Abstract
Differentiation between autoimmune pancreatitis and pancreatic cancer is sometimes difficult. It has been reported that serum IgG4 concentrations are significantly elevated and particularly high (>135 mg/dL) in autoimmune pancreatitis. Measurement of serum IgG4 has become a useful tool for differentiating between autoimmune pancreatitis and pancreatic cancer. However, we present a 74-year-old female with a markedly elevated serum IgG4 (433 mg/dL) who underwent pancreaticoduodenectomy for pancreatic cancer. Elevated serum IgG4 levels continued after the resection. On histology, adenocarcinoma of the pancreas accompanied with moderate lymphoplasmacytic infiltration infiltrated the lower bile duct and duodenum, but there were no findings of autoimmune pancreatitis. Although a small metastasis was detected in one parapancreatic lymph node, regional lymph nodes were swollen. Abundant IgG4-positive plasma cells infiltrated the cancerous areas of the pancreas, but only a few IgG4-positive plasma cells were detected in the noncancerous areas. Pancreatic cancer cells were not immunoreactive for IgG4. An abundant infiltration of IgG4-positive plasma cells was detected in the swollen regional lymph nodes and in the duodenal mucosa. We believe that the serum IgG4 level was elevated in this patient with pancreatic cancer as the result of an IgG4-related systemic disease that had no clinical manifestations other than lymphadenopathy.
Keywords: IgG4, Pancreatic cancer, Autoimmune pancreatitis
INTRODUCTION
Autoimmune pancreatitis is a unique form of pancreatitis, histopathologically characterized by dense lymphoplasmacytic infiltration and fibrosis of the pancreas with obliterative phlebitis[1,2]. Patients with this disease usually have a swollen pancreas with delayed enhancement on computed tomography (CT), irregular narrowing of the main pancreatic duct on endoscopic retrograde pancreatography, and a favorable response to steroid therapy[1-4]. Autoimmune pancreatitis has been frequently mistaken for pancreatic cancer for 3 major reasons: most patients are elderly (average age of 59[4]-61[5] years); painless obstructive jaundice is present due to the associated sclerosing cholangitis (65%[4]-86%[6]); and there is a mass in the pancreas.
In 2001, Hamano et al[7] reported that serum IgG4 concentrations were significantly elevated and particularly high in autoimmune pancreatitis and were closely associated with disease activity. They measured serum IgG4 levels in 20 patients with autoimmune pancreatitis and 70 patients with pancreatic cancer. A cutoff value for the serum IgG4 level of 135 mg per deciliter resulted in a high rate of accuracy (97%), sensitivity (95%), and specificity (97%) for the differentiation of autoimmune pancreatitis from pancreatic cancer. Since this report, IgG4 has become a serological marker of autoimmune pancreatitis[8,9]. Hirano et al[10] also stated that serum IgG4 concentrations are helpful for differentiating between autoimmune pancreatitis and pancreatic cancer, as none of their 23 patients with pancreatic cancer had a high serum IgG4 level. Here, we present a resected case of pancreatic cancer that had a high serum IgG4 level and discuss the possible mechanisms for the elevated serum IgG4 levels in this patient.
CASE REPORT
A 74-year-old female was admitted to our hospital in May 2005 complaining of jaundice. She had no history of other illness or alcohol abuse. On admission, there was no swelling of the salivary glands and the cervical lymph nodes were not palpable. Laboratory data included: total bilirubin 7.4 mg/dL (normal: 0.2-1.1 mg/dL), alkaline phosphatase 1466 IU/L (115-359 IU/L), aspartate aminotransferase 236 IU/L (11-25 IU/L), and alanine aminotransferase 431 IU/L (4-27 IU/L). Serum levels of tumor markers DUPAN 2 and Span 1 were elevated to 7526 U/mL (< 150 U/mL) and 101 U/mL (< 30 U/mL), respectively.
Abdominal ultrasonography demonstrated a 4 cm-sized hypoechoic mass in the pancreatic head with dilatation of the intrahepatic and extrahepatic bile ducts and the main pancreatic duct. On helical CT scan, the mass was hypoattenuated to the pancreas in the early phase, but attenuation increased in the delayed phase. Based on the CT finding of delayed enhancement, we examined the serological findings, suspecting autoimmune pancreatitis. Although the serum IgG was 1560 mg/dL (870-1700 mg/dL) and the antinuclear antibody test (ANA) was negative, the serum IgG4 measured by single radial immunodiffusion (The Binding Site, Birmingham, UK) was elevated to 433 mg/dL.
Endoscopic retrograde cholangiopancreatography showed stenosis of the lower portion of the common bile duct and the main pancreatic duct near the major duodenal papilla, and endoscopic nasobiliary drainage was performed. Histological examination of the biopsy specimen from the irregular erosive duodenal mucosa around the major duodenal papilla under duodenoscopy revealed infiltration of adenocarcinoma.
Given the diagnosis of pancreatic head cancer, a pancreaticoduodenectomy was performed. Histological examination revealed that moderately differentiated tubular adenocarcinoma arising in the head of the pancreas infiltrated to the lower bile duct and duodenum. A small histological metastasis was detected in one parapancreatic lymph node. Although moderate lymphoplasmacytic infiltration was observed in the cancerous areas, no findings of autoimmune pancreatitis were detected in the noncancerous areas of the pancreas.
The specimens resected during pancreaticoduodenectomy were fixed in 10% formaldehyde. Serial sections (4 μm thick) were cut from paraffin embedded tissue blocks. Sections of the pancreas, including the extrahepatic bile duct, gallbladder, duodenum, stomach and regional lymph nodes were immunostained using anti-CD4-T (Novocastra, Newcastle upon Tyne, UK), CD8-T (DakoCytomation, Glostrup, Denmark), and IgG4 (The Binding Site) with avidin-biotin-peroxidase complex (ABC). The number of immunohistochemically identified IgG4 positive plasma cells was counted per high power field (hpf). Four fields were analyzed per section. Immunohistochemically, abundant IgG4-positive plasma cells (> 20/hpf) and CD4- or CD8-positive T lymphocytes were seen infiltrating the cancerous lesion of the pancreas (Figure 1A), but only a few IgG4-positive plasma cells (< 3/hpf) were detected in the noncancerous areas of the pancreas (Figure 1B). Pancreatic cancer cells were not immunoreactive for IgG4. Regional lymph nodes were swollen up to 1.5 cm in diameter, and infiltration of abundant IgG4-positive plasma cells (> 20/hpf) was detected in almost all regional lymph nodes (Figure 1C). Abundant IgG4-positive plasma cells infiltrated the duodenum (Figure 1D), but there were only a few IgG4-positive plasma cells (< 3/hpf) detected in the extrahepatic bile duct, gallbladder, and gastric mucosa.
Figure 1.
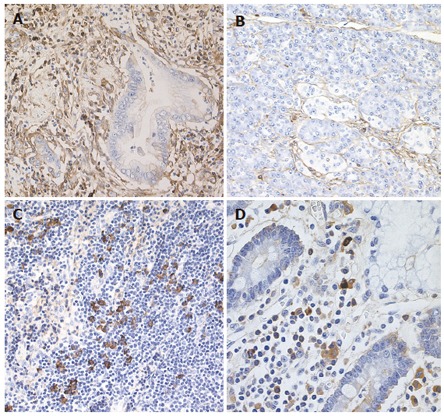
Immunostaining of IgG4 in the resected specimens. Abundant IgG4-positive plasma cells were detected in the cancerous lesion of the pancreas (A) but only a few IgG4-positive plasma cells were seen in the noncancerous areas of the pancreas (B). Infiltration of abundant IgG4-positive plasma cells was detected in regional lymph node (C) and the duodenal mucosa (D).
The patient was given chemotherapy as an outpatient and was well 6 mo later. Since the operation, her serum IgG4 levels have continued to be elevated (Figure 2).
Figure 2.
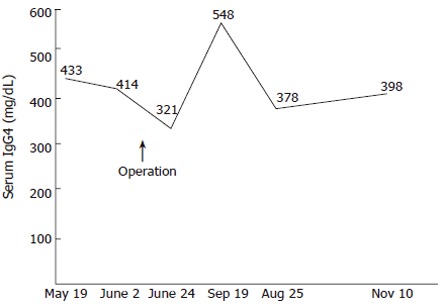
Course of serum IgG4 levels in this patient. Elevated serum IgG4 levels continued after operation.
DISCUSSION
Clinically, patients with autoimmune pancreatitis and pancreatic cancer share many features, including being elderly, having painless jaundice, new-onset diabetes mellitus, and elevation of tumor markers[1-4]. Radiologically, focal swelling of the pancreas, the “double-duct sign” representing stricture in both the biliary and pancreatic ducts, and angiographic abnormalities seen in pancreatic cancer patients are sometimes also observed in patients with autoimmune pancreatitis[1-4]. As autoimmune pancreatitis responds dramatically to steroid therapy[1-4,11], an accurate diagnosis of autoimmune pancreatitis can avoid unnecessary laparotomy or pancreatic resection. Imaging findings, such as a mass showing delayed enhancement and a capsule-like rim on dynamic CT, and a narrowing of the pancreatic duct associated with a less dilated upstream pancreatic duct, are all useful in differentiating pancreatic cancer from autoimmune pancreatitis[3,4]. Histopathological approach on biopsy is necessary in some cases, but it is sometimes difficult to obtain sufficient pancreatic tissue biopsy. Since Hamano et al[7] reported that serum IgG4 concentrations were significantly elevated and particularly high in autoimmune pancreatitis, measurement of serum IgG4 has become a useful tool in the differentiation of autoimmune pancreatitis and pancreatic cancer[8-10].
However, the present case had a histologically proven pancreatic cancer and displayed marked elevation of serum IgG4 concentration. The patient did not have any pathological conditions such as atopic dermatitis, parasitic disease, pemphigus vulgaris and foliaceus, which are sometimes associated with elevated serum IgG4 levels[7,9]. Interestingly, elevated serum IgG4 levels continued after the resection of the pancreatic cancer. Characteristic histological feature of autoimmune pancreas include dense lymphoplasmacytic infiltration and fibrosis of the pancreas[1,2]. In our patient, moderate lymphoplasmacytic infiltration was observed in the cancerous lesion, but an inflammatory process was not detected in the noncancerous areas of the pancreas. That is, there were no histological findings that suggested autoimmune pancreatitis in this patient’s pancreas. Immunohistochemically, pancreatic cancer cells were not positive for IgG4. Abundant IgG4-positive plasma cells infiltrated the cancerous areas of the pancreas, but only a few IgG4-positive plasma cells were detected in the noncancerous areas of the pancreas. Furthermore, many IgG4-positive plasma cells infiltrated almost all of the swollen regional lymph nodes and the duodenum.
Autoimmune pancreatitis is sometimes associated with sclerosing cholangitis, sclerosing sialadenitis, retroperitoneal fibrosis, and lymphadenopathy[12,13]. During follow-up of sclerosing sialadenitis, some patients developed autoimmune pancreatitis[13]. We have reported that lymphoplasmacytic infiltration with fibrosis was observed in the peripancreatic retroperitoneal tissue, biliary tract, and salivary glands, as well as in the pancreas of patients with autoimmune pancreatitis[13]. Furthermore, an abundant infiltration (> 30/hpf) of IgG4-positive plasma cells together with CD4- or CD8-positive T lymphocytes was observed in the various organs of patients with autoimmune pancreatitis, including the peripancreatic retroperitoneal tissue, biliary tract, salivary glands, gastric mucosa, lymph nodes, and pancreas, but an abundant infiltration of IgG4-positive plasma cells was not detected in the organs of patients with chronic alcoholic pancreatitis[13-15]. We therefore proposed the existence of a novel clinicopathological entity, an IgG4-related systemic disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis, and retroperitoneal fibrosis with lymphadenopathy, characterized by extensive IgG4-positive plasma cell and CD4- or CD8-positive T lymphocyte infiltration of organs[14,15]. We also suggested that autoimmune pancreatitis is not simply a pancreatitis but that, in fact, it is a pancreatic lesion reflecting an IgG4-related systemic disease, and that in some cases only organs other than the pancreas might be clinically involved[14,15].
In our patient, the IgG4-positive plasma cells infiltrating the lymph nodes were as abundant as those in the lymph nodes of patients with autoimmune pancreatitis, and elevation of serum IgG4 levels continued after the resection. We believe that the serum IgG4 level was elevated in this patient with pancreatic cancer as the result of an IgG4-related systemic disease that had no clinical manifestations other than lymphadenopathy. Furthermore, this case calls into question the specificity of elevated IgG4 levels for benign disease, and suggests that serum IgG4 levels should be interpreted with caution in patients with a mass in the pancreas but no histological documentation of either carcinoma or pancreatitis.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
References
- 1.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–2699. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605–1616. doi: 10.1111/j.1572-0241.2004.30336.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264–1269. doi: 10.1053/gast.2002.33022. [DOI] [PubMed] [Google Scholar]
- 6.Takayama M, Hamano H, Ochi Y, Saegusa H, Komatsu K, Muraki T, Arakura N, Imai Y, Hasebe O, Kawa S. Recurrent attacks of autoimmune pancreatitis result in pancreatic stone formation. Am J Gastroenterol. 2004;99:932–937. doi: 10.1111/j.1572-0241.2004.04162.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 8.Chen RY, Adams DB. IgG4 levels in non-Japanese patients with autoimmune sclerosing pancreatitis. N Engl J Med. 2002;346:1919. doi: 10.1056/NEJM200206133462420. [DOI] [PubMed] [Google Scholar]
- 9.Pezzilli R, Corinaldesi R. IgG4 as a serological marker of autoimmune pancreatitis: the latest news. JOP. 2004;5:531–533. [PubMed] [Google Scholar]
- 10.Hirano K, Komatsu Y, Yamamoto N, Nakai Y, Sasahira N, Toda N, Isayama H, Tada M, Kawabe T, Omata M. Pancreatic mass lesions associated with raised concentration of IgG4. Am J Gastroenterol. 2004;99:2038–2040. doi: 10.1111/j.1572-0241.2004.40215.x. [DOI] [PubMed] [Google Scholar]
- 11.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Morphological changes after steroid therapy in autoimmune pancreatitis. Scand J Gastroenterol. 2004;39:1154–1158. doi: 10.1080/00365520410008033. [DOI] [PubMed] [Google Scholar]
- 12.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Extrapancreatic lesions in autoimmune pancreatitis. J Clin Gastroenterol. 2005;39:904–907. doi: 10.1097/01.mcg.0000180629.77066.6c. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683–687. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 15.Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006;6:132–137. doi: 10.1159/000090033. [DOI] [PubMed] [Google Scholar]


