Abstract
AIM: To evaluate the 5-lipoxygenases (Loxs) expression level in human colorectal cancer specimens in order to determine its clinicopathologic significance in human tumorigenesis.
METHODS: The relative quantity of 5-Lox mRNA in paired 91 colorectal tumor and adjacent normal mucosa samples was determined by real time quantitative PCR. Additionally, the expression of 5-Lox and cyclooxygenase (Cox)-2 proteins was also examined using immunohistochemical staining methods.
RESULTS: There was a marked increase in 5-Lox mRNA levels in the tumor compared with paired normal mucosa samples (P < 0.0001). Sixty six (72.5%) tumors showed high 5-Lox mRNA levels. The positivity rate of 5-Lox and Cox-2 protein expression was 68.7% and 79.1% respectively. There was a significant association between tumoral 5-Lox mRNA level and tumor size (Rho = 0.392, P = 0.0002), depth or vessel invasion.
CONCLUSION: These results suggest that 5-Lox is up-regulated in colorectal cancer and that inhibition of its expression might be valuable in the prevention and treatment of colorectal cancer.
Keywords: Arachidonic acid, 5-Lipoxygenase, Cyclooxygenase-2, Real time PCR, Immunohisto-chemistry, Colorectal cancer
INTRODUCTION
Evidence from epidemiological and animal studies suggests that a high-fat consumption is associated with an increased incidence and growth of tumors at several specific organ sites, including breast, pancreas, colon, and prostate[1]. In this respect, it has been demonstrated that the metabolism of arachidonic acid, a polyunsaturated fatty acid by either the Cyclooxygenase (Cox) pathway or the Lipoxygenase (Lox) pathway, generates a host of pro-inflammatory substances called eicosanoids including prostaglandins, thromboxanes, leukotrienes, etc., that act as potent autocrine and paracrine regulators of cell biology[2]. These substances are known to modulate diverse physiological and pathological responses, including growth and invasiveness of tumor cells as well as suppression of immune surveillance[2].
To date, two Cox isozymes have been identified, the constitutive Cox-1, and the inducible Cox-2[3-5]. For Lox, three major isoforms have been characterized in human tissue, according to the oxygenation sites in the substrate arachidonic acid. These enzymes are named, 5-, 12-, and 15-Lox[4-7]. Over the last two decades, a large body of evidence from experimental and clinical studies has shown that Cox-1/-2 play an important role in the promotion and progression of many types of human neoplasms[3,8-11]. And most importantly, specific inhibition of these enzymes is reported to exhibit a dramatic anti-neoplastic activity[12-15]. Although most attention has focused on prostaglandins (PGs) and other Cox-derived metabolites, emerging evidence now suggests that Lox-catalyzed products, Leukotrienes (LTs) and Hydroxyeicosatetraenoic acids (HETEs) also exert profound biological effects on the development and progression of human cancers[7]. An increase in expression of Lox and their metabolites has been detected in a variety of human cancer cell lines and tissues including those of the prostate[16], bladder[17], breast[18], lung[18], colon[5,19], pancreas[1,20], oral[21] and esophagus[22]; and this over-expression was reported to be significantly associated with tumor cell proliferation, resistance to apoptosis, and angiogenesis[23-25]. Moreover, the direct inhibition of 5-Lox or 12-Lox, was found to markedly suppress the tumor cell growth[2,17,18,26]. Nonetheless, some investigators also have demonstrated a down-regulation of 15-Lox-1/-2 in human colorectal[27,28] and prostate tumors[29] compared with adjacent normal tissues. The clinicopathologic significance of Lox expression is less clearly defined in human colorectal cancer. This observation prompted us to evaluate the expression level of 5-Lox and, determine its correlation with clinical and pathological features of colorectal cancer.
MATERIALS AND METHODS
Patients and tissue samples
Ninety one patients who underwent surgical resection for colorectal cancer in our institution between January and December 2004 were included in this study. These consisted of 53 men and 38 women with a mean age of 64 ± 11 years; 49 colon cancers and 42 rectal cancers. The clinicopathologic parameters were determined according to the International Union Against Cancer Tumor-Node-Metastasis (TNM) classification of malignant tumors[30] and, the tumor size represents the greatest dimension measured after formalin fixation. Immediately after surgery, a small piece of colorectal tumor samples and matched adjacent normal mucosa (taken from the borders of the surgical specimen), were separately placed directly in RNA stabilization reagent (RNAlater, Qiagen) and stored frozen at -80°C until further analysis. Additionally, 67 formalin-fixed, paraffin-embedded tumor tissue blocks obtained from the same patients were also available for immunohistochemical studies.
This study was approved by the Institutional Review Board of the Tokyo Medical and Dental University, and written informed consent was obtained from all patients.
RNA extraction and cDNA synthesis
Total RNA for each sample was extracted using the RNeasy mini kit (Qiagen) according to the manufacturers’ instructions. RNA was quantified by measurement of A260 and A280 using a UV spectrophotometer (Beckman, Life Science); and, its quality was determined by electrophoresis through agarose gels and visualization of the 18S and 28S RNA bands under UV light. For cDNA synthesis, 10 μg of total RNA was reverse-transcribed into cDNA samples using Superscript II RNase H-Reverse transcriptase (Invitrogen) according to the manufacturer’ protocol.
Real time PCR
Real time quantitative Polymerase Chain Reaction (RTQ-PCR) analysis was done by using the 7300 Real Time PCR System (Applied Biosystems). Primers and probe sequences were designed using the Primer Express software (Applied Biosystems) as follows: 5-Lox primers: GGGCATGGAGAGCAAAGAAG and ACCTCGGCCGTGAACGT, probe: TACTTCTACCGGGACGACGGGCTCCT; ACTB-1 primers: TGAGCGCGGCTACAGCTT and TCCTTAATGTCACGCACGATTT; probe: ACCACCACGGCCGAGCGG.
The PCR reaction was carried out with the TaqMan Universal PCR Master Mix (Applied Biosystems) using 1 µL of cDNA in a 24 µL final reaction volume. The thermal cycling conditions were as follows: 50°C for 2 min, 95°C for 10 min and 45 cycles of 15 seconds denaturation at 95°C and 1 min annealing at 60°C. Each sample was run in duplicate for both target gene and endogenous gene.
The amount of 5-Lox (target) normalized to ACTB-1 (endogenous control) and relative to HCT15 (calibrator), was determined by the comparative CT method[31] using the Relative Quantification (ddCT) Study Software (7300 Sequence Detection System version 1.2.1, Applied Biosystems).
The human colon adenocarcinoma cell line HCT15 used as a calibrator in this study, was kindly provided by Cell Resource Center for Biomedical Research Institute (Tohoku University, Miyagi, Japan).
Immunohistochemical evaluation of 5-Lox expression
A Universal Immuno-enzyme Polymer method (Nichirei simple staining) was performed for immunohistochemical staining as we previously described[32]. Anti-5-Lox polyclonal antibody (Cayman Chemical, Ann Arbor, MI; dilution: 1:250) and anti-Cox-2 monoclonal antibody (Cayman Chemical, Ann Arbor, MI; dilution: 1:250) were used as the primary antibody for 2 h incubation at room temperature.
The immunoreactivity of 5-Lox/Cox-2 protein in the tumor cells was determined according to the procedure previously described[32]. Briefly, staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), 3 (strong). Extent of staining was scored as 0 (0%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%) according to the percentage of positive staining area in relation to the whole carcinoma area. Then, the sum of intensity and extent score was regarded as the final staining score for 5-Lox or Cox-2; and, tumors having a final score of ≥ 3 were considered to be positive[32].
Statistical analysis
Median value of normal samples was used as the cut-off point to distinguish between low and high levels of mRNA expression. Nonparametric Wilcoxon and Mann-Whitney U tests were used to evaluate differences in 5-Lox mRNA expression levels between groups. The correlations with clinicopathologic parameters were assessed with a Chi-square test for categorical variables and a Spearman rank test for continuous variables. The strength of association between 5-Lox and Cox-2 staining scores was determined by the Spearman rank test. The difference was considered significant at P < 0.05. All analyses were performed with the statistical software package Stat View (version 5.0).
RESULTS
5-Lox mRNA expression and its association with clinicopathologic parameters
The median value of 5-Lox mRNA expression (relative quantity, RQ) in normal and tumor samples was 36.094 (minimum: 5.966, maximum: 131.859) and 61.113 (minimum: 6.228, maximum: 224.773), respectively. Both Wilcoxon and Mann-Whitney U tests, showed a significant difference in mRNA levels between the two groups of samples (P < 0.0001; Figure 1). Using the median RQ of normal samples as the cut off point, we found that 66 of 91 tumor specimens (72.5%) showed high 5-Lox mRNA level. In Table 1, the associations between 5-Lox mRNA expression status and various clinicopathologic parameters are shown. 5-Lox expression was significantly correlated with tumor size (Rho = 0.392, P = 0.0002; Figure 2), depth of invasion (P = 0.0208), lymphatic invasion (P = 0.0297), and venous invasion (P = 0.0057). There was no significant correlation with patient age and gender, tumor site, histological type, lymph node metastasis and TNM stage.
Figure 1.
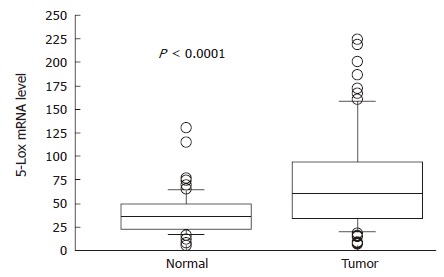
Differential 5-Lox mRNA relative quantity between matched normal mucosa and tumor tissue samples. Boxes: lower and upper quartiles (median inside); bars: at end of whiskers; individual plots: values outside the range of the whiskers.
Table 1.
Correlation of clinicopathologic parameters with 5-Lox mRNA expression
| Clinicopathologic parameters | n |
5- Lox expression |
P | |
| Low | High | |||
| All cases: | 91 | 25 (27.47%) | 66 (72.53%) | |
| Age: | 91 | Rho = 0.018 | NS1 | |
| Gender: | ||||
| Male | 53 | 15 | 38 | |
| Female | 38 | 10 | 28 | NS |
| Tumor site: | ||||
| Colon | 49 | 13 | 36 | |
| Rectum | 42 | 12 | 30 | NS |
| Tumor size: | 91 | Rho = 0.392 | 0.00021 | |
| Histology: | ||||
| Well | 49 | 11 | 38 | |
| Moderate | 34 | 13 | 21 | |
| Poor | 8 | 1 | 7 | NS |
| Depth of invasion: | ||||
| pT1 | 11 | 7 | 4 | |
| pT2 | 18 | 6 | 12 | |
| pT3 | 43 | 9 | 34 | |
| pT4 | 19 | 3 | 16 | 0.021 |
| Lymph node metastasis: | ||||
| Yes | 45 | 16 | 29 | |
| No | 46 | 9 | 37 | NS |
| Lymphatic invasion: | ||||
| Yes | 66 | 14 | 52 | |
| No | 25 | 11 | 14 | 0.030 |
| Venous invasion: | ||||
| Yes | 72 | 15 | 57 | |
| No | 19 | 10 | 9 | 0.006 |
| TNM stage: | ||||
| I | 12 | 2 | 10 | |
| II | 31 | 7 | 24 | |
| III | 38 | 15 | 23 | |
| IV | 10 | 1 | 9 | NS |
Spearman rank test P value; NS: Not Significant (P > 0.05).
Figure 2.
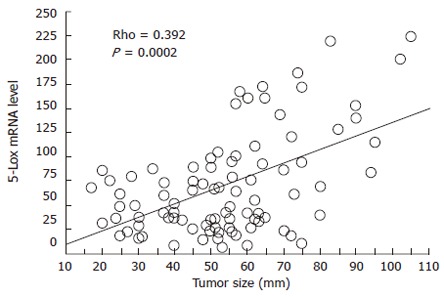
Linear regression plot showing a significant positive correlation between tumoral 5-Lox mRNA level and tumor size.
5-Lox and Cox-2 protein expression
Immunoreactivity of 5-Lox protein was found in the tumor epithelial cells within the cytoplasm, nucleus and nuclear membrane. In contrast, Cox-2 expression was mainly cytoplasmic. Occasionally, little staining was found in the stromal cells or tissue and the adjacent normal mucosa particularly for 5-Lox. Representatives of the staining patterns are shown in Figure 3.
Figure 3.
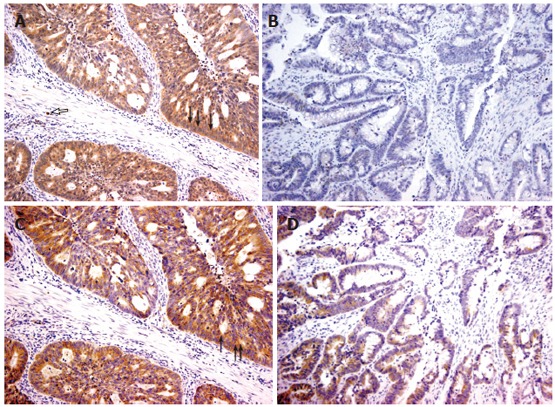
Representative cases of 5-Lox/Cox-2 immunostaining in colorectal cancer (x 200). A: Positive, diffuse, strong cytoplasmic and focal nuclear membrane 5-Lox staining in epithelial tumor cells (black arrow) and stromal cells (white arrow); B: Negative, weak 5-Lox staining. The same samples are shown with strong (C) and moderate (D) Cox-2 staining.
The positivity rate (staining score of 3-7) for 5-Lox and Cox-2 expression was 68.7% and 79.1%, respectively. The Spearman rank test, showed a significant correlation between 5-Lox and Cox-2 staining scores (Rho = 0.277, P = 0.0246); and, 5-Lox mRNA levels versus protein staining scores (Rho = 0.380, P = 0.0025). Forty four samples stained concurrently positive for both proteins, and had a greater mean tumor size when compared with the group of other samples (58.273 ± 19.966 vs 46.391 ± 20.221, P = 0.0476; Figure 4).
Figure 4.
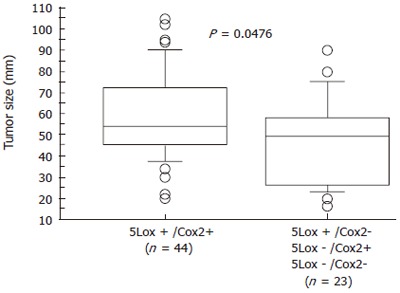
Differential tumor size between a group of concurrent 5-Lox/Cox-2 positive samples and a group of others (5Lox+/Cox2-; 5Lox-/Cox2+; 5Lox-/Cox2-).
DISCUSSION
In the current study, we demonstrated that 5-Lox expression is significantly up-regulated in human colorectal tumor compared with the adjacent normal mucosa. Similar results have been reported for various tumors including those of the colon and rectum[19], esophagus[22], pancreas[1], oral[21], bladder[17], and prostate[16]. However, according to a PubMed search using the keywords 5-Lipoxygenase, and cancer, this is the first study to examine the expression levels and clinicopathologic significance of this gene in a large series of surgical specimens.
Our most important finding was the positive correlation found between 5-Lox expression level with tumor size, depth and vessels invasion. This observation is similar to our previous data indicating that gastric[33] or colorectal[32] tumors with higher Cox-2 level, grow more larger and deeper. It also provides further support to the concept that 5-Lox and Cox-2 display similarities in expression and function in human cancer such as proangiogenic and anti-apoptotic properties[3] and a substrate preference. In our series, the fact that concurrent 5-Lox and Cox-2 positive tumors had the largest mean size, may be considered as the consequence of a combined effect of the two metabolic pathways that remove a pro-apoptotic substrate, arachidonic acid[9], and generate more proangiogenic, anti-apoptotic products, eicosanoids; leading to an increase tumor cells growth. The absence of a statistically significant correlation between 5-Lox over-expression and more aggressive tumor behavior such as lymph node metastasis, confirmed the observation by Ohd et al[19] that positive 5-Lox expression was not a strong predictor of survival in a study of 61 colorectal cancer patients[19].
In contrast to Cox-2, little is known about the mechanisms by which lipoxygenases contribute to tumor growth and progression; but, emerging evidence from several experimental studies suggest that these enzymes and their products, similarly to Cox-2 and PGs, may act on tumor cells through inhibition of apoptosis, increase of cell proliferation and stimulation of angiogenesis[23-25]. Recently, Hoque et al[25] using esophageal cancer cell lines, demonstrated that 5-Lox inhibitors caused a dose- and time-dependant reduction in cell viability and induced apoptosis, which was associated with the level of 5-Lox expression and LTB4 production in these cell lines[25]. According to Romano et al[24], the regulation of vascular endothelial growth factor (VEGF) release and mRNA levels by 5-Lox activity in malignant mesothelial cells was a crucial mechanism of 5-Lox actions on proliferation and apoptosis[24]. Similarly, Ye et al[34] in a mice inflammation-associated colon cancer formation model study, found that 5-Lox expression was accompanied by an up-regulation of matrix metalloproteinase (MMP) -2 activity and VEGF expression, both of which are key angiogenic factors for tumorigenesis[34].
Regarding other Lox family enzymes, little is known about 12-Lox and, a great controversy exists in the literature as to the involvement of 15-Lox in human carcinogenesis. Ikawa et al[5] reported that 15-Lox-1 was highly expressed in human colorectal tumor compared with adjacent normal mucosa[5]. On the contrary, other investigators have extensively demonstrated not only a down-regulation, but also potential tumor-suppressor functions of 15-Lox-1/-2 in colorectal[27,28] and prostate[29] tumors.
Nevertheless, many questions still remain unanswered, including the exact contribution of individual enzymes as well as their interactions. Further studies are warranted. Finally, it is worthy to notice that several studies have consistently indicated that pharmacologic agents that specifically inhibit the Lox and/or Cox metabolic pathways, exhibit a dramatic anti-neoplastic activity in addition to their classic anti-inflammatory effect[7,12-15,35]. And, despite the recent withdrawal of Vioxx (rofecoxib) from the market and warning about other Cox-2 inhibitors over risk of cardiovascular side effects[36], these pathways still remain an exciting and promising research area for cancer chemoprevention and treatment. Particularly the class of dual 5-Lox/Cox inhibitors such as licofelone (previously named ML3000) has emerged as an effective and well-tolerated therapy that could offer safety advantages over Cox inhibition alone[4,37,38].
In conclusion, our data suggest that 5-Lox over-expression may influence the development of colorectal cancer. Therefore, inhibition of this metabolic pathway might provide an effective therapeutic option for colorectal cancer prevention and treatment.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu WF
References
- 1.Hennig R, Ding XZ, Tong WG, Schneider MB, Standop J, Friess H, Büchler MW, Pour PM, Adrian TE. 5-Lipoxygenase and leukotriene B(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. Am J Pathol. 2002;161:421–428. doi: 10.1016/S0002-9440(10)64198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romano M, Claria J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. FASEB J. 2003;17:1986–1995. doi: 10.1096/fj.03-0053rev. [DOI] [PubMed] [Google Scholar]
- 4.Leval Xd, Julemont F, Delarge J, Pirotte B, Dogne JM. New trends in dual 5-LOX/COX inhibition. Curr Med Chem. 2002;9:941–962. doi: 10.2174/0929867024606713. [DOI] [PubMed] [Google Scholar]
- 5.Ikawa H, Kamitani H, Calvo BF, Foley JF, Eling TE. Expression of 15-lipoxygenase-1 in human colorectal cancer. Cancer Res. 1999;59:360–366. [PubMed] [Google Scholar]
- 6.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 7.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kelloff GJ. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1999;8:467–483. [PubMed] [Google Scholar]
- 8.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128:1445–1461. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002;190:279–286. doi: 10.1002/jcp.10068. [DOI] [PubMed] [Google Scholar]
- 10.Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- 11.Hase T, Yoshimura R, Matsuyama M, Kawahito Y, Wada S, Tsuchida K, Sano H, Nakatani T. Cyclooxygenase-1 and -2 in human testicular tumours. Eur J Cancer. 2003;39:2043–2049. doi: 10.1016/s0959-8049(03)00485-4. [DOI] [PubMed] [Google Scholar]
- 12.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 13.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 14.Reardon DA, Quinn JA, Vredenburgh J, Rich JN, Gururangan S, Badruddoja M, Herndon JE 2nd, Dowell JM, Friedman AH, Friedman HS. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer. 2005;103:329–338. doi: 10.1002/cncr.20776. [DOI] [PubMed] [Google Scholar]
- 15.Milella M, Gelibter A, Di Cosimo S, Bria E, Ruggeri EM, Carlini P, Malaguti P, Pellicciotta M, Terzoli E, Cognetti F. Pilot study of celecoxib and infusional 5-fluorouracil as second-line treatment for advanced pancreatic carcinoma. Cancer. 2004;101:133–138. doi: 10.1002/cncr.20338. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–743. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura R, Matsuyama M, Tsuchida K, Kawahito Y, Sano H, Nakatani T. Expression of lipoxygenase in human bladder carcinoma and growth inhibition by its inhibitors. J Urol. 2003;170:1994–1999. doi: 10.1097/01.ju.0000080296.54262.c8. [DOI] [PubMed] [Google Scholar]
- 18.Hong SH, Avis I, Vos MD, Martínez A, Treston AM, Mulshine JL. Relationship of arachidonic acid metabolizing enzyme expression in epithelial cancer cell lines to the growth effect of selective biochemical inhibitors. Cancer Res. 1999;59:2223–2228. [PubMed] [Google Scholar]
- 19.Ohd JF, Nielsen CK, Campbell J, Landberg G, Löfberg H, Sjölander A. Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomas. Gastroenterology. 2003;124:57–70. doi: 10.1053/gast.2003.50011. [DOI] [PubMed] [Google Scholar]
- 20.Hennig R, Grippo P, Ding XZ, Rao SM, Buchler MW, Friess H, Talamonti MS, Bell RH, Adrian TE. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005;65:6011–6016. doi: 10.1158/0008-5472.CAN-04-4090. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Sood S, Wang S, Fang M, Wang P, Sun Z, Yang CS, Chen X. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Wang S, Wu N, Sood S, Wang P, Jin Z, Beer DG, Giordano TJ, Lin Y, Shih WC, et al. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clin Cancer Res. 2004;10:6703–6709. doi: 10.1158/1078-0432.CCR-04-0838. [DOI] [PubMed] [Google Scholar]
- 23.Avis I, Hong SH, Martinez A, Moody T, Choi YH, Trepel J, Das R, Jett M, Mulshine JL. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J. 2001;15:2007–2009. doi: 10.1096/fj.00-0866fje. [DOI] [PubMed] [Google Scholar]
- 24.Romano M, Catalano A, Nutini M, D'Urbano E, Crescenzi C, Claria J, Libner R, Davi G, Procopio A. 5-lipoxygenase regulates malignant mesothelial cell survival: involvement of vascular endothelial growth factor. FASEB J. 2001;15:2326–2336. doi: 10.1096/fj.01-0150com. [DOI] [PubMed] [Google Scholar]
- 25.Hoque A, Lippman SM, Wu TT, Xu Y, Liang ZD, Swisher S, Zhang H, Cao L, Ajani JA, Xu XC. Increased 5-lipoxygenase expression and induction of apoptosis by its inhibitors in esophageal cancer: a potential target for prevention. Carcinogenesis. 2005;26:785–791. doi: 10.1093/carcin/bgi026. [DOI] [PubMed] [Google Scholar]
- 26.Matsuyama M, Yoshimura R, Tsuchida K, Takemoto Y, Segawa Y, Shinnka T, Kawahito Y, Sano H, Nakatani T. Lipoxygenase inhibitors prevent urological cancer cell growth. Int J Mol Med. 2004;13:665–668. [PubMed] [Google Scholar]
- 27.Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, Spindler SA, Greenson JK, Normolle D, Hasan AA, Lawrence TS, et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20:1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 28.Heslin MJ, Hawkins A, Boedefeld W, Arnoletti JP, Frolov A, Soong R, Urist MM, Bland KI. Tumor-associated down-regulation of 15-lipoxygenase-1 is reversed by celecoxib in colorectal cancer. Ann Surg. 2005;241:941–946; discussion 941-946;. doi: 10.1097/01.sla.0000164177.95620.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack GS, Brash AR, Olson SJ, Manning S, Coffey CS, Smith JA Jr, Shappell SB. Reduced 15-lipoxygenase-2 immunostaining in prostate adenocarcinoma: correlation with grade and expression in high-grade prostatic intraepithelial neoplasia. Hum Pathol. 2000;31:1146–1154. doi: 10.1053/hupa.2000.16670. [DOI] [PubMed] [Google Scholar]
- 30.Hermanek P, Sobin LH. UICC/TNM classification of malignant tumors. 4th ed. New York: Springer-verlag; 1987. pp. 47–49. [Google Scholar]
- 31.Chotteau-Lelièvre A, Révillion F, Lhotellier V, Hornez L, Desbiens X, Cabaret V, de Launoit Y, Peyrat JP. Prognostic value of ERM gene expression in human primary breast cancers. Clin Cancer Res. 2004;10:7297–7303. doi: 10.1158/1078-0432.CCR-04-0593. [DOI] [PubMed] [Google Scholar]
- 32.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–8471. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 33.Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [PubMed] [Google Scholar]
- 34.Ye YN, Liu ES, Shin VY, Wu WK, Cho CH. Contributory role of 5-lipoxygenase and its association with angiogenesis in the promotion of inflammation-associated colonic tumorigenesis by cigarette smoking. Toxicology. 2004;203:179–188. doi: 10.1016/j.tox.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GK, Weitzman A, O'Reilly E, Brail L, de Alwis DP, Cleverly A, Barile-Thiem B, Vinciguerra V, Budman DR. Phase I and pharmacokinetic study of LY293111, an orally bioavailable LTB4 receptor antagonist, in patients with advanced solid tumors. J Clin Oncol. 2005;23:5365–5373. doi: 10.1200/JCO.2005.02.766. [DOI] [PubMed] [Google Scholar]
- 36.Krotz F, Schiele TM, Klauss V, Sohn HY. Selective COX-2 inhibitors and risk of myocardial infarction. J Vasc Res. 2005;42:312–324. doi: 10.1159/000086459. [DOI] [PubMed] [Google Scholar]
- 37.Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 38.Alvaro-Gracia JM. Licofelone--clinical update on a novel LOX/COX inhibitor for the treatment of osteoarthritis. Rheumatology (Oxford) 2004;43 Suppl 1:i21–i25. doi: 10.1093/rheumatology/keh105. [DOI] [PubMed] [Google Scholar]


