.1093/ajcn/68.4.866. [DOI] [PubMed] [Google Scholar]
- 20.Abbate R, Sardi I, Pepe G, Marcucci R, Brunelli T, Prisco D, Fatini C, Capanni M, Simonetti I, Gensini GF. The high prevalence of thermolabile 5-10 methylenetetrahydrofolate reductase (MTHFR) in Italians is not associated to an increased risk for coronary artery disease (CAD) Thromb Haemost. 1998;79:727–730. [PubMed] [Google Scholar]
- 21.Kono S, Chen K. Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci. 2005;96:535–542. doi: 10.1111/j.1349-7006.2005.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Marchand L, Wilkens LR, Kolonel LN, Henderson BE. The MTHFR C677T polymorphism and colorectal cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14:1198–1203. doi: 10.1158/1055-9965.EPI-04-0840. [DOI] [PubMed] [Google Scholar]
- 23.Keku T, Millikan R, Worley K, Winkel S, Eaton A, Biscocho L, Martin C, Sandler R. 5,10-Methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and whites. Cancer Epidemiol Biomarkers Prev. 2002;11:1611–1621. [PubMed] [Google Scholar]
- 24.Ulvik A, Vollset SE, Hansen S, Gislefoss R, Jellum E, Ueland PM. Colorectal cancer and the methylenetetrahydrofolate reductase 677C -> T and methionine synthase 2756A -> G polymorphisms: a study of 2,168 case-control pairs from the JANUS cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:2175–2180. [PubMed] [Google Scholar]
- 25.Martínez ME, Henning SM, Alberts DS. Folate and colorectal neoplasia: relation between plasma and dietary markers of folate and adenoma recurrence. Am J Clin Nutr. 2004;79:691–697. doi: 10.1093/ajcn/79.4.691. [DOI] [PubMed] [Google Scholar]
- 26.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 27.Refsum H, Ueland PM, Nygård O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 28.den Heijer M, Koster T, Blom HJ, Bos GM, Briet E, Reitsma PH, Vandenbroucke JP, Rosendaal FR. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334:759–762. doi: 10.1056/NEJM199603213341203. [DOI] [PubMed] [Google Scholar]
- 29.Simioni P, Prandoni P, Burlina A, Tormene D, Sardella C, Ferrari V, Benedetti L, Girolami A. Hyperhomocysteinemia and deep-vein thrombosis. A case-control study. Thromb Haemost. 1996;76:883–886. [PubMed] [Google Scholar]
- 30.Makris M. Hyperhomocysteinemia and thrombosis. Clin Lab Haematol. 2000;22:133–143. doi: 10.1046/j.1365-2257.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 31.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–V224. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]


