Abstract
Propylthiouracyl (PTU)-related liver toxicity is likely to occur in about 1% of treated patients. In case of acute or subacute hepatitis, liver failure may occur in about one third. We report two further cases of PTU-induced subacute hepatitis, in whom the delay between occurrence of liver damage after the initiation of treatment, the underestimation of its severity and the delayed withdrawal of the drug were all likely responsible for liver failure. The high incidence of liver toxicity related to PTU, its potential severity and delayed occurrence after initiation of treatment are in favor of monthly alanine aminotransferase monitoring, at least during the first six months of therapy.
Keywords: Hepatitis, Drug-induced liver toxicity, Propylthiouracil, Alanine aminotransferase
INTRODUCTION
Propylthiouracil (PTU)-related severe hepatitis remains a relatively rare occurrence[1,2] even if a transient increase in liver function tests is observed following the initiation of treatment in 15% to 28% of cases[2-4]. Before 1998, only 25 cases of PTU-induced severe liver injury were reported[5] and 83 cases with 28 deaths were included in a recent review of literature[6]. We here report two additional cases of PTU-induced severe liver injury, one was complicated by liver failure with hepatic encephalopathy. The late occurrence of liver toxicity and the underestimation of liver damage together with delayed drug withdrawal likely play an important role in their severity.
CASE REPORT
Case 1
A 35-year old woman was referred to our unit on February 4, 2004 for a two-month history of jaundice and pruritus. She was treated for hyperthyroidism with thiamazol, thiamazol and levothyroxin in 1997-1999. In early July 2003 three months following an uneventful delivery, she presented with features of relapsing hyperthyroidism, with her thyroid-stimulating hormone (TSH) unmeasurable (normal range: 0.2-3.5 microU/mL), T4 > 6 ng/dL (normal range: 0.8-2 ng/mL) and a strong positivity of antithyroid antibodies. She was treated with PTU (150 mg per day during the first 2 mo followed by 100 mg per day). In November 2003, she experienced upper abdominal discomfort, nausea and vomiting together with a weight-loss of 4 Kg. Thyroid testing performed on December 16 showed: TSH: 0.04, T4: O.95, free T3: 9.71. Liver biochemistry showed an increase in aspartate aminotransferase (AST) at 495 IU/L (normal value < 33) and ALT at 1575 IU/mL (normal value < 63). The total serum bilirubin level was 10 mg/dL. Despite this worsening in liver function test (LFT), therapy with PTU was however continued till its delayed withdrawal on January 21, 2004. She was referred to our unit on February 4, 2004 due to jaundice and fatigue together with the persistence of upper gastrointestinal (GI) symptoms.
She was deeply icteric with a hepatomegaly 4 cm under the right costal margin. Liver biochemistry showed alanine aminotransferase (ALT): 458 IU/L, AST: 307 IU/L, alkaline phosphatase (Alk. Phos): 139 IU/L (normal value < 94 IU/L), lactate dehydrogenase (LDH): 239 IU/L (normal value < 192 IU/L), and total s. bilirubin at 22.2 mg/dL. Serology for hepatitis A, B, C, CMV, EBV and herpes was negative. There was a slight positivity of pANCA and cANCA (1/160). At the time of admission, TSH was < 0.01, T4: 3.5 and anti-TPO antibodies: 665 (normal value < 100 U/mL). Upper abdominal ultrasound was unremarkable. Magnetic resonance imaging showed the presence of bands of necrosis scattered throughout the liver parenchyma. At transjugular hepatic vein catheterisation, the hepatic venous pressure was 9 mmHg suggestive of post-sinusoidal portal hypertension. A transvenous liver biopsy specimen showed replacement of the normal architecture by areas of multilobular necrosis infiltrated by mononuclear inflammatory cells and regenerating bile ducts (Figure 1A). The few preserved portal structures were enlarged and infiltrated, also showing interface hepatitis. Shortly after admission the patient exhibited features of hepatic encephalopathy with confusion, tremor and agitation which lasted for 8 d. EEG showed features compatible with severe liver encephalopathy (grade 3 of Child’s EEG scoring system). The biochemical condition then slowly improved, hyperthyroidism was treated with thiamazol (20 mg per day followed by 10 mg per day). The patient was discharged after 13 d, while her clinical and biochemical resolution occurred within 4 mo.
Figure 1.
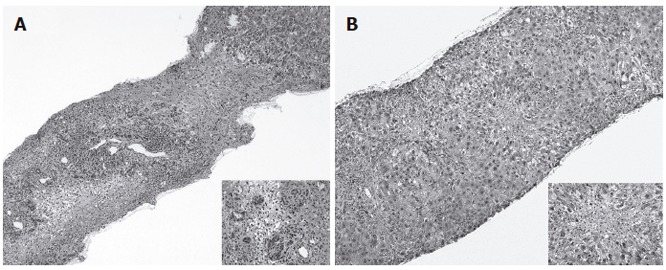
Liver histology of case 1 showing a picture of panlobular necrosis infiltrated with dense inflammatory infiltrates and ductular proliferation at higher magnification (close-up) (A), liver histology of case 2 showing enlarged and infiltrated portal tracts with mild interface hepatitis with prominent lobular necrosis in the centrilobular regions (close-up) (B).
Case 2
A 43-year old female was referred to our unit on March 6, 2004 for a history of 10-d jaundice, fever and upper GI symptoms. She had no previous medical history except for hyperthyroidism diagnosed in August 2003. The condition was first treated with thiamazol which was however rapidly withdrawn due to the occurrence of skin rash. A treatment with PTU (100 mg per day) was initiated in early October 2003, levothyroxin (75 mg/d) was added shortly therafter. At clinical examination upon referral, the patient was icteric with no sign of hyperthyroidism. The liver was felt 3 cm under the right costal margin. Liver biochemistry showed: total s. bilirubin: 10.9 mg/dL, AST: 2310 IU/L, ALT: 5040 IU/L. Serology was negative for hepatitis A and B as well as for antinuclear and smooth muscle antibodies. CMV-IgM as well as EBV-IgM antibodies were slightly positive. TSH was 4.09 micro U/mL, T4: 1.2, free T3: 1.9. Anti-TPO and anti-TG antibodies were both negative. Upper abdominal ultrasound showed a normal size hyperechogenic liver parenchyma. A transcutaneous liver biopsy was obtained which showed widely enlarged portal tracts infiltrated with lymphocytes and neutrophils together with ductular proliferation. Interface hepatitis was clearly visible and there were prominent areas of centrilobular necrosis infiltrated with lymphocytes in the lobules (Figure 1B). Acidophilic bodies were scattered into the parenchyma. Immunostaining for both EBV and CMV was negative. Following drug withdrawal the clinical condition of the patient progressively improved together with the normalisation of liver function tests which occurred after 8 wk of follow-up. Thyroid testing remained in the normal range.
DISCUSSION
We reported two cases of severe PTU-induced hepatitis, one appearing 5 mo after the initiation of therapy and the other one after 6 mo. Liver histology of the first case showed multilobular necrosis, the disease ran a subfulminant course complicated by hepatic encephalopathy and liver failure. In the second case characterised by morphological features of centrilobular necrosis, the clinical and biological evolution was less severe, but ran a prolonged disabling course. The diagnosis of PTU-induced hepatitis was based on the occurrence of liver damage within the first few months of therapy, the absence of previous history and/or other causes of liver disease and the recovery following its discontinuation.
Changes in liver function tests occurring in hyperthyroidism have been known for a long time. Abnormal liver function tests can be observed in 15%-76% of the cases. The pathophysiology of these changes remains unknown, likely involving multifactorial factors including liver congestion, increased liver oxygen consumption, reduced bile acid synthesis, and/or glycuronyl-transferase inhibition[3,7]. In this setting liver histology only shows non-specific changes[8].
On the contrary, PTU-induced acute hepatitis occurs in 0.1%-1.2% of treated patients[4,9,10]. This rate is much higher than that observed in the large context of liver immuno-allergic toxicity (1/10 000 to 1/100 000 or even to 1/1 000 000 for the majority of drugs)[11], and much lower than that predicted by an asymptomatic increase in ALT which is observed in about 14%-28% of cases during the first week of therapy[2,10]. In the presence of acute hepatitis the mortality may be as high as 25%[12] and so far only 28 lethal cases have been reported in the English literature[6], with two additional cases successfully transplanted[12,13]. As in our case reports, histological features liver necrosis of variable severity with or without cholestasis[10], suggesting the role of an immuno-allergic type of toxicity irrespective of the doses of drug used[5].
In our cases, the delay between the initiation of therapy and the occurrence of symptoms of hepatitis was 5 and 6 mo, respectively. This is in agreement with previous reports[3,14,15]. The majority of cases are however observed during the first 6 mo of therapy[1].The positivity of antineutrophil cytoplasmic antibody (ANCA) testing is not surprising since PTU is a potential causative agent of ANCA positive vasculitis[16].
In our first observation thiamazol was substituted with PTU without side effect as in another reported case in which carbimazole was used as a substitute to PTU[17]. Convincing descriptions of thiamazol and PTU hepatic cross-toxicity are indeed lacking, even if cross-sensitivity may occur[18] and if the rate of untoward cross-reaction between carbimazole and PTU can reach 15%[18].
In conclusion, this report reinforces the warning against the potential for PTU to induce severe life-threatening hepatitis. The incidence of transient increase in liver function tests at the initiation of treatment reaches about 28%, while the incidence of severe toxicity is about 1%, a risk being much higher than that observed in the majority of compounds considered as potential hepatotoxins. Due to this high incidence of liver toxicity and the rather short delay between the initiation of treatment and the occurrence of hepatitis as well as its relatively slow “subacute” progression, ALT monitoring may be cost-effective in reducing the severity of the liver side effect. The duration of optimal biochemical follow-up remains to be further evaluated in large prospective trials but should be about six months, during which the highest rate of PTU-induced hepatitis is observed.
Footnotes
S- Editor Liu Y L- Editor Wang XL E- Editor Bi L
References
- 1.Vitug AC, Goldman JM. Hepatotoxicity from antithyroid drugs. Horm Res. 1985;21:229–234. doi: 10.1159/000180054. [DOI] [PubMed] [Google Scholar]
- 2.Liaw YF, Huang MJ, Fan KD, Li KL, Wu SS, Chen TJ. Hepatic injury during propylthiouracil therapy in patients with hyperthyroidism. A cohort study. Ann Intern Med. 1993;118:424–428. doi: 10.7326/0003-4819-118-6-199303150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Gürlek A, Cobankara V, Bayraktar M. Liver tests in hyperthyroidism: effect of antithyroid therapy. J Clin Gastroenterol. 1997;24:180–183. doi: 10.1097/00004836-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Huang MJ, Li KL, Wei JS, Wu SS, Fan KD, Liaw YF. Sequential liver and bone biochemical changes in hyperthyroidism: prospective controlled follow-up study. Am J Gastroenterol. 1994;89:1071–1076. [PubMed] [Google Scholar]
- 5.Ichiki Y, Akahoshi M, Yamashita N, Morita C, Maruyama T, Horiuchi T, Hayashida K, Ishibashi H, Niho Y. Propylthiouracil-induced severe hepatitis: a case report and review of the literature. J Gastroenterol. 1998;33:747–750. doi: 10.1007/s005350050167. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz JK, Rossi GV, Vallejos HA, Brenet RW, Lopez IB, Escribano AA. Fulminant hepatic failure associated with propylthiouracil. Ann Pharmacother. 2003;37:224–228. doi: 10.1177/106002800303700213. [DOI] [PubMed] [Google Scholar]
- 7.Beckett GJ, Kellett HA, Gow SM, Hussey AJ, Hayes JD, Toft AD. Raised plasma glutathione S-transferase values in hyperthyroidism and in hypothyroid patients receiving thyroxine replacement: evidence for hepatic damage. Br Med J (Clin Res Ed) 1985;291:427–431. doi: 10.1136/bmj.291.6493.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Steenbergen W, Fevery J, De Vos R, Leyten R, Heirwegh KP, De Groote J. Thyroid hormones and the hepatic handling of bilirubin. I. Effects of hypothyroidism and hyperthyroidism on the hepatic transport of bilirubin mono- and diconjugates in the Wistar rat. Hepatology. 1989;9:314–321. doi: 10.1002/hep.1840090225. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DS: Treatment of thyrotoxicosis. In : Braverman L.E, Utiger RD (eds). Werner and Ingbar's the thyroid. Philadelphia: Lippincott-Raven; 1996. p. 474. [Google Scholar]
- 10.Kim HJ, Kim BH, Han YS, Yang I, Kim KJ, Dong SH, Kim HJ, Chang YW, Lee JI, Chang R. The incidence and clinical characteristics of symptomatic propylthiouracil-induced hepatic injury in patients with hyperthyroidism: a single-center retrospective study. Am J Gastroenterol. 2001;96:165–169. doi: 10.1111/j.1572-0241.2001.03469.x. [DOI] [PubMed] [Google Scholar]
- 11.Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22:145–155. doi: 10.1055/s-2002-30105. [DOI] [PubMed] [Google Scholar]
- 12.Williams KV, Nayak S, Becker D, Reyes J, Burmeister LA. Fifty years of experience with propylthiouracil-associated hepatotoxicity: what have we learned. J Clin Endocrinol Metab. 1997;82:1727–1733. doi: 10.1210/jcem.82.6.4011. [DOI] [PubMed] [Google Scholar]
- 13.Morris CV, Goldstein RM, Cofer JB, Solomon H, Klintmalm GB. An unusual presentation of fulminant hepatic failure secondary to propylthiouracil therapy. Clin Transpl. 1989:311. [PubMed] [Google Scholar]
- 14.Eisen MJ. Fulminant hepatitis during treatment with propylthiouracil. N Engl J Med. 1953;249:814–816. doi: 10.1056/NEJM195311122492007. [DOI] [PubMed] [Google Scholar]
- 15.Lock DR, Sthoeger ZM. Severe hepatotoxicity on beginning propylthiouracil therapy. J Clin Gastroenterol. 1997;24:267–269. doi: 10.1097/00004836-199706000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Gunton JE, Stiel J, Caterson RJ, McElduff A. Clinical case seminar: Anti-thyroid drugs and antineutrophil cytoplasmic antibody positive vasculitis. A case report and review of the literature. J Clin Endocrinol Metab. 1999;84:13–16. doi: 10.1210/jcem.84.1.0013. [DOI] [PubMed] [Google Scholar]
- 17.Kontoleon P, Ilias I, Koutras DA, Kontogiannis D, Papapetrou PD. Successful treatment with carbimazole of a hyperthyroid pregnancy with hepatic impairment after propylthiouracil administration: a case report. Clin Exp Obstet Gynecol. 2002;29:304–305. [PubMed] [Google Scholar]
- 18.Meyer-Gessner M, Benker G, Olbricht T, Windeck R, Cissewski K, Reiners C, Reinwein D. [Side effects of antithyroid therapy of hyperthyroidism. A study of 1256 continuously treated patients] Dtsch Med Wochenschr. 1989;114:166–171. doi: 10.1055/s-2008-1066570. [DOI] [PubMed] [Google Scholar]


