Abstract
AIM: To investigate the expression of insulin-like growth factor binding protein-2 (IGFBP-2) in gastric carcinoma and its clinical significance and to explore its relationship with cell proliferation.
METHODS: Expressions of IGFBP-2 and Ki-67 in 118 cases of gastric carcinoma and 40 cases of normal gastric mucosa were detected by EnVision immunohistochemical technique.
RESULTS: Expression of IGFBP-2 in gastric carcinoma was higher than that in normal gastric mucosa (P < 0.01). There was no difference between high- and low-grade gastric carcinoma (P > 0.05). Expression of IGFBP-2 in advanced gastric carcinoma was higher than that in early gastric carcinoma (P < 0.05). Expression of IGFBP-2 in gastric carcinoma with lymph node metastasis was higher than that without lymph node metastasis (P < 0.01). IGFBP-2 expression was a positively related to the clinical stage of gastric carcinoma (P < 0.01). There was a positive correlation between IGFBP-2 and Ki-67 (P < 0.05).
CONCLUSION: IGFBP-2 may be involved in carcino-genesis and progression of gastric carcinoma by promoting cell proliferation.
Keywords: Gastric carcinoma, Insulin-like growth factor binding protein, Cell proliferation, Immunohistochemistry
INTRODUCTION
The insulin-like growth factor (IGF) system plays a crucial role in normal cell proliferation and malignant transformation[1,2]. It comprises IGF-Iand IGF-II, the typeIand II receptors[3], and a family of IGF binding proteins (IGFBPs) that specifically bind to IGFs[4]. The IGFBPs are a family of binding proteins involved in the regulation of tissue development through their interactions with IGFs. By sequestering free IGFs and reducing their bioavailability for interaction with IGFRs, IGFBPs are able to modulate cellular growth and differentiation at a local level[5]. IGFBP-2 is the second most abundant IGFBP in the circulation and can be found in a variety of mammalian fluids and tissues. IGFBP-2 was initially thought to mainly bind to IGFs (IGF-Iand IGF-II) through its IGF-binding motif, and thereby inhibits IGF-mediated mitogenic activities[6]. However, recent studies have shown that IGFBP-2 also increases the tumorigenic potential and mitogenesis in some cancer cells[7-9], suggesting that IGFBP-2 possesses multifaceted functions.
So far, only one study focusing on the expression of IGFBPs in gastric cancer cells[10] is available. In this study, we used immunohistochemical method to clarify the expression and localization of IGFBP2 in malignant and normal gastric tissues. The aim was to determine whether IGFBP-2 plays a role during gastric carcinogenesis and to clarify the correlation of IGFBP2 expression with cell proliferation.
MATERIALS AND METHODS
Tissue samples and histopathological study
Paraffin blocks from gastrectomy specimens were obtained from the archives of Department of Pathology, Affiliated Yijishan Hospital of Wannan Medical College, between May 2004 and June 2005. Selected cases were classified as cardia carcinoma, fundus/body and antrum carcinomas according to their location, following the criteria described in a previous study[11]. Mean age and gender were also recorded. Carcinomas were divided into two types, namely early and advanced carcinoma. The depth of wall penetration and nodal status were also recorded. The 1997 version of the International Union Against Cancer (UICC) pTNM system was employed to stage the carcinomas[12].
Immunohistochemistry
Monoclonal antibody against IGFBP2 diluted 1:150 (c-18; Santa Cruz Biotechnology, Inc., Santa Cruz, UK) was used in immunohistochemistry studies. This antibody is specific and does not crossreact with other isoforms of IGFBP. For each procedure, the samples were chosen randomly from each group. The 4-μm thick sections of phosphate buffered saline (PBS)/formalin-fixed and paraffin wax-embedded specimens were routinely dewaxed in xylene, rehydrated in a graded series of ethanol, and washed in distilled water. Antigen retrieval was achieved by placing the specimens in 0.01 mol/L citrate buffer at pH 6.0 and exposing them to repeated microwave heating for 10 min at 450 W. The buffer was replenished after each interval because of evaporation. The specimens were cooled at room temperature for 15 min and washed in sterile water for 5 min and then in PBS at pH 7.6 for 5 min. Endogenous peroxidase or phosphatase activities were quenched in 0.3% H2O2 for 30 min, followed by blocking non-specific antibody binding in 10% goat serum for 30 min at room temperature. Tissue sections were incubated overnight at 4°C in a humidifier with primary goat anti-IGFBP2. Mayer’s hematoxylin nuclear stain was used as a counter stain. Negative controls were performed using conjugate alone.
Assessment of immunohistochemical staining
Immunohistochemical staining results were assessed semi-quantitatively using the Busund score system[13]. Expression of IGFBP2 was then graded semi-quantitatively and classified into one of the four grades. The staining was scored as: 0: no staining; 1: weak staining; 2: strong staining of 25% tumor cells or moderate staining of < 80%; 3: strong staining of 25%-50% or moderate staining of > 80%; 4: strong staining of > 50% tumor cells. In each case, 10 high power fields of representative areas were counted. The maximal staining intensity was typically higher in those cases where more cells were positive. Most positive cells showed cytoplasmic staining. Slides were examined and scored independently by two of the authors, blinded to other pathological information. The Ki-67 labeling index (KI) was calculated as the percentage of positive tumor nuclei divided by the total number of tumor cells examined. At least 1000 tumor cells per specimen were examined in 5 randomly selected fields by light microscopy (× 400).
Statistical analysis
Data are presented as mean ± SD. Individual groups were compared using the parametric Student’s t test. Association between IGFBP2 expression and different clinical parameters in malignant tissue was tested by nonparametric t test. The relation between IGFBP2 expression and Ki-67 was tested by Spearman’s correlation coefficient. For all these statistical analyses, P < 0.05 was considered statistically significant.
RESULTS
Clinicopathological features
The clinical and pathological characteristics of 118 patients are summarized in Table 1.
Table 1.
Clinical and pathological characteristics of patients with early gastric carcinoma (n = 118)
| Characteristic | n |
| All patients | 118 |
| Gender | |
| Male/Female | 71/47 |
| Age (yr) | |
| Median (Range) | 53.2 (24-76) |
| Tumor site | |
| Cardia | 21 |
| Fundus/body | 43 |
| Antrum | 54 |
| Histological type | |
| Differentiated | 56 |
| Undifferentiated | 62 |
| Lymph node metastasis | |
| Positive | 67 |
| Negative | 51 |
| pTNM stage | |
| I-II | 52 |
| III-IV | 66 |
| Depth wall penetration | |
| T1-T2 | 48 |
| T3-T4 | 70 |
Immunohistochemical expression of IGFBP2 in normal gastric mucosa and carcinoma
Among the 118 tumor samples, 40 specimens of normal mucosa (≥ 10 cm distant from cancer) showed a weak pancytoplasmatic expression of IGFBP2 (Figure 1). The immunohistochemical staining score of IGFBP2 was judged as 0, 1 or 2. There was an over-expression of IGFBP2 in carcinoma. Pancytoplasmatic staining or more diffuse granular staining was observed in cytoplasm of neoplastic cells (Figures 2 and 3). IGFBP2 expression score was 2.61 ± 0.94 in gastric carcinoma and 1.06 ± 0.64 in normal human gastric mucosa respectively. There was a significant difference between carcinoma and normal mucosa (t = 5.223, P < 0.01) as shown in Table 2.
Figure 1.
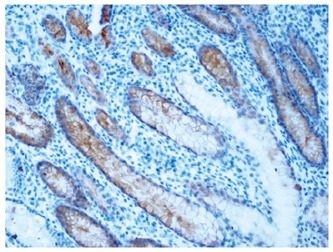
IGFBP2 expression showing a weak pancytoplasmatic staining in normal gastric mucosa (× 200).
Figure 2.
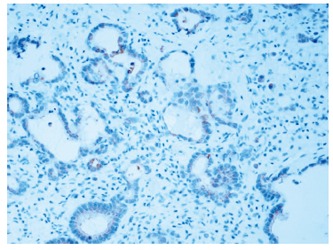
IGFBP2 expression showing a weak cytoplasmatic staining in the tumor cells (× 200).
Figure 3.
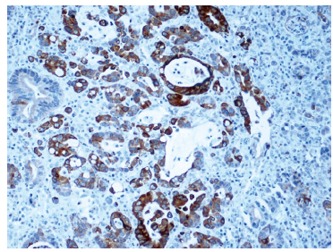
IGFBP2 expression showing a more diffuse granular cytoplasmatic staining in the tumor cells (× 200).
Table 2.
Quantitative score of IGFBP2 expression in normal gastric mucosa and gastric carcinoma
| Group | Quantitative score of IGFBP2 expression | P |
| (mean ± SD) | ||
| Normal mucosa | 1.06 ± 0.64 | < 0.01 |
| Carcinoma | 2.61 ± 0.94 |
Correlation between IGFBP2 expression and clinicopath-ological parameters of gastric carcinoma patients
A significant difference was observed in IGFBP2 expression and tumor depth of wall penetration (P = 0.019). Although immunohistochemical staining score of IGFBP2 expression in undifferentiated carcinoma (2.72 ± 0.88) was higher than that in differentiated carcinoma (2.31 ± 0.82), the difference was not significant (P = 0.336).
A significant difference was also observed in IGFBP2 expression and nodal metastasis (P = 0.0048). The immunohistochemical staining score of IGFBP2 expression in stagesIand II (2.02 ± 0.93) was lower than that in stages III and IV (2.98 ± 0.87) (P = 0.0027) (Table 3).
Table 3.
Summary of quantitative score of IGFBP2 expression in gastric carcinoma according to clinical and pathological parameters (mean ± SD)
| Parameter | Quantitative score of IGFBP2 expression | P |
| Gender Male Female | 2.05 ± 0.82 2.35 ± 0.81 | > 0.05 |
| Tumor site Cardia Fundus/body Antrum | 2.19 ± 0.98 2.31 ± 0.89 2.18 ± 0.95 | > 0.05 |
| Histological type Differentiated Undifferentiated | 2.31 ± 0.82 2.72 ± 0.88 | > 0.05 |
| Lymph node metastasis Positive Negative | 2.16 ± 0.98 2.81 ± 0.91 | < 0.01 |
| pTNM stage I-II III-IV | 2.02 ± 0.93 2.98 ± 0.87 | < 0.01 |
| Depth wall penetration T1-T2 T3-T4 | 2.08 ± 0.97 2.79 ± 0.84 | < 0.05 |
No significant difference was observed in the distribution of immunohistochemical staining scores of IGFBP2 expression, gender of patients and location of the tumor (P = 0.235) (Table 3).
Correlation between expressions of IGFBP2 and Ki-67
The expression of Ki-67 was localized to the nuclei of neoplastic cells (Figure 4). As shown in Figure 5, a significant positive correlation was observed between the immunohistochemical staining score of IGFBP2 and the proliferating index of Ki-67 (P = 0.005).
Figure 4.
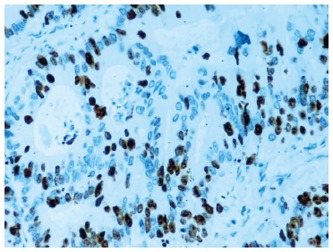
Ki-67 expression showing a strong nuclear staining in the tumor cells (×200).
Figure 5.
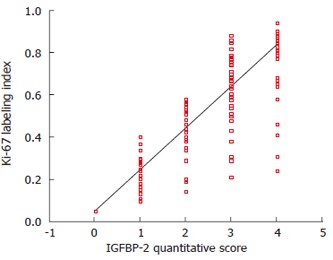
The positive correlation between IGFBP2 expression and Ki-67 in gastric cancer (Spearman's correlation coefficient, P = 0.005).
DISCUSSION
In this study, we determined the expression of IGFBP2 in 118 specimens of gastric carcinoma. To our knowledge, the expression of IGFBP2 in gastric carcinoma has not been evaluated by immunohistochemistry. Normal gastric epithelial tissue was negative or weakly positive and localized to the cytoplasm of gastric mucosa. Weak, moderate to strong expression of IGFBP2 was observed in gastric carcinoma with diffuse granular staining or strong pancytoplasmatic staining. This finding is in accordance with previous reports showing that IGFBP2 can augment IGF1-induced mitogenicity of cancer cells[14].
Serum IGFBP-2 concentrations in combination with carcinoembryonic antigen determinations are highly prognostic for metastasis and recurrence of colon cancers, and their use in cancer surveillance is suggested[15,16]. In ovarian cancer patients, serum IGFBP-2 levels are positively correlated with the ovarian tumor marker CA 125[17]. Some studies showed that serum IGFBP-2 levels in patients with prostate carcinoma are elevated and significantly correlated with PSA levels, suggesting that serum IGFBP-2 measurement in patients with low serum PSA levels can be used to monitor prostate tumor burden[18-20]. IGFBP-2 is consistently elevated in cerebrospinal fluid (CSF) of patients with malignant tumors of the central nervous system (CNS), whereas in patients with peripheral tumors, normal IGFBP-2 levels have been found in CSF[21], suggesting that IGFBP-2 in CSF can be considered a specific marker for CNS tumors. Busund et al[13] reported that IGFBP-2 is not significantly expressed in normal and hyperplasia glandular cells of breast. Atypical hyperplasia slightly increases cytoplasmic expression of IGFBP-2, and carcinoma in situ shows a distinctive membrane and cytoplasmic expression. Infiltrating carcinomas strongly express cytoplasmic IGFBP-2, suggesting that IGFBP-2 might be an independent indicator of malignancy. Richardsen et al[22] have found a significant over-expression of IGFBP-2 in prostatic intraepithelial neoplasia (PIN) and in more than 90% of cancers regardless of their grade. All cases of benign prostatic hyperplasia (BPH) are almost negatively or very weakly stained. In the present study, the immunohistochemical staining score of IGFBP-2 in gastric carcinomas was higher than that in normal mucosa, suggesting that over-expression of IGFBP-2 may have a stimulatory role in the pathogenesis of gastric cancer.
IGFBP-2, the second most abundant IGFBP in circulation, has traditionally been thought as a growth inhibitor. In transgenic mouse models, for example, IGFBP-2 over-expression through the CMV-promoter results in significantly reduced weight gain, presumably through IGFBP-2 sequestering of IGFs by reducing the bioavailability of these growth factors for their receptors[23,24]. Over-expression of IGFBP-2 in human embryonic kidney fibroblasts inhibits cell proliferation, which can be reversed by the addition of exogenous IGFs, suggesting that IGFBP-2 has an inhibitory effect on IGF action[25]. Conversely, expression of IGFBP-2 has also been found to be positively correlated with tumor grade, including hepatoblastoma, mammary and ovarian cancer, Wilm’s and adrenal tumors as well as prostate cancer[26], indicating that IGFBP-2 might have cancer-promoting properties[8]. Recently, Song et al[27] used a yeast two-hybrid system to identify a binding partner of IGFBP-2, which was named invasion inhibitory protein 45 (IIp45), and found that IGFBP-2 could stimulate glioblastoma multiform (GBM) cell invasion, but IIp45 inhibits GBM cell invasion. Because IGFBP-2 carries the Tg domain and the RGD motif, IGFBP-2 may stimulate cell invasion via the integrin-mediated signaling pathway and IIp45 may inhibit IGFBP-2-stimulated invasion by interacting with the Tg-RGD region of IGFBP-2. Indeed, IGFBP2 has been recently found to interact with the alpha v beta 3 and alpha 5 beta 1 integrins[28,29]. In addition, IGFBP2 can stimulate the growth of prostate cancer cells, an effect that can be blocked by MAP kinase and PI3-kinase inhibitors[26]. IGFBP2 is co-expressed with the vascular endothelial growth factor in pseudopalisading glioma cells surrounding tumor necrosis[30]. These findings suggest that IGFBP2 plays a key role in human cancer development.
We examined the correlation between the immuno-histochemical staining score of IGFBP-2 and several clinicopathological factors. The immunostaining score of IGFBP-2 in carcinoma with T3-T4 penetration was higher than that with T1-T2 penetration. Higher immunohistochemical staining score of IGFBP-2 significantly correlated with lymph node metastasis and clinical stage. These findings suggest that IGFBP-2 might be a marker of invasive gastric carcinoma. However, in contrast to other tumors, no relationship was found between the immunohistochemical staining score of IGFBP-2 in gastric carcinoma and tumor grade. This discrepancy might be possibly attributed to the different materials studied.
Hormone status as measured by estrogen receptor (ER) and progesterone receptor (PR) correlates with IGFBP-2 in breast carcinoma[13]. However, the relationship between proliferation and IGFBP-2 expression in various tumors including gastric carcinoma has not been analyzed. Therefore, we carried out the present study to investigate the association of proliferative activity with IGFBP-2 expression in gastric carcinoma. Cell proliferative activity is important for evaluating tumor growth. Monoclonal antibodies that recognize Ki-67 can label all proliferating cells in G1, S, G2 and M phases of the cell cycle and have been widely used to estimate the proliferative fraction of neoplasms[31]. Our results reveal that the immunohistochemical staining score of IGFBP-2 is positively correlated with KI. These findings suggest that IGFBP-2 might play an important role in the progression of gastric carcinoma by promoting cell proliferative activity.
In conclusion, increased IGFBP-2 expression is related to malignant transformation of gastric carcinoma and plays an important role in the progression of gastric carcinoma. Although our data provide evidence for a role of IGFBP-2 in predicting biologic behavior in a relatively large series of gastric carcinoma, further studies are needed to establish the relationship between IGFBP-2 expression in this tumor and survival of patients with this tumor.
Footnotes
Supported by the Department of Education and Natural Science Foundation of Anhui Province, China, No. 2006KJ412B
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
References
- 1.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth. Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 2.Werner H, LeRoith D. The role of the insulin-like growth factor system in human cancer. Adv Cancer Res. 1996;68:183–223. doi: 10.1016/s0065-230x(08)60354-1. [DOI] [PubMed] [Google Scholar]
- 3.Werner H, Le Roith D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncog. 1997;8:71–92. doi: 10.1615/critrevoncog.v8.i1.40. [DOI] [PubMed] [Google Scholar]
- 4.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 5.Wetterau LA, Moore MG, Lee KW, Shim ML, Cohen P. Novel aspects of the insulin-like growth factor binding proteins. Mol Genet Metab. 1999;68:161–181. doi: 10.1006/mgme.1999.2920. [DOI] [PubMed] [Google Scholar]
- 6.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 7.Menouny M, Binoux M, Babajko S. IGFBP-2 expression in a human cell line is associated with increased IGFBP-3 proteolysis, decreased IGFBP-1 expression and increased tumorigenicity. Int J Cancer. 1998;77:874–879. doi: 10.1002/(sici)1097-0215(19980911)77:6<874::aid-ijc13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Hoeflich A, Fettscher O, Lahm H, Blum WF, Kolb HJ, Engelhardt D, Wolf E, Weber MM. Overexpression of insulin-like growth factor-binding protein-2 results in increased tumorigenic potential in Y-1 adrenocortical tumor cells. Cancer Res. 2000;60:834–838. [PubMed] [Google Scholar]
- 9.Slootweg MC, Ohlsson C, Salles JP, de Vries CP, Netelenbos JC. Insulin-like growth factor binding proteins-2 and -3 stimulate growth hormone receptor binding and mitogenesis in rat osteosarcoma cells. Endocrinology. 1995;136:4210–4217. doi: 10.1210/endo.136.10.7545101. [DOI] [PubMed] [Google Scholar]
- 10.Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257–2263. doi: 10.1016/s0959-8049(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 11.Pinto-De-Sousa J, David L, Seixas M, Pimenta A. Clinicopathologic profiles and prognosis of gastric carcinomas from the cardia, fundus/body and antrum. Dig Surg. 2001;18:102–110. doi: 10.1159/000050109. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LE, Whitekind CH. TNM classification of malignant tumours. International Union Against Cancer. 5th ed. New York: John Wiley & Sons; 2001. p. 98. [Google Scholar]
- 13.Busund LT, Richardsen E, Busund R, Ukkonen T, Bjørnsen T, Busch C, Stalsberg H. Significant expression of IGFBP2 in breast cancer compared with benign lesions. J Clin Pathol. 2005;58:361–366. doi: 10.1136/jcp.2004.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee D, Favoni RE, Lupu R, Cullen KJ, Lebovic GS, Huff KK, Lee PD, Lee YL, Powell DR, Dickson RB. The insulin-like growth factor binding protein BP-25 is expressed by human breast cancer cells. Biochem Biophys Res Commun. 1989;158:38–44. doi: 10.1016/s0006-291x(89)80173-1. [DOI] [PubMed] [Google Scholar]
- 15.Renehan AG, Jones J, Potten CS, Shalet SM, O'Dwyer ST. Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br J Cancer. 2000;83:1344–1350. doi: 10.1054/bjoc.2000.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renehan AG, Painter JE, O'Halloran D, Atkin WS, Potten CS, O'Dwyer ST, Shalet SM. Circulating insulin-like growth factor II and colorectal adenomas. J Clin Endocrinol Metab. 2000;85:3402–3408. doi: 10.1210/jcem.85.9.6770. [DOI] [PubMed] [Google Scholar]
- 17.Flyvbjerg A, Mogensen O, Mogensen B, Nielsen OS. Elevated serum insulin-like growth factor-binding protein 2 (IGFBP-2) and decreased IGFBP-3 in epithelial ovarian cancer: correlation with cancer antigen 125 and tumor-associated trypsin inhibitor. J Clin Endocrinol Metab. 1997;82:2308–2313. doi: 10.1210/jcem.82.7.4085. [DOI] [PubMed] [Google Scholar]
- 18.Cohen P, Peehl DM, Stamey TA, Wilson KF, Clemmons DR, Rosenfeld RG. Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab. 1993;76:1031–1035. doi: 10.1210/jcem.76.4.7682560. [DOI] [PubMed] [Google Scholar]
- 19.Kanety H, Madjar Y, Dagan Y, Levi J, Papa MZ, Pariente C, Goldwasser B, Karasik A. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77:229–233. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 20.Ho PJ, Baxter RC. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin Endocrinol (Oxf) 1997;46:333–342. [PubMed] [Google Scholar]
- 21.Müller HL, Oh Y, Lehrnbecher T, Blum WF, Rosenfeld RG. Insulin-like growth factor-binding protein-2 concentrations in cerebrospinal fluid and serum of children with malignant solid tumors or acute leukemia. J Clin Endocrinol Metab. 1994;79:428–434. doi: 10.1210/jcem.79.2.7519190. [DOI] [PubMed] [Google Scholar]
- 22.Richardsen E, Ukkonen T, Bjørnsen T, Mortensen E, Egevad L, Busch C. Overexpression of IGBFB2 is a marker for malignant transformation in prostate epithelium. Virchows Arch. 2003;442:329–335. doi: 10.1007/s00428-003-0786-2. [DOI] [PubMed] [Google Scholar]
- 23.Hoeflich A, Wu M, Mohan S, Föll J, Wanke R, Froehlich T, Arnold GJ, Lahm H, Kolb HJ, Wolf E. Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology. 1999;140:5488–5496. doi: 10.1210/endo.140.12.7169. [DOI] [PubMed] [Google Scholar]
- 24.Schneider MR, Lahm H, Wu M, Hoeflich A, Wolf E. Transgenic mouse models for studying the functions of insulin-like growth factor-binding proteins. FASEB J. 2000;14:629–640. doi: 10.1096/fasebj.14.5.629. [DOI] [PubMed] [Google Scholar]
- 25.Höflich A, Lahm H, Blum W, Kolb H, Wolf E. Insulin-like growth factor-binding protein-2 inhibits proliferation of human embryonic kidney fibroblasts and of IGF-responsive colon carcinoma cell lines. FEBS Lett. 1998;434:329–334. doi: 10.1016/s0014-5793(98)01011-4. [DOI] [PubMed] [Google Scholar]
- 26.Moore MG, Wetterau LA, Francis MJ, Peehl DM, Cohen P. Novel stimulatory role for insulin-like growth factor binding protein-2 in prostate cancer cells. Int J Cancer. 2003;105:14–19. doi: 10.1002/ijc.11015. [DOI] [PubMed] [Google Scholar]
- 27.Song SW, Fuller GN, Khan A, Kong S, Shen W, Taylor E, Ramdas L, Lang FF, Zhang W. IIp45, an insulin-like growth factor binding protein 2 (IGFBP-2) binding protein, antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl Acad Sci USA. 2003;100:13970–13975. doi: 10.1073/pnas.2332186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira JJ, Meyer T, Docherty SE, Reid HH, Marshall J, Thompson EW, Rossjohn J, Price JT. Bimolecular interaction of insulin-like growth factor (IGF) binding protein-2 with alphavbeta3 negatively modulates IGF-I-mediated migration and tumor growth. Cancer Res. 2004;64:977–984. doi: 10.1158/0008-5472.can-03-3056. [DOI] [PubMed] [Google Scholar]
- 29.Schütt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32:859–868. doi: 10.1677/jme.0.0320859. [DOI] [PubMed] [Google Scholar]
- 30.Godard S, Getz G, Delorenzi M, Farmer P, Kobayashi H, Desbaillets I, Nozaki M, Diserens AC, Hamou MF, Dietrich PY, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613–6625. [PubMed] [Google Scholar]
- 31.Sato T, Yoshinaga K, Okabe S, Okawa T, Higuchi T, Enomoto M, Takizawa T, Sugihara K. Cyclooxygenase-2 expression and its relationship with proliferation of colorectal adenomas. Jpn J Clin Oncol. 2003;33:631–635. doi: 10.1093/jjco/hyg114. [DOI] [PubMed] [Google Scholar]


