Abstract
AIM: To study whether hemoglobin could amplify colon cancer cell proliferation via reactive oxygen species (ROS) production.
METHODS: Colon cancer cell line HT-29 was grown in the conventional method using RPMI1640 media. The viability of the cells was measured using the colorimetric MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay after adding hemoglobin. We determined reactive oxygen species levels to be indicators of oxidative stress in HT 29 cell lines with and without hemoglobin and/or 5-fluorouracil (5-FU), 5’-deoxy-5-fluorouridine (5-DFUR) using fluorometric dichlorofluorescin diacetate (DCFH-DA) assay.
RESULTS: Cellular proliferation was increased with hemoglobin in a concentration-dependent manner. A significant increment on ROS levels was found in HT 29 cells following hemoglobin incubation. The cytotoxic effects of 5-FU and 5-DFUR were significantly blunted by administration of hemoglobin. There was a slight increase of peroxiredoxin 1, superoxide dismutase 1 concentration according to different hemoglobin concentrations.
CONCLUSION: Hemoglobin has a cellular proliferative effect on HT-29 colon cancer cell line by production of ROS. Also, hemoglobin abates cytotoxic effects of chemotherapeutic agents such as 5-FU and 5-DFUR.
Keywords: Colon cancer, Hemoglobin, Reactive oxygen species
INTRODUCTION
Colon cancer is an important public health issue[1]. There are nearly one million cases of colon cancer diagnosed worldwide each year. The increasing trend of this cancer is prominent in Asian countries, including Korea[2,3]. Until the present day, scientists have made an intensive effort to find a provocative factor of this major cancer. Many epidemiologic studies indicate that a western style diet is associated with a high incidence of colon cancer[4-6]. An especially high protein consumption as in the western-style diet is regarded as a major factor in inducing colon cancer. There is consistent evidence that high meat consumption, in particular red meat, confers an increased risk of this cancer. However, recent large prospective epidemiologic studies that hypothesized a strong relationship between red meat consumption and colorectal cancer development have revealed inconsistent results.
Hemoglobin is a complex of heme and globin, which contribute an important role of oxygen delivery processes to individual tissues[7]. Intake of these particular molecules contributes nutritional buildup as an iron and protein supplementation. Hemoglobin inside food is already in an oxidized form, and so cannot be used as an oxygen delivery porter. Recently, interesting results about the carcinogenic effects of dietary haemin were reported, documenting that dietary haemin increases the number of aberrant crypt foci in rat colon mucosa[8-10]. It is important to understand how reactive oxygen species (ROS) are formed in the gut lumen and which biological potency they may have, since intracellular reactions with active oxygen can result in the initiation and progression of carcinogenesis by induction of gene mutations, chromosomal damage and cytotoxic effects[11-13]. Furthermore, active oxygen regulates expression of genes active during cell differentiation and growth and therefore, probably plays an important role in the promotion phase of tumor generation. In the colon, iron is expected to increase the production of ROS from peroxides via the Fenton reaction, which may be the cause of cellular toxicity and even pro-mutagenic lesions[14].
The aim of this study was to investigate whether hemoglobin could be classified as a proliferative agent for colon cancer cells by causing reactive oxygen species release. For this purpose, we have studied the cellular viability of differentiated colon cell line HT29 after administration of hemoglobin at different concentrations. ROS production was investigated in each step. Additionally, we examined the protective effect of hemoglobin on the cytotoxicity of chemotherapeutic agents.
MATERIALS AND METHODS
Reagents and antibodies
Human hemoglobin, 5-fluorouracil (5-FU), 5’-deoxy-5-fluorouridine (5-DFUR) were obtained from Sigma (St. Louis, MO, USA). 2’, 7’-Dichlorofluorescein diacetate (DCFH-DA) was purchased from Calbiochem (Meudon, France). Antibodies to human peroxiredoxin 1 and superoxide dismutase 1 were purchased from Labfrontier (Seoul, Korea).
Cell culture
The human colon cancer cell line HT-29 and Lovo was established from Korean Cell Line Bank. To compare the effect on normal fibroblast, we used CCD-33Co normal colonic fibroblast cell line purchased from American Type Culture Collection (Catalog No. CRL-1539). Cells were maintained in stocks of liquid nitrogen, thawed and grown in tissue culture flasks with RPMI 1640 (Gibco BRL, NY, USA) supplemented with 100 mL/L fetal bovine serum and 10 g/L penicillin/streptomycin at 37°C in a 50 mL/L CO2 incubator. The cultured cells were trypsinized with fresh 2.5 g/L trypsin solution, trypsin was removed and the culture let sit at 37°C until the cells detached (about 5 min). Fresh media was added, aspirated and dispensed into new flasks. Subculture was done every 4-6 d.
MTT assay
A freshly prepared cell suspension was serially diluted in RPMI 1640 containing 100 mL/L FBS to give a cell density ranging 109/L to 1011/L counted by hemocytometer. After 24 h, the culture medium was replaced with a fresh medium containing hemoglobin, 5-FU, 5-DFUR or combination thereof. Six duplicate wells were set up in each sample. The cells not treated with the drugs served as control cells. After incubation time passed, 20 mL dimethylthiazol diphenyl tetrazolium bromide (MTT, 3 g/L) was added to each well and incubated at 37°C for 3 h. After removal of the medium, MTT stabilization solution (DMSO:ethanol = 1:1) was added, then shaken for 10 min until all crystal was dissolved. Then, optical density (OD) was detected in a microplate reader at 550 nm wavelength using an ELISA reader (EMAX ED927, Molecular Devices Inc., USA). The negative control well had no cells and was used as zero point of absorbance. Each assay was performed in triplicate. The following formula was used: cell proliferation inhibited (%) = [1-(A of the experimental samples/A of the control)] × 100%. Cell growth curve was completed using time as the abscissa and a value (mean ± SD) as the ordinate.
Measurement of ROS
Human hemoglobin was dissolved in RPMI 1640. HT 29 cells were incubated with 5-FU or 5’-fluoro-2’-deoxyuridine (5-FDUR) at 37°C in suspension culture at different concentrations 1 to 2 μg or for different incubation periods. These samples were processed for analysis of ROS by usual flowcytometric techniques. Briefly, cells were harvested, washed twice with PBS and resuspended in serum-free medium. They were incubated with 50 μmol/L 2’,7’-dichlorofluoroscein diacetate (Calbiochem, Meudon, France) for 2 h at 37°C and washed with ice-cold HEPES/saline and placed on ice. Fluorescence was measured by flowcytometry (Becton Diskinson, San Jose, USA). As a positive control, cells were separately treated with H2O2 and processed for ROS detection.
Immunoblot analysis
Harvested cell line extracts were homogenized with ice-cold lysis buffer (20 mmol/L HEPES pH 7.2, 150 mmol/L sodium chloride, 1% Triton X-100, 1 mmol/L EDTA, 1 mmol/L EGTA, 10 mg/L leupeptin, 10 mg/L aprotinine, 0.1 mmol/L DTT and 1 mmol/L phenylmethylsulfonyl fluoride). For the immunoblot analysis, extracted proteins of 10 μg were denatured by heating at 95°C for 10 min with Laemmli cooking buffer and separated on 120 g/L SDS polyacrylamide gel electrophoresis. The resolved protein bands were transferred onto a PVDF membrane (Amersham Biosciences, UK) and blocked non-specific binding site by immersing the membrane in 30 mL/L skim milk, 120 g/L Tween 20 in TBS for 2 h. The membrane was incubated with 1:2000 diluted primary antibody for peroxiredoxin I (Difco, USA) and superoxide dismutase 1 for 1 h at room temperature on an orbital shaker. The blotted membrane was then incubated using secondary antibody (anti-rabbit IgG, 1:3000) for 1 h at room temperature on an orbital shaker. Detection was performed with the ECL system.
Statistical analysis
Data shown in figures represent mean ± SEM. Unless otherwise stated, these means were calculated from the means of triplicate replicates obtained in at least three independent experiments. Statistical evaluation was performed with the Prism program version 6.0. Depending on sample size and type of experiment, repeated measures of ANOVA or one-way ANOVAs were used to determine the significance of the experimental variables.
RESULTS
Proliferation effect of hemoglobin
Cell growth was determined by MTT assay. HT-29 cell line, the main cell of colon cancer, and Lovo cell line and CCL-33Co cell line were objected to confirm the difference in proliferation dependant on the types of cells. It has been verified, as demonstrated in Figure 1, when injected with hemoglobin, proliferation in all three types of cell lines was much greater. We saw the time effects in HT-29 cell line. These effects began to display after 30 min of treatment and were most obvious after approximately 24 h (Figure 1A). After various adjustments of the concentration of the hemoglobin, the results showed that the higher the concentration, the greater the proliferation (Figure 1).
Figure 1.
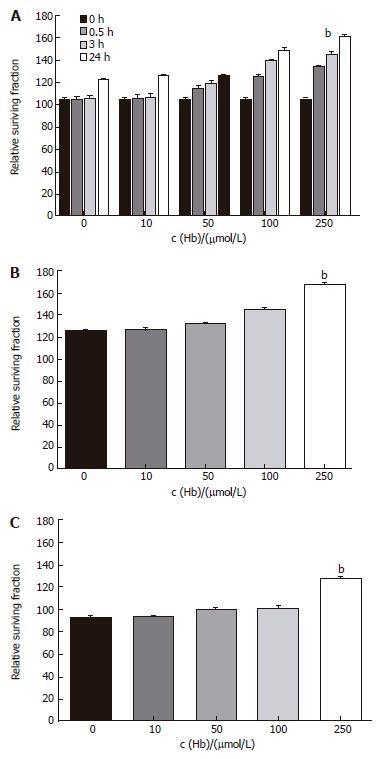
Time-course and dose-response effects of hemoglobin on apoptosis of colorectal cancer cell lines. A: HT-29 cell line. bP = 0.000 vs 0 μmol/L; B: Lovo cell line. bP = 0.000 vs 0 μmol/L; C: CCL-33Co cell line. bP = 0.000 vs 0 μmol/L.
ROS production by hemoglobin
Flowcytometry using DEPC was conducted to measure the total quantity of ROS, which occurs during the administration of hemoglobin. As expected, the amount of ROS production showed a significant increase dependent on the concentration of hemoglobin (Figure 2), and ROS production increased subject to the amount of time following increment.
Figure 2.
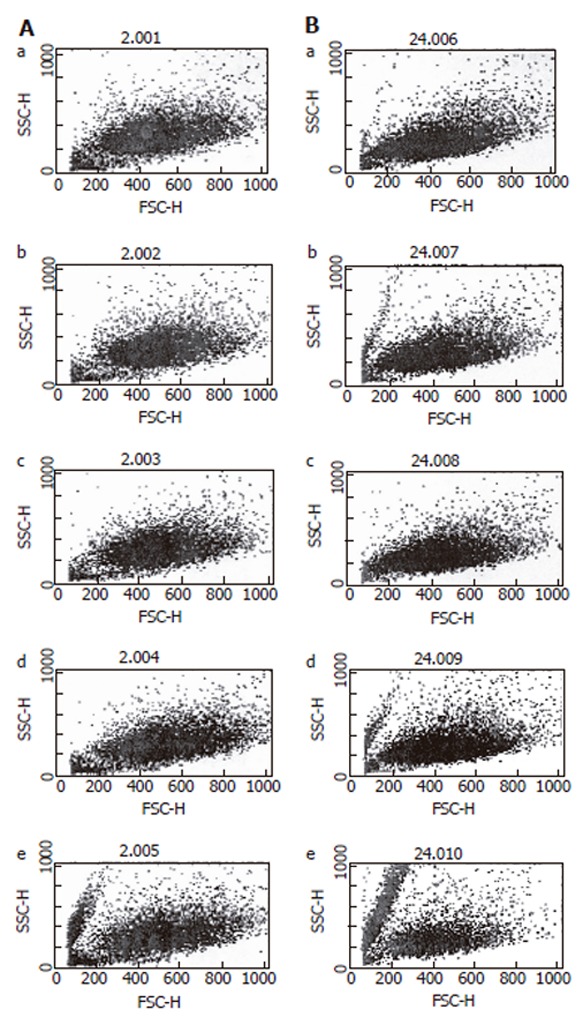
Flowcytometry of reactive oxygen species on HT-29 cells. A: After 3 h of hemoglobin administration; B: After 24 h of hemoglobin administration. a: Hb 0 μmol/L; b: Hb 10 μmol/L; c: Hb 50 μmol/L; d: Hb 100 μmol/L; e: Hb 250 μmol/L.
The restraint of proliferation by anti-cancer medicines
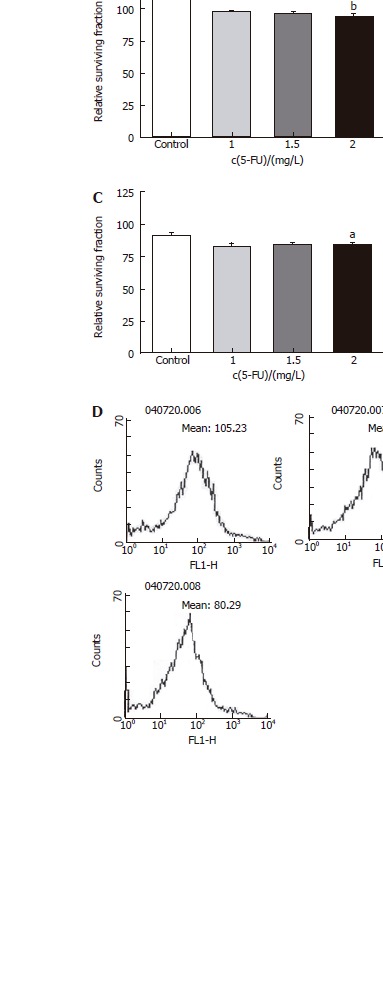
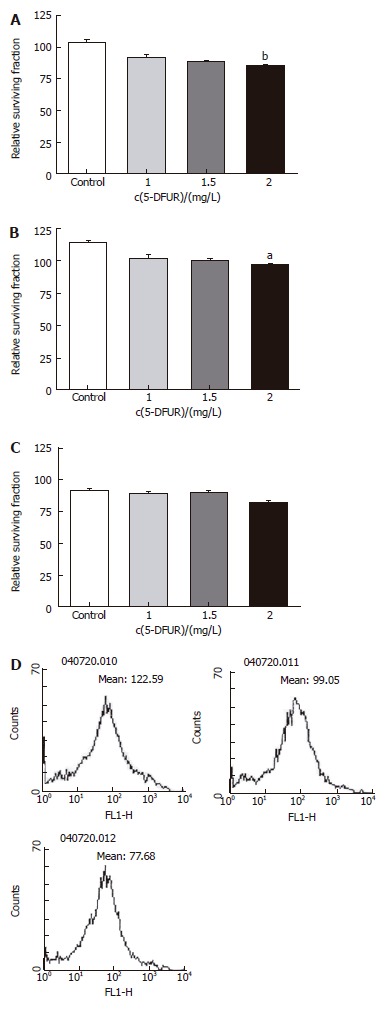
Two drugs (5-FU, an anti-cancer drug most commonly used in treatment of colon cancer, and 5-DFUR, the activating form of capecitabine which is presently used in metastatic colon cancer) were added to verify the constraint of proliferation effects. Twenty-four hours after drug application at varying concentrations, it was found that the higher the density, the greater was the constraint of proliferation in all three cell types (Figures 3 and 4). Also by flowcytometry, the anti-cancer medication decreases ROS production. Hence, it is believed that administration of anti-cancer drugs is effective in reducing ROS production (Figures 3D and 4D).
Figure 3.
Relative surviving fraction of cell lines at 24 h after simultaneously adding each concentration of 5-fluorouracil. A: HT-29 cell line. bP = 0.001 vs Control; B: Lovo cell line. bP = 0.003 vs Control; C: CCL-33Co cell line. aP = 0.039 vs Control.
Figure 4.
Relative surviving fraction of cell lines at 24 h after simultaneously adding each concentration of 5-DFUR. A: HT-29 cell line. bP = 0.001 vs Control; B: Lovo cell line. bP = 0.003 vs Control; C: CCL-33Co cell line. bP = 0.010 vs Control.
Influence of hemoglobin on anti-cancer agents
Twenty-four hours after simultaneously adding 5-FU, 5-DFUR and hemoglobin at various concentrations into three cell lines, results showed evidence of a weakening in the decreased proliferation as compared to using only anti-cancer drugs. Results were proportionate to the given densities of hemoglobin (Figures 5 and 6).
Figure 5.
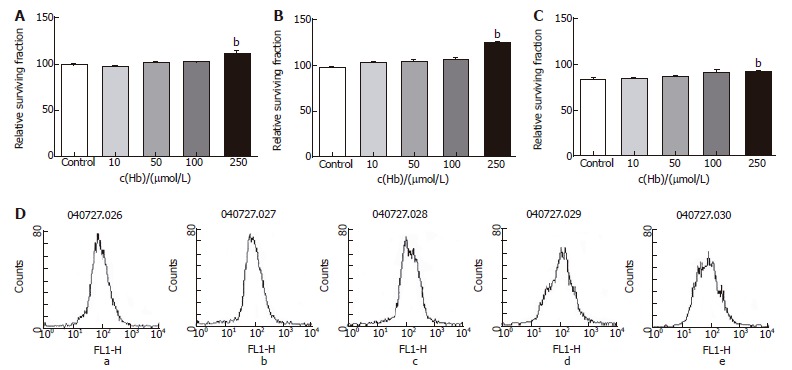
Relative surviving fraction of cell lines after adding 5-FU (1 mg/L) and each concentration of hemoglobin. A: HT-29 cell line. bP = 0.004 vs Control; B: Lovo cell line. bP = 0.000 vs Control; C: CCL-33Co cell line. bP = 0.005 vs Control; D: Flowcytometry of HT-29 cell line. a: Hb 0 μmol/L; b: Hb 10 μmol/L; c: Hb 50 μmol/L; d: Hb 100 μmol/L; e: Hb 250 μmol/L.
Figure 6.
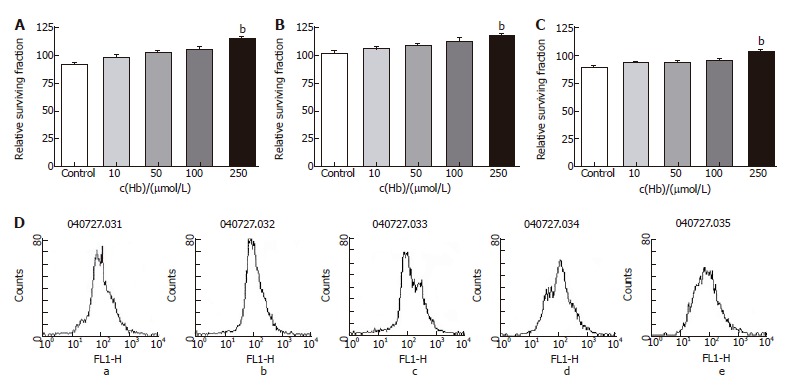
Relative surviving fraction of cell lines after adding 5-DFUR (1 mg/L) and each concentration of hemoglobin. A: HT-29 cell line. bP = 0.000 vs Control; B: Lovo cell line. bP = 0.006 vs Control; C: CCL-33Co cell line. bP = 0.000 vs Control; D: Flowcytometry of HT-29. a: Hb 0 μmol/L; b: Hb 10 μmol/L; c: Hb 50 μmol/L; d: Hb 100 μmol/L; e: Hb 250 μmol/L.
Expression of superoxide dismutase and peroxiredoxin
The occurrence of superoxide dismutase, the most important rate-limiting enzyme in ROS production in vivo, and peroxiredoxin, which is a producer of hydrogen peroxide (prominent type of ROS in cancer cells), was compared by the amount of hemoglobin added. Twenty four hours following treatment, the rate of occurrence of the two types of enzymes increased proportionately to the density of hemoglobin. However, the variation was only slight and less than expected (Figures 7 and 8).
Figure 7.
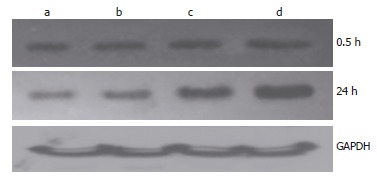
Change of Prx1 expression after administration of hemoglobin. a: Hb 0 μmol/L; b: Hb 10 μmol/L; c: Hb 100 μmol/L; d: Hb 250 μmol/L.
Figure 8.
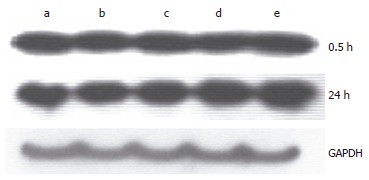
Change of SOD1 expression after administration of hemoglobin. a: Hb 0 μmol/L; b: Hb 10 μmol/L; c: Hb 50 μmol/L; d: Hb 100 μmol/L; e: Hb 250 μmol/L.
DISCUSSION
High intake of meat is believed to be one of the main factors of high incidence of colon cancer among Western countries as compared with Asian countries. There are many reports dealing with which components and nutrients inside meat are considered most risky. There are plenty of reports about nutrients such as iron[15], folate[16], cholesterol[17], calcium[18], bile salt[19], heterocyclic amines[20] and vitamin D[21] etc. related with various gastrointestinal malignancies. It is generally known that red meat has more carcinogenic content than white meat. Due to the fact that some cancer patients believe red meat induces cancerous growths or encourages the recurrence thereof, they consume little to no red meats. Occasionally, malnutrition occurs as a result of extreme or reckless diet changes[22]. However, conclusions about carcinogenic effects of red meat are not confirmed to date, especially the medical basis is insufficient. Therefore, in vitro research is required to effectively prove these carcinogenic effects.
The term ‘red meat’ refers mainly to mammal flesh such as beef, pork and lamb, etc. as with most edible meats, whereas the term ‘white meat’ refers mainly to foul. The ruddy coloring of red meat reflects the density of myoglobin that is found in muscle tissue and some roles of hemoglobin are also included. Hemoglobin is a tetramer consisting of two alpha chains and two beta chains. Since each unit can combine with oxygen wherein iron is present, 1 hemoglobin can carry 4 oxygen molecules. The main function of hemoglobin is to carry oxygen, in addition to the storage of iron and the place providing globin. This type of function occurs only in vivo and exists within the bloodstream. When hemoglobin is inducted into the digestive tract, it is dissolved by digestive enzymes as are other proteins and absorbed as a protein and other nutrients. There are a few reports on the carcinogenic effects of heme or hemoglobin among the components of meat[10,23-27]. Sesink et al[28] stated that dietary heme effects on colon epithelial hyperproliferation are hindered by calcium. They administered pure hemin in a meal of an F344 female rat. They announced that the resulting effect of a low calcium and heme containing diet increases colon wall aberrant crypt foci (ACF). Into an experimental model by Pierre et al[10] heme and hemoglobin were injected and the size and number of ACF were compared and measured with fecal thiobarbaturic acid reactive substances (TBAR). In the case of hemoglobin, the number of ACF and the amount of TBAR increased. From these results, hemoglobin was announced as a potent promoter of colorectal carcinogenesis. Glei et al[14] observed the increase of DNA strand break after the iron overload injected Fe-NTA (ferric-nitrilotriacetate) which was synthesized from the ferric nitrate and nitrilotriacetic acid into HT29 clone 19A cell line. Also, it was reported that when peroxide was added, more DNA strand breaks occurred with increasing peroxide concentrations. With these findings, iron content within a hemoglobin containing diet increased the DNA genotoxicity. We proceeded with this study under the assumption that hemoglobin components of dietary red meat affects cell proliferation of the cancerous and normal colonic cells via production of ROS. As expected, the results of this study confirmed the leading effect of hemoglobin. By the amount of hemoglobin dealt with, not only the cellular proliferation increased but also the amount of ROS production increased supporting predictions thereof. The proliferation effect of hemoglobin on normal colonic fibroblast was noted similarly in cancer cell lines. It is assumed that it presents the effect that exposure of hemoglobin leads to normal colon proliferation as well and produces ROS, which forms much more DNA breaks and also helps in becoming susceptible to other carcinogenic stimuli. Enzymes related with ROS production such as peroxiredoxin 1[29] and superoxide dismutase 1[30] also increased after hemoglobin application but the effect was minimal compared with expectations. These results suggested that production of enzymes was not a major factor in the process of colon cancer cell proliferation via ROS production.
5-FU and 5-DFUR are major chemotherapeutic agents in the treatment of colorectal cancer[31,32]. We used these drugs to identify adverse effects of hemoglobin on chemotherapy. Cellular proliferation was decreased 12 h after adding the 5-FU or 5-DFUR and ROS production was decreased as expected. The results with 5-FU was similar with that of 5-DFUR. These reactions were affected by administration of hemoglobin in a concentration-dependent manner in all three cell lines. ROS production was also decreased with hemoglobin. It is interpreted that hemoglobin administration restricted the cytotoxic effect of anti-cancer drugs in colon cancer cells and normal colonic fibroblasts.
In conclusion, hemoglobin inside red meat has a promoting effect on cellular proliferation in cancer cells and in normal colonic fibroblast cells by release of ROS. Furthermore, this phenomenon reduces the cytotoxicity of anticancer drugs, such as 5-FU and 5-DFUR, to colon cancer cells, which could be an adverse factor during chemotherapy in a clinical setting.
Footnotes
Supported by 2003 the Aventis-Cheiljedang Grant Award offered from Korean society of Coloproctology, No: 2003-005
S- Editor Pan BR L- Editor Karam SM E- Editor Bi L
References
- 1.Ransohoff DF. Colon cancer screening in 2005: status and challenges. Gastroenterology. 2005;128:1685–1695. doi: 10.1053/j.gastro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of health and welfare of Korea Reports of cancer incidence 07-27. Available from: http: //www.mohw.go.kr/index.jsp [Google Scholar]
- 3.Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685–4688. doi: 10.3748/wjg.v11.i30.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson LR. Meat consumption, cancer risk and population groups within New Zealand. Mutat Res. 2002;506-507:215–224. doi: 10.1016/s0027-5107(02)00168-9. [DOI] [PubMed] [Google Scholar]
- 5.Matos E, Brandani A. Review on meat consumption and cancer in South America. Mutat Res. 2002;506-507:243–249. doi: 10.1016/s0027-5107(02)00171-9. [DOI] [PubMed] [Google Scholar]
- 6.Nkondjock A, Ghadirian P. Associated nutritional risk of breast and colon cancers: a population-based case-control study in Montreal, Canada. Cancer Lett. 2005;223:85–91. doi: 10.1016/j.canlet.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Young GP, Rose IS, St John DJ. Haem in the gut. I. Fate of haemoproteins and the absorption of haem. J Gastroenterol Hepatol. 1989;4:537–545. doi: 10.1111/j.1440-1746.1989.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 8.Shivshankar P, Devi SC. Screening of stimulatory effects of dietary risk factors on mouse intestinal cell kinetics. World J Gastroenterol. 2005;11:242–248. doi: 10.3748/wjg.v11.i2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Hong EK. Aberrant crypt foci in the background mucosa of colorectal adenocarcinoma. Cancer Res Treatment. 2001;33:216–224. doi: 10.4143/crt.2001.33.3.216. [DOI] [PubMed] [Google Scholar]
- 10.Pierre F, Taché S, Petit CR, Van der Meer R, Corpet DE. Meat and cancer: haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis. 2003;24:1683–1690. doi: 10.1093/carcin/bgg130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 12.Forsberg L, de Faire U, Morgenstern R. Oxidative stress, human genetic variation, and disease. Arch Biochem Biophys. 2001;389:84–93. doi: 10.1006/abbi.2001.2295. [DOI] [PubMed] [Google Scholar]
- 13.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glei M, Latunde-Dada GO, Klinder A, Becker TW, Hermann U, Voigt K, Pool-Zobel BL. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res. 2002;519:151–161. doi: 10.1016/s1383-5718(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee DH, Jacobs Jr DR, Folsom AR. A hypothesis: interaction between supplemental iron intake and fermentation affecting the risk of colon cancer. The Iowa Women's Health Study. Nutr Cancer. 2004;48:1–5. doi: 10.1207/s15327914nc4801_1. [DOI] [PubMed] [Google Scholar]
- 16.Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–2709. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Toyoshima H, Kojima M, Tokudome S, Hayakawa N, Watanabe Y, et al. Serum oxidized low-density lipoprotein levels and risk of colorectal cancer: a case-control study nested in the Japan Collaborative Cohort Study. Cancer Epidemiol Biomarkers Prev. 2004;13:1781–1787. [PubMed] [Google Scholar]
- 18.Gross MD. Vitamin D and calcium in the prevention of prostate and colon cancer: new approaches for the identification of needs. J Nutr. 2005;135:326–331. doi: 10.1093/jn/135.2.326. [DOI] [PubMed] [Google Scholar]
- 19.Alberts DS, Martínez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, Guillen J, Krutzsch M, Batta AK, Salen G, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005;97:846–853. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- 20.Pence BC, Landers M, Dunn DM, Shen CL, Miller MF. Feeding of a well-cooked beef diet containing a high heterocyclic amine content enhances colon and stomach carcinogenesis in 1,2-dimethylhydrazine-treated rats. Nutr Cancer. 1998;30:220–226. doi: 10.1080/01635589809514667. [DOI] [PubMed] [Google Scholar]
- 21.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr. 2004;134:3463S–3471S. doi: 10.1093/jn/134.12.3463S. [DOI] [PubMed] [Google Scholar]
- 22.Lee KS, Ahn HS, Hwang LI, Lee YS, Koo BH. Utilization of alternative therapies in cancer patients. J Korean Cancer Assoc. 1998;30:203–213. [Google Scholar]
- 23.Glei M, Klenow S, Sauer J, Wegewitz U, Richter K, Pool-Zobel BL. Hemoglobin and hemin induce DNA damage in human colon tumor cells HT29 clone 19A and in primary human colonocytes. Mutat Res. 2006;594:162–171. doi: 10.1016/j.mrfmmm.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Anderson KE, Harnack LJ, Folsom AR, Jacobs DR Jr. Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women's Health Study. J Natl Cancer Inst. 2004;96:403–407. doi: 10.1093/jnci/djh047. [DOI] [PubMed] [Google Scholar]
- 25.Pierre F, Freeman A, Taché S, Van der Meer R, Corpet DE. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colons. J Nutr. 2004;134:2711–2716. doi: 10.1093/jn/134.10.2711. [DOI] [PubMed] [Google Scholar]
- 26.Lakshmi VM, Clapper ML, Chang WC, Zenser TV. Hemin potentiates nitric oxide-mediated nitrosation of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) to 2-nitrosoamino-3-methylimidazo[4,5-f]quinoline. Chem Res Toxicol. 2005;18:528–535. doi: 10.1021/tx049792r. [DOI] [PubMed] [Google Scholar]
- 27.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002;132:3522S–3525S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 28.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: dietary haem-induced colonic cytotoxicity and epithelial hyperproliferation are inhibited by calcium. Carcinogenesis. 2001;22:1653–1659. doi: 10.1093/carcin/22.10.1653. [DOI] [PubMed] [Google Scholar]
- 29.Noh DY, Ahn SJ, Lee RA, Kim SW, Park IA, Chae HZ. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res. 2001;21:2085–2090. [PubMed] [Google Scholar]
- 30.St Clair D, Zhao Y, Chaiswing L, Oberley T. Modulation of skin tumorigenesis by SOD. Biomed Pharmacother. 2005;59:209–214. doi: 10.1016/j.biopha.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Reddy GK. Efficacy of adjuvant capecitabine compared with bolus 5-fluorouracil/leucovorin regimen in dukes C colon cancer: results from the X-ACT trial. Clin Colorectal Cancer. 2004;4:87–88. doi: 10.1016/s1533-0028(11)70105-0. [DOI] [PubMed] [Google Scholar]
- 32.Kim IY. Improving outcomes with chemotherapy in colorectal cancer: current options, current evidence. J Korean Soc Coloproctol. 2006;22:137–148. [Google Scholar]


