Abstract
AIM: To report a case of severe idiopathic gastroparesis in complete absence of Kit-positive gastric interstitial cells of Cajal (ICC).
METHODS: Gastric tissue from a patient with severe idiopathic gastroparesis unresponsive to medical treatment and requiring surgery was analyzed by conventional histology and immunohistochemistry.
RESULTS: Gastric pacemaker cells expressing Kit receptor had completely disappeared while the local level of stem cell factor, the essential ligand for its development and maintenance, was increased. No signs of cell death were observed in the pacemaker region.
CONCLUSION: These results are consistent with the hypothesis that a lack of Kit expression may lead to impaired functioning of ICC. Total gastrectomy proves to be curative.
Keywords: C-kit, Gastroparesis, Interstitial cells of Cajal, Stem cell factor
INTRODUCTION
Gastroparesis syndrome is a clinical entity characterized by chronic nausea, epigastric discomfort and recurrent vomiting, in the absence of mechanical obstruction[1]. Gastroparesis may be either primary (idiopathic) or secondary, i.e. associated with illnesses or specific disorders that are the likely cause[2]. Apart from common secondary causes of gastroparesis, such as diabetes mellitus and gastric surgery, most cases are idiopathic. Although in recent years some previously-labeled “idiopathic” gastroparesis cases have been shown to have a probable causal relationship with prior viral infections[3] or myoenteric plexus ganglionitis[4], in most cases a definite cause or even a possible link remains undetected.
Indirect evidence from electrogastrographic[5-7] and manometric[8,9] studies suggests that, at least in some patients, gastric hypomotility may be due to alteration of mechanisms controlling the motor activity of the stomach. Interstitial cells of Cajal (ICC), whose importance has been suspected for decades due to their close anatomical relationship with smooth muscle cells and terminal varicosities of enteric nerves[10-12], are the pacemaker cells in the gastrointestinal tract[13-16] and can mediate input from the enteric nervous system[17,18]. It may thus be expected that disruption of ICC networks leads to disordered gastrointestinal motility in the affected segments[19]. Research into ICC biology has been greatly stimulated by the discovery that they express the proto-oncogene c-kit[20,21], and activation of Kit tyrosine kinase receptor signaling by the natural ligand stem cell factor (SCF or steel)[22,23] is necessary for development and maintenance of ICC phenotype[15,24].
Since ICC can express Kit, immunohistochemical analysis using anti-Kit antibodies provides an efficient means of identifying them in a variety of species, including humans[25-27]. In the last few years several reports have demonstrated that ICC abnormalities are associated with some human upper-gut pathological conditions[28,29], although few studies in gastroparetic patients to date are available.
An abnormal reduction of pacemaker cells in the stomach has been recently described in a murine model of gastroparesis[30] and in a case of idiopathic gastroparesis[31]. Here we report a case of severe idiopathic gastroparesis with complete absence of Kit-positive gastric ICC.
MATERIALS AND METHODS
Case history
A 39-year old woman was investigated for a long-lasting complaint of severe dyspeptic symptoms. Her past history included repeated upper GI series and endoscopic examinations, always unremarkable in 1989-1995. She was treated with antacids, sucralfate, clebopride and ranitidine, with little or no success. In 1996 she received our attention for persisting dyspeptic symptoms associated with gastro-esophageal reflux. Physical examination and blood chemistry were unremarkable. An upper panendoscopy showed antral erythema and erosions, and histologic specimens revealed the presence of active gastritis associated with H pylori colonization. After eradication therapy, the patient continued to complain of severe post-prandial discomfort and epigastric fullness, sometimes associated with food regurgitation. Esophageal manometry and pH-metry were normal. Scintigraphic gastric emptying of a 300 kcal standard meal (Tc-albumin labeled egg white)[32] was severely delayed (the t½ at 120 min was 474, normal values 60-150). Abdominal ultrasound scans, small bowel enema and nuclear magnetic resonance of the brain and brainstem were normal. No autonomic dysfunction was documented. Therapeutic courses of high dose cisapride and domperidone were ineffective, as were oral and intravenous erythromycin.
In 1997, since the symptoms continued to worsen with the appearance of vomiting and weight loss, a percutaneous jejunostomy was thus required for feeding. However, in March 2000, due to a poor quality of life, she was again admitted and an upper gastrointestinal manometry[33] showed almost no fasting and post-prandial gastric motility, whereas duodenojejunal motor activity was within normal limits. A total gastrectomy with esophago-jejunal anastomosis and Roux-en-Y reconstruction was carried out. The post-surgical period was uneventful and she was discharged after 10 d. She subsequently enjoyed a good health, regained body weight, and did not complain of abdominal problems at follow-up.
Histology and immunohistochemistry
Multiple full-thickness samples from the stomach (fundus, corpus, and antrum) were obtained at surgery, and processed for conventional histological examination (H&E, Gomori's trichrome) and immunohistochemistry. The myenteric plexuses were assessed with a rabbit polyclonal anti-S-100 antibody (Dako, Carpinteria, CA, USA, dilution 1:4000), a monoclonal anti-CD56 antibody (clone 1B6, Novocastra Laboratories, Newcastle-upon-Tyne, UK, dilution 1:50), a rabbit polyclonal anti-neuron specific enolase (NSE) antibody (Dako, dilution 1:1000), and a monoclonal anti-neurofilament antibody (clone 2F11, Immunon, Shandon, Pittsburgh, PA, prediluted). Expression of Kit and SCF was assessed using a rabbit polyclonal antibody (Dako, dilution 1:50) and a goat polyclonal antiserum produced against a peptide mapping at the amino terminus of SCF of human origin (Santa Cruz Biotechnology, Santa Cruz, CA, dilution 1:100), respectively. Briefly, consecutive formalin-fixed and paraffin-embedded sections were dewaxed and rehydrated through alcohol series up to distilled water. Sections were subjected to heat-induced epitope retrieval (CD56, neurofilaments, Kit and SCF) by immersion in a heat-resistant container filled with citrate buffer solution (pH 6.0) placed in a pressure cooker and micro-waved for 20 min. Endogenous peroxidase activity was suppressed by incubation with 3% solution of H2O2 for 5 min. Immunostaining for CD56, Kit and SCF was done with a peroxidase-based visualization DAKO EnVisionTM kit, following the manufacturer’s recommendations. Immunostaining for S100, NSE, and neurofilaments was performed with a peroxidase-based visualization DAKO LSAB® kit, following the manufacturer’s recommendations. Diaminobenzidine tetrahydrochloride was used as chromogen. The slides were then counterstained with Mayer hematoxylin for 5 s, dehydrated and mounted in Clarion (Biomeda, Foster City, CA, USA).
To determine the non-specific staining, peptides blocking polyclonal antibody binding were used or sections were incubated in the absence of primary antibody. In these cases, no immunostaining was detected. Kit-positive mast cells serving as an internal control, were counted under a light microscope at × 200 magnification within a square micrometer and expressed as mean (SE) per surface area (1 mm2). Quantification of Kit-positive ICC and nervous structures was performed by manual procedures under optical microscope (Carl Zeiss-Axioscop, Germany) and computer-assisted image analysis system (Immagini&Computer, Bareggio, Milano, Italy) using the Image Pro Plus program (Media, Cybernetics, Silver Spring, MD, USA). Five areas (1 mm2) were selected from similar regions in patient and control tissues. The area of immunopositive cells was calculated and expressed as a mean percentage of the total area of the image.
SCF staining patterns were evaluated using the immunoreactive score (IRS) proposed by Remmele[34] in which IRS = staining intensity (SI) × percentage of positive cells (PP). SI was determined as 0 = negative, 1 = weak, 2 = moderate, 3 = strong. PP was defined as 0 = negative, 1 = 1%-20% positive cells, 2 = 21%-50% positive cells, 3 = 51%-100% positive cells. Ten visual fields from different areas of each specimen were chosen at random for IRS evaluation, and the average (SE) was calculated.
Apoptosis detection
Apoptosis was detected by transferase-mediated digoxigenin-tagged 16-desoxy-uridine-triphosphate nick-end labeling (TUNEL) assay, using the DeathEndTM colorimetric TUNEL system (Promega, Madison, WI, USA). After deparaffinization and rehydration, sections were digested with 20 mg/L proteinase K (Sigma, st Louis, MO, USA) for 15 min at room temperature, and then incubated with a terminal transferase plus nucleotide mixture for 1.5 h at 37°C. After exposure to antidigoxigenin peroxidase for 45 min, diaminobenzidine (Sigma) was used as chromogen to detect TUNEL-positive cells, and the sections were counterstained with hematoxylin.
Controls
Control gastric tissue was obtained from 4 specimens of normal stomach taken during pancreasectomy for tumors of the pancreatic head.
Determination of SCF concentrations in sera
Serum samples collected from the patient prior to surgery and 10 age-matched healthy female volunteers, were assayed for soluble SCF using a commercially-available ELISA kit (R&D Systems, Abingdon, UK), following the manufacturer’s instructions. All samples were evaluated in duplicate. The lower threshold of detection of this assay is 9 ng/L.
RESULTS
Histological examination
The mucosal, submucosal, and smooth muscle architecture appeared normal at conventional H&E staining. Trichrome stain revealed mild and scattered fibrosis of the submucosa plus some fibrosis of the external longitudinal mucosal layer as well. Neither intranuclear or viral inclusions nor any acute inflammatory cells were observed.
Immunohistochemistry
The submucous and myenteric plexus cells were abnormal in number and distribution, with ganglion cells reduced in number and size compared to normal counterparts (1.4% vs 3.9%). Kit immunodetection findings showed that scattered Kit-positive cells (identified as mast cells and used as an internal control) were observed in the patient's tissue, numerically distributed as in normal tissue (24.9/mm2 ± 2.6/mm2 vs 25.7/mm2 ± 3.8 /mm2) (Figures 1A and 1B). By contrast, in both parietal cells and intrinsic innervation locations Kit immunoreactivity was completly absent in the patient's tissue (Figures 1D and 1F), whereas in control tissues Kit was intensely expressed in 10% of mucosal parietal cells and at the level of the intrinsic innervation (1.8%) (Figures 1C and 1E). Since previous studies have shown that functional Kit-positive ICC are disappeared in the murine small bowel when Kit receptors are blocked[24], we used a TUNEL method to determine whether ICC undergo apoptosis in gastroparesis. No evidence for apoptosis was observed (data not shown).
Figure 1.
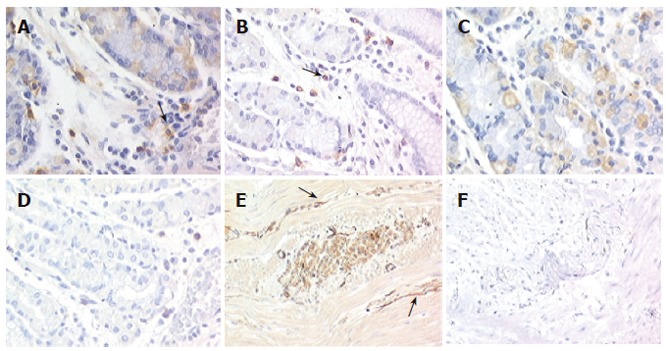
Expression of Kit in normal gastric tissue and gastroparesis (x 200). In both normal (A) and gastroparetic stomach (B), Kit was detected on mast cells (arrows) used as an internal control; In normal gastric samples, positivity is shown on parietal cells (C) and at the intrinsic innervation level (E, arrows), whereas in gastroparesis no staining is present at either levels (D, F).
Intracytoplasmatic expression of SCF was intense with membrane reinforcement in 70% of the patient’s parietal cells and weaker with no membrane reinforcement in controls (Figures 2A and 2B), while the positivity for SCF, absent at the intrinsic innervation level, was diffuse throughout the tunica muscularis, in both gastroparesis and normal tissues. In order to make quantitative comparisons of SCF staining patterns in normal and diseases tissues, immunoreactive scores were determined as described by Remmele et al[34] and subjected to statistical analysis, which showed significantly elevated levels of SCF in the patient’s tissue sample (Figure 3).
Figure 2.
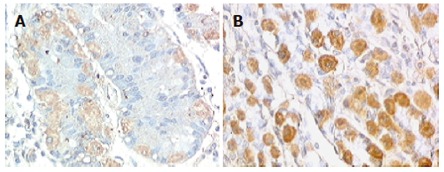
Expression of SCF in normal gastric tissue (A) and gastroparesis (B) (x 200). Intense intracytoplasmatic expression of SCF with membrane reinforcement in gastroparesis parietal cells was present.
Figure 3.
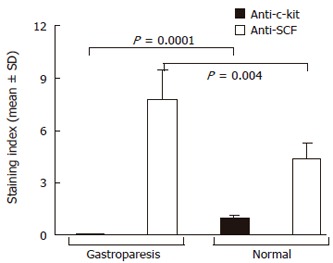
Quantitative evaluation of SCF expression on parietal cells in gastric tissue specimens from control subjects and the patient with gastroparesis. Differences in the immunoreactive score, obtained as described in the text, were assessed by t-test.
Serum levels of soluble SCF
Serum levels of SCF in the patient (332.7 ng/L) were below the range detected in controls (mean: 741 ng/L, range: 558-1441 ng/L).
DISCUSSION
Little is known about the pathogenesis of gastroparesis, and apart from causes secondary to well-known conditions (diabetes mellitus, gastric surgery) only sporadic cases of idiopathic myenteric ganglionitis[4] or infective causes (bacterial, viral) preceding the onset of symptoms have been identified[35,36]. Unfortunately, pathological studies of idiopathic gastroparesis are rare, and the early ones revealed no abnormalities of the smooth muscle or the myenteric plexus[37,38]. More recent evidence (with the introduction of Kit immunodetection) points to a reduction of gastric ICC in idiopathic gastroparesis, both in a murine model[30] and in a single case report[31], although the potential role in this condition of SCF, the critical factor for Kit signaling in ICC[39], has not been investigated.
We report a case of severe gastroparesis refractory to diverse therapeutic measures, which eventually required total gastrectomy to relieve the symptoms, and in which there was complete disruption of the Kit-positive ICC network together with a reduction in number and size of ganglion cells in the submucous and myenteric plexus vs normal counterparts. No signs of apoptosis in the pacemaker and myenteric regions were observed, suggesting that degenerative injuries are not involved in these abnormalities. To the best of our knowledge, a total lack of detectable ICC has never been described in a “functional” gastrointestinal disorder. Since the gastric ICC network is essential for slow wave generation and plays a key role in neurotrasmission of cholinergic excitation and inhibition due to nitric oxyde[17,40], this complete disruption likely explains the significant symptoms, the slow gastric emptying and the lack of response to therapeutic interventions. The lack of detectable Kit-positive ICC may be a primary event or secondary to a loss of connected signaling molecules. Previous studies have shown that ICC can be influenced to abandon the normal course of development, lose pacemaker function, and radically change its morphology if Kit is blocked or if an unsuitable form of SCF is presented[21,41].
The binding of SCF to Kit receptors is essential to initiate a signaling pathway in ICC, which is essential for their normal development and rhythmic activity in the gastrointestinal tract[16,42-45]. We also investigated the expression of SCF in the patient’s mucosa, and found that intracytoplasmatic and membrane SCF levels were particularly high in parietal cells vs controls. In addition, as in the normal counterpart, gastroparesis tissue showed diffuse positivity for SCF throughout the tunica muscularis, potentially in close proximity to ICC.
The steel locus encodes two distinct isoforms of SCF, both of which are synthesized as transmembrane proteins expressed at the cell surface[46]. However, one isoform, known as soluble SCF, is rapidly cleaved by proteolytic processing and released, while the other, known as membrane-bound SCF isoform, lacking the proteolytic cleavage site, releases soluble SCF much more slowly[47,48]. Soluble and membrane-bound isoforms of SCF are expressed in the gastrointestinal tract, but the role of each isoform in supporting ICC development is unknown. However, since spontaneous Steel-Dickie mutant mice that express soluble SCF exclusively do not display spontaneous, rhythmic electric slow wave activity, and ICC are not present in the myenteric plexus[21], membrane-bound SCF more likely plays an essential role in development and/or maintenance of ICC. Since membrane-bound SCF expression was not only retained but even increased in gastric parietal cells and supporting cells potentially in close proximity to ICC in the patient’stissue specimen, we can postulate that in this case of gastroparesis a defective expression of Kit receptor by ICC rather than the absence of these cells leads to accumulation of SCF in this case of gastroparesis. Studies showing that mice harboring inactivating mutations in the c-kit gene lack ICC and their gastrointestinal tract fails to display any slow wave-type action potential[16,24] are in favour of this hypothesis. Since the blocking of Kit receptors in the mouse causes transdifferentiation of ICC to a smooth muscle phenotype[35], this inherent plasticity between ICC and smooth muscle cells might underlie this clinical condition. If verified, this hypothesis might be exploited, because if ICC do not die in gastroparesis but redifferentiate, it might be possible to create conditions that would shift the phenotype back toward ICC.
Besides the increased local accumulation of SCF in this case of gastroparesis, the soluble circulating form estimated by specific ELISA in the serum, was found to be decreased in our patient compared with healthy subjects. This decreased SCF serum concentration is difficult to explain and correlate with the disease, because several cell types, such as stromal cells, fibroblasts and endothelial cells, present in different tissues as well as in the gastrointestinal tract, express and release SCF and may contribute to the circulating soluble form. Moreover, SCF is thought to act locally, close to the site of production, where the concentration is likely to be much higher[49].
This patient has undergone numerous treatments with several different prokinetic drugs, but none of them proved to be effective, as is often the case in this condition[50]. Recently, electrical stimulation of enteric nerves and/or pacing of gastric slow wave activity have been attempted in gastroparesis refractory to medical treatment[51]. Although reports appear to be encouraging (decreased frequency of vomiting and gastrointestinal symptoms, and improved quality of life)[52], it must be kept in mind that this is still to be considered an experimental option available only in a few research centers worldwide. Moreover, it should also take into consideration that, in some cases, pacing the stomach may not be effective in improving emptying when the primary cause of abnormal motility is due to insufficient excitation by the enteric nerves.
The above considerations also leave little room for other therapeutic options, such as injecting botulinum toxin into the pyloric sphincter[53], and a surgical approach may be necessary, and is possible if small bowel function is intact. Only limited data on surgical treatment of gastroparesis are available, showing that complete gastrectomy may be effective for surgical gastroparesis, whereas a more cautious approach is required for the diabetic or idiopathic forms[54].
In conclusion, we report a case of severe idiopathic gastroparesis, which was likely due to impaired gastric motility, the absence of ICC, or more likely due to their functional absence (i.e., ICC were present but not expressing Kit receptors, and therefore not functioning). Since this is the first reported abnormality, these findings may be useful for a better understanding of the pathophysiology of “idiopathic” gastroparesis and perhaps, for a more targeted therapeutic approach. Moreover, there is evidence that enteric abnormalities are also found in other so-called “functional” disorders (such as irritable bowel syndrome)[55], thus leading to a reconsideration of the classification of these entities, in which organic abnormalities are increasingly found.
Footnotes
Supported by a grant from MIUR (Roma,Italy) to G Emanuelli and partly by a grant from Regione Piemonte to G Bellone
S- Editor Wang GP L- Editor Wang XL E- Editor Liu WF
References
- 1.O'Donovan D, Feinle-Bisset C, Jones K, Horowitz M. Idiopathic and Diabetic Gastroparesis. Curr Treat Options Gastroenterol. 2003;6:299–309. doi: 10.1007/s11938-003-0022-9. [DOI] [PubMed] [Google Scholar]
- 2.Hornbuckle K, Barnett JL. The diagnosis and work-up of the patient with gastroparesis. J Clin Gastroenterol. 2000;30:117–124. doi: 10.1097/00004836-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Sigurdsson L, Flores A, Putnam PE, Hyman PE, Di Lorenzo C. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751–754. doi: 10.1016/s0022-3476(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 4.De Giorgio R, Barbara G, Stanghellini V, Cogliandro RF, Arrigoni A, Santini D, Ceccarelli C, Salvioli B, Rossini FP, Corinaldesi R. Idiopathic myenteric ganglionitis underlying intractable vomiting in a young adult. Eur J Gastroenterol Hepatol. 2000;12:613–616. doi: 10.1097/00042737-200012060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Telander RL, Morgan KG, Kreulen DL, Schmalz PF, Kelly KA, Szurszewski JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978;75:497–501. [PubMed] [Google Scholar]
- 6.Bortolotti M, Sarti P, Barbara L. Gastric myoelectrical activity in patients with chronic idiopathic gastroparesis. J Gastrointest Motil. 1990;2:104–108. [Google Scholar]
- 7.Chen JD, McCallum RW. Clinical applications of electrogastrography. Am J Gastroenterol. 1993;88:1324–1336. [PubMed] [Google Scholar]
- 8.Malagelada JR, Camilleri M, Stanghellini V. Manometric diagnosis of gastrointestinal motility disorders. New York: Thieme; 1986. [Google Scholar]
- 9.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94–99. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 10.Faussone Pellegrini MS, Cortesini C, Romagnoli P. [Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal's interstitial cells] Arch Ital Anat Embriol. 1977;82:157–177. [PubMed] [Google Scholar]
- 11.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells. Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 12.Suzuki N, Prosser CL, Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Physiol. 1986;250:G287–G294. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]
- 13.Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 16.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 17.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 19.Huizinga JD. Neural injury, repair, and adaptation in the GI tract. IV. Pathophysiology of GI motility related to interstitial cells of Cajal. Am J Physiol. 1998;275:G381–G386. doi: 10.1152/ajpgi.1998.275.3.G381. [DOI] [PubMed] [Google Scholar]
- 20.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 21.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 22.Williams DE, Eisenman J, Baird A, Rauch C, Van Ness K, March CJ, Park LS, Martin U, Mochizuki DY, Boswell HS. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990;63:167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- 23.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 24.Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–148. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- 25.Yamataka A, Kato Y, Tibboel D, Murata Y, Sueyoshi N, Fujimoto T, Nishiye H, Miyano T. A lack of intestinal pacemaker (c-kit) in aganglionic bowel of patients with Hirschsprung's disease. J Pediatr Surg. 1995;30:441–444. doi: 10.1016/0022-3468(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 26.Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996;111:279–288. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- 27.Horisawa M, Watanabe Y, Torihashi S. Distribution of c-Kit immunopositive cells in normal human colon and in Hirschsprung's disease. J Pediatr Surg. 1998;33:1209–1214. doi: 10.1016/s0022-3468(98)90152-x. [DOI] [PubMed] [Google Scholar]
- 28.Isozaki K, Hirota S, Miyagawa J, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol. 1997;92:332–334. [PubMed] [Google Scholar]
- 29.Ohshiro K, Yamataka A, Kobayashi H, Hirai S, Miyahara K, Sueyoshi N, Suda K, Miyano T. Idiopathic gastric perforation in neonates and abnormal distribution of intestinal pacemaker cells. J Pediatr Surg. 2000;35:673–676. doi: 10.1053/jpsu.2000.5940. [DOI] [PubMed] [Google Scholar]
- 30.Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 31.Zárate N, Mearin F, Wang XY, Hewlett B, Huizinga JD, Malagelada JR. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52:966–970. doi: 10.1136/gut.52.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanghellini V, Tosetti C, Corinaldesi R. Standards for non-invasive methods for gastrointestinal motility: scintigraphy. A position statement from the Gruppo Italiano di Studio Motilità Apparato Digerente (GISMAD) Dig Liver Dis. 2000;32:447–452. doi: 10.1016/s1590-8658(00)80267-4. [DOI] [PubMed] [Google Scholar]
- 33.Bassotti G, Bucaneve G, Furno P, Morelli A, Del Favero A. Double-blind, placebo-controlled study on effects of diclofenac sodium and indomethacin on postprandial gastric motility in man. Dig Dis Sci. 1998;43:1172–1176. doi: 10.1023/a:1018883102636. [DOI] [PubMed] [Google Scholar]
- 34.Remmele W, Hildebrand U, Hienz HA, Klein PJ, Vierbuchen M, Behnken LJ, Heicke B, Scheidt E. Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A Pathol Anat Histopathol. 1986;409:127–147. doi: 10.1007/BF00708323. [DOI] [PubMed] [Google Scholar]
- 35.Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501–1504. [PubMed] [Google Scholar]
- 36.Pande H, Lacy BE, Crowell MD. Inflammatory causes of gastroparesis: report of five cases. Dig Dis Sci. 2002;47:2664–2668. doi: 10.1023/a:1021036601462. [DOI] [PubMed] [Google Scholar]
- 37.You CH, Chey WY, Lee KY, Menguy R, Bortoff A. Gastric and small intestinal myoelectric dysrhythmia associated with chronic intractable nausea and vomiting. Ann Intern Med. 1981;95:449–451. doi: 10.7326/0003-4819-95-4-449. [DOI] [PubMed] [Google Scholar]
- 38.Shellito PC, Warshaw AL. Idiopathic intermittent gastroparesis and its surgical alleviation. Am J Surg. 1984;148:408–412. doi: 10.1016/0002-9610(84)90483-5. [DOI] [PubMed] [Google Scholar]
- 39.Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384–401. doi: 10.1002/(SICI)1097-4547(20000201)59:3<384::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 41.Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol. 1997;505(Pt 1):241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rich A, Miller SM, Gibbons SJ, Malysz J, Szurszewski JH, Farrugia G. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313–G320. doi: 10.1152/ajpgi.00093.2002. [DOI] [PubMed] [Google Scholar]
- 44.Klüppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of cajal in the mammalian small intestine. Dev Dyn. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Liu LW, Thuneberg L, Huizinga JD. Development of pacemaker activity and interstitial cells of Cajal in the neonatal mouse small intestine. Dev Dyn. 1998;213:271–282. doi: 10.1002/(SICI)1097-0177(199811)213:3<271::AID-AJA4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandiella A, Bosenberg MW, Huang EJ, Besmer P, Massagué J. Cleavage of membrane-anchored growth factors involves distinct protease activities regulated through common mechanisms. J Biol Chem. 1992;267:24028–24033. [PubMed] [Google Scholar]
- 48.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- 49.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 50.Camilleri M. Appraisal of medium- and long-term treatment of gastroparesis and chronic intestinal dysmotility. Am J Gastroenterol. 1994;89:1769–1774. [PubMed] [Google Scholar]
- 51.Tougas G, Huizinga JD. Gastric pacing as a treatment for intractable gastroparesis: shocking news. Gastroenterology. 1998;114:598–601. doi: 10.1016/s0016-5085(98)70544-x. [DOI] [PubMed] [Google Scholar]
- 52.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 53.Miller LS, Szych GA, Kantor SB, Bromer MQ, Knight LC, Maurer AH, Fisher RS, Parkman HP. Treatment of idiopathic gastroparesis with injection of botulinum toxin into the pyloric sphincter muscle. Am J Gastroenterol. 2002;97:1653–1660. doi: 10.1111/j.1572-0241.2002.05823.x. [DOI] [PubMed] [Google Scholar]
- 54.Jones MP, Maganti K. A systematic review of surgical therapy for gastroparesis. Am J Gastroenterol. 2003;98:2122–2129. doi: 10.1111/j.1572-0241.2003.07721.x. [DOI] [PubMed] [Google Scholar]
- 55.Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]


