Abstract
AIM: To investigate the role that the hedgehog (Hh) signaling pathway, which includes sonic hedgehog (Shh), Patched (Ptc), Smoothened (Smo) and Gli-1, plays in human gastrointestinal stromal tumors (GISTs).
METHODS: Surgically resected specimens from patients with GISTs, leiomyomas and schwannomas were examined by immunohistochemical staining for aberrant expression of hedgehog signaling components, Shh, Ptc, Smo and Gli-1, respectively.
RESULTS: In GISTs, 58.1% (18 of 31), 77.4% (24 of 31), 80.6% (25 of 31) and 58.1% (18 of 31) of the specimens stained positive for Shh, Ptc, Smo and Gli-1, respectively. In leiomyomas, 92.3% (12 of 13), 92.3% (12 of 13), 69.2% (9 of 13) and 92.3% (12 of 13) stained positive for Shh, Ptc, Smo and Gli-1, respectively. In schwannomas, 83.3% (5 of 6), 83.3% (5 of 6), 83.3% (5 of 6) and 100% (6 of 6) stained positive for Shh, Ptc, Smo and Gli-1, respectively. Immunohistochemistry revealed that the expressions of Shh and Gli-1 were significantly higher in leiomyomas than in GISTs (P < 0.05, respectively). Shh expression strongly correlated with the grade of tumor risk category and with tumor size (P < 0.05, respectively). However, the expressions of Ptc and Smo did not correlate with histopathological differentiation.
CONCLUSION: These results suggest that the Hh signaling pathway may play an important role in myogenic differentiation and the malignant potential of human intestinal stromal tumors.
Keywords: Gastrointestinal stromal tumor, Leiomyoma, Schwannoma, Hedgehog, Immunohistochemistry
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors of the gastrointestinal tract that occur from the esophagus to the anus, including in the omentum, mesentery and retroperitoneum[1]. Small GISTs are often detected during surgery for other conditions, gastroscopy or routine X-ray[1,2]. Some GISTs present with bleeding, perforation, pain, obstruction or a combination of these symptoms[3,4]. These tumors have a wide clinical spectrum from benign, incidentally detected nodules to malignant tumors[1] categorized into four risk groups: very low, low, intermediate and high[5]. Traditionally, primary mesenchymal spindle cell tumors of the gastrointestinal (GI) tract have been uniformly classified as smooth muscle tumors (e.g., leiomyomas, cellular leiomyomas, or leiomyosarcomas). Tumors with epithelioid cytologic features have been designated as leiomyoblastomas or epithelioid leiomyosarcomas[6]. Recently it has been postulated that GISTs originate from Cajal cells in the gastrointestinal tract, which are thought to be pacemaker cells that regulate intestinal motility[7,8]. Thus, GISTs differ from leiomyomas and schwannomas, which are of mesenchymal cell origin. Further, GISTs are characterized by the frequent expression of the bone marrow leukocytic progenitor cell antigen CD34[9] and c-kit proto-oncogene[8]. They also show a remarkable variability in their differentiation, and can be roughly divided into four major categories according to the phenotypic features of the tumors: smooth muscle type, neural type, combined type and uncommitted type[10]. Although there are many studies about GISTs, their mechanisms of tumorigenesis, progression and differentiation remain unknown.
The Hedgehog (Hh) gene was initially isolated from Drosophila embryonic segments, and it controls patterning of imaginal disc-derived adult structures such as the eye, the appendage and the abdominal cuticle[11-13]. The mammalian Hh gene, Sonic hedgehog (Shh), is important in the patterning of many tissues and structures such as gastrointestinal epithelium, neurons, smooth muscle tissue, and bone[14-16]. Shh also plays a role in the development of the endoderm, mesoderm and ectoderm[17,18]. The response to the Hh signal is controlled by two transmembrane proteins, the tumor-suppressor Patched (Ptc) and the proto-oncogene Smoothened (Smo)[13]. Smo is a member of the seven transmembrane-receptor family[11] and its activity is suppressed by the twelve-span transmembrane Ptc. Hh stimulation releases this inhibition, leading to Smo activation of a transcriptional response[13]. Downstream targets of the pathway in vertebrates include Gli-1, which is associated with development of basal cell carcinomas and medulloblastomas[19].
There is ample evidence suggesting that the Hh signaling pathway is involved in tumor growth and differentiation. However, there are no studies that examine the expression of Hh pathway components in stromal tumors of the GI tract or the role of the Hh signaling pathway in the etiology of these tumors. Therefore, the purpose of this study is to investigate the expression of Hh pathway signaling proteins in intestinal stromal tumors.
MATERIALS AND METHODS
Tumor classification and selection
A total of 31 GISTs (all cases of stomach), 13 leiomyomas (5 cases of oesophagus, 6 of stomach and 2 of large intestine), and 6 schwannomas (5 cases of stomach and 1 of large intestine) were obtained from patients at Nagasaki University Hospital between 1997 and 2004. The tumor sizes of GISTs were 0.8-8.0 cm in diameter, leiomyomas were 0.1-2.5 cm, and schwannomas were 0.6-4.0 cm. In this study, GISTs were defined and selected as those tumors expressing both c-kit and CD34 surface antigens. Further, we classified smooth muscle actin expressing tumors into smooth muscle (M) type GISTs, S-100 protein expressing tumors into neurogenic (N) type GISTs, both smooth muscle actin and S-100 protein expressing tumors into committed type GISTs, and those expressing only c-kit and CD34 into uncommitted (UN) type GISTs[10]. And we classified histomorphologically tumors with epithelioid cell form into epithelioid cell (EP) type GISTs, spindle cell form into spindle cell (SP) type GISTs, both epithelioid and spindle cell form tumors into mixed (MIX) type GISTs [20].
We also categorized GISTs into four groups according to their malignant potential[5]. The number of mitoses was determined by counting 50 high-power fields (× 400) under Nikon (Tokyo, Japan) E400 microscope. Leiomyomas were defined and selected as tumors expressing smooth muscle actin and not expressing c-kit and CD34. Schwannomas were defined and selected as tumors expressing S-100 protein and not expressing c-kit and CD34. Tumor identification and classification were determined by two independent pathologists (T. Nakayama and I. Sekine), and cases of questionable diagnosis were omitted from this study.
Immunohistochemical staining
Formalin-fixed paraffin-embedded tissues were cut into 4-μm sections, deparaffinized in xylene, and rehydrated in PBS. Deparaffinized sections were preincubated with normal bovine serum to prevent non-specific binding and then incubated overnight at 4°C with an optimal dilution (0.1 mg/L) of a primary polyclonal goat antibody against human Shh (N-19), Ptc (C-20), Smo (N-19) and Gli-1 (C-18). Each antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The slides for Shh, Ptc, Smo and Gli-1 were then sequentially incubated with an alkaline phosphatase-conjugated donkey anti-goat immunoglobulin antibody, and the reaction products were visualized using a mixture of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chloride (BCIP/NBT; BRL, Gaithersburg, MD, USA). Primary antibodies preabsorbed with excess antigen peptides or recombinant protein were used as negative controls. Basal cell carcinoma tissue served as the internal positive control for Shh, Ptc, Smo and Gli-1 immunoreactivity. Immunohistochemical analyses were performed independently by two investigators (T. Nakayama and A. Yoshizaki). Shh, Ptc, Smo and Gli-1 expression was classified into two categories depending on the percentage of cells stained: -, 0%-15% positive tumor cells; +, > 15% positive tumor cells.
Statistical analysis
The Stat View II program (Abacus Concepts, Inc., Berkeley, CA, USA) was used for statistical analyses. Analyses comparing the levels of Shh, Ptc, Smo and Gli-1 expression were performed using the Mann-Whitney, Kruskal-Wallis and Spearman’s tests. P < 0.05 was taken as significant.
RESULTS
The results from the immunohistochemical analysis of Hh pathway components in human intestinal stromal tumors are summarized in Table 1. Shh expression was heterogenous and localized to the cytoplasm of cells in GISTs (Figure 1A). Ptc and Smo were localized to the cytoplasm and cell membrane, and Gli-1 was localized to the cytoplasm and nucleus of GIST tumor cells (Figure 1B-D). Immunohistochemical stainings of Hh pathway components in leiomyomas and schwannomas are shown in Figures 2 and 3, respectively. The four proteins of the Hh pathway that we examined showed patterns of expression similar to that observed in GISTs. While only 58.1% (18 of 31) of the GISTs were positive for Shh, nearly all leiomyomas and schwannomas were positive (92.3%, 12 of 13, P < 0.05). The following results were observed for the other proteins: 77.4% (24 of 31), 92.3% (12 of 13), 83.3% (5 of 6) of the GISTs, leiomyomas and schwannomas were positive for Ptc, respectively; 80.6% (25 of 31), 69.2% (9 of 13), 83.3% (5 of 6) of the GISTs, leiomyomas and schwannomas were positive for Smo, respectively; and 58.1% (18 of 31), 92.3% (12 of 13), 100.0% (6 of 6) of the GISTs, leiomyomas and schwannomas were positive for Gli-1, respectively. Though schwannomas expressed high levels of each protein examined, they had no statistical correlation with GISTs or leiomyomas.
Table 1.
Aberrant expression of Hedgehog pathway signaling proteins in intestinal stromal tumors n (%)
|
Shh |
Ptc |
Smo |
Gli-1 |
|||||||
| n | + | - | + | - | + | - | + | - | ||
| GIST | 31 | 18 (58.1) | 13 (41.9) | 24 (77.4) | 7 (22.6) | 25 (80.6) | 6 (19.4) | 18 (58.1) | 13 (41.9) | |
| I | GIST, M | 7 (22.6) | 4 (57.1) | 3 (42.9) | 5 (71.4) | 2 (28.6) | 6 (85.7) | 1 (14.3) | 4 (57.1) | 3 (42.9) |
| GIST, N | 8 (25.8) | 5 (62.5) | 3 (37.5) | 5 (62.5) | 3 (37.5) | 4 (50.0) | 4 (50.0) | 3 (37.5) | 5 (62.5) | |
| GIST, UN | 16 (51.6) | 9 (56.3) | 7 (43.8) | 14 (87.5) | 2 (12.5) | 15 (93.8) | 1 (6.3) c | 11 (68.8) | 5 (31.3) | |
| II | GIST, EP | 5 (16.1) | 3 (60.0) | 2 (40.0) | 4 (80.0) | 1 (20.0) | 3 (60.0) | 2 (40.0) | 2 (40.0) | 3 (60.0) |
| GIST, MIX | 3 (9.7) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 3 (100) | 0 (0.0) | 2 (66.7) | 1 (33.3) | |
| GIST, SP | 23 (74.2) | 13 (56.5) | 10 (43.4) | 17 (73.9) | 6 (26.1) | 19 (82.6) | 4 (17.4) | 14 (60.9) | 9 (39.1) | |
| Leiomyoma | 13 | 12 (92.3) | 1 (7.7) a | 12 (92.3) | 1 (7.7) | 9 (69.2) | 4 (30.8) | 12 (92.3) | 1 (7.7) a | |
| Schwannoma | 6 | 5 (83.3) | 1 (16.7) | 5 (83.3) | 1 (16.7) | 5 (83.3) | 1 (16.7) | 6 (100) | 0 (0.0) | |
n (%): Tumor cases followed by percentage (%) of total cases.
P < 0.05 between Leiomyoma and GIST in Shh or Gli-1;
P < 0.05 between GIST, UN and GIST, N.
Figure 1.
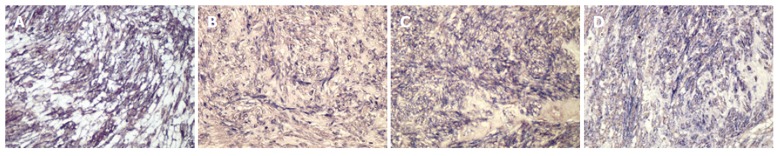
Immunohistochemical staining of Hh signaling components. Alkaline phosphatase reaction products demonstrating Shh (A), Ptc (B), Smo (C) and Gli-1 (D) expression. Shh is expressed in the cytoplasm, Ptc and Smo are expressed in both the cytoplasm and cell membrane, and Gli-1 is expressed in both the cytoplasm and nucleus of GIST cells (x 200).
Figure 2.

Immunohistochemical staining of human intestinal leiomyomas. Alkaline phosphatase reaction products demonstrating Shh (A), Ptc (B), Smo (C) and Gli-1 (D) expression (x 200).
Figure 3.

Immunohistochemical staining of human intestinal schwannomas. Alkaline phosphatase reaction products demonstrating Shh (A), Ptc (B), Smo (C) and Gli-1 (D) expression (x 200).
Immunohistochemical analyses of Hh pathway components in GISTs classified by cellular differentiation are shown in Table 1. In this study there was no case where a GIST was composed of combined types of cellular differentiation. The following results were observed: Shh was detected in 56.3% (9 of 16), 57.1% (4 of 7) and 62.5% (5 of 8) of UN, M and N type of GISTs; Ptc was detected in 87.5% (14 of 16), 71.4% (5 of 7) and 62.5% (5 of 8), respectively; Smo was detected in 93.8% (15 of 16), 85.7% (6 of 7) and 50.0% (4 of 8), respectively; and Gli-1 was detected in 68.8% (11 of 16), 57.1% (4 of 7) and 37.5% (3 of 8), respectively. The expression of Smo was significantly lower in UN type than in N type of GISTs (P < 0.05). And immunohistochemical analyses of Hh pathway components in GISTs classified histomorphologically by cellular form are shown in Table 1, also. In all GISTs, 16.1% (5 of 31) of EP cell type, 9.7% (3 of 31) of MIX cell type and 74.2% (23 of 31) of SP cell type were included, respectively. Each Hh pathway component was detected variably in different cellular type of GISTs. However, there was no correlation between the expression of Hh pathway components and cellular subtype of GISTs.
The results from immunohistochemical analysis of Hh pathway components with regard to the malignant potential of GISTs are summarized in Table 2. There were no correlations between mitosis counts and the expression levels of Hh pathway components. In contrast, the results suggested that lower levels of Shh expression correlated with lower risk GIST categories (P < 0.05) and smaller tumor sizes (P < 0.05).
Table 2.
Expression of Hedgehog pathway components in various categories of intestinal stromal tumors n (%)
|
Shh |
Ptc |
Smo |
Gli-1 |
||||||
| n | + | - | + | - | + | - | + | - | |
| GIST | 31 | 18 (58.1) | 13 (41.9) | 24 (77.4) | 7 (22.6) | 25 (80.6) | 6 (19.4) | 18 (58.1) | 13 (41.9) |
| Risk categories | P < 0.05 | NS | NS | NS | |||||
| High | 4 | 1 (25.0) | 3 (75.0) | 3 (75.0) | 1 (25.0) | 3 (75.0) | 1 (25.0) | 3 (75.0) | 1 (25.0) |
| Intermediate | 6 | 3 (50.0) | 3 (50.0) | 5 (83.3) | 1 (16.7) | 4 (66.7) | 2 (33.3) | 2 (33.3) | 4 (66.7) |
| Low | 16 | 9 (56.3) | 7 (43.8) | 11 (68.8) | 5 (31.3) | 13 (81.3) | 3 (18.8) | 9 (56.3) | 7 (43.8) |
| Very low | 5 | 5 (100) | 0 (0.0) | 5 (100) | 0 (0.0) | 5 (100) | 0 (0.0) | 4 (80.0) | 1 (20.0) |
| Tumor size (cm in diameter) | P < 0.05 | NS | NS | NS | |||||
| n ≤ 2 | 8 | 7 (87.5) | 1 (12.5) | 7 (87.5) | 1 (12.5) | 8 (100) | 0 (0.0) | 6 (75.0) | 2 (25.0) |
| 2 < n ≤ 5 | 18 | 10 (55.6) | 8 (44.4) | 14 (77.8) | 4 (22.2) | 13 (72.2) | 5 (27.8) | 8 (44.4) | 10 (55.6) |
| 5 < n | 5 | 1 (20.0) | 4 (80.0) | 3 (60.0) | 2 (40.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 1 (20.0) |
| Mitosis counts1 | NS | NS | NS | NS | |||||
| 0-5 | 23 | 13 (56.5) | 10 (43.5) | 17 (73.9) | 6 (26.1) | 18 (78.3) | 5 (21.7) | 14 (60.9) | 9 (39.1) |
| 6-10 | 3 | 2 (66.7) | 1 (33.3) | 3 (100) | 0 (0.0) | 3 (100) | 0 (0.0) | 2 (66.7) | 1 (33.3) |
| 11-28 | 5 | 3 (60.0) | 2 (40.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 1 (20.0) | 2 (40.0) | 3 (60.0) |
n (%): Tumor cases followed by percentage (%) of total cases; NS: Not significant;
Numbers per 50 areas in high-power field (× 400).
DISCUSSION
Recent studies have shown that the Hh pathway plays important roles in cell differentiation, tissue patterning and embryonic development[16,19,21]. However, the role of the Hh pathway in human intestinal stromal tumors is still unclear. We investigated the expression of Shh, Ptc, Smo and Gli-1 in three types of intestinal stromal tumors (GISTs, leiomyomas and schwannomas) using immunohistochemical techniques. Our data demonstrated that Shh and Gli-1 were expressed at higher levels in leiomyomas than in GISTs. It suggested that Shh and Gli-1 expressions were correlated with myogenic differentiation. However, in the subclassification of GISTs, myogenic differentiation did not show expression of Shh or Gli-1. Moreover, the consequence of low expression of Smo in neuronal GISTs is not clear yet. Thus, future studies will address the role of the Hh pathway in the differentiation of intestinal stromal tumors.
Abrogation of the Hh pathway can also lead to tumorigenesis. In this pathway, Gli-1, which is involved in controlling cell proliferation and angiogenesis, is a key target of oncogenic action[3,13,22]. Loss of function Ptc mutations and gain of function Smo mutations are mechanisms of tumorigenesis in many types of tumors such as basal cell carcinomas, medulloblastomas, astrocytomas, small cell lung carcinomas and pancreatic cancers[19,23-25].
In this study, the expression of Shh correlated with low risk categories and small tumor sizes. It suggests that expression of Shh reduces the risk of malignant GISTs. Normally, Shh releases Smo from Ptc suppression to induce Gli-1 expression and activation[11,13,19]. Then by a negative feedback mechanism, Gli-1 suppresses the expression of Shh, which results in decrease of Gli-1. However, our data did not show a concomitant decrease in Gli-1 expression in tumors that expressed low levels of Shh. In fact, we observed high Gli-1 levels in larger tumors of high risk categories when Shh expression was low. We hypothesize that Gli-1 may be up-regulated by pathways other than the Hh pathway, or mutation of an Hh pathway component could disrupt the feedback mechanism in high risk GISTs. Thus, future studies will examine Hh pathway components in high risk GIST tumors. In conclusion, our study suggests that the Hh pathway may play important roles in myogenic differentiation and the malignant potential of human intestinal stromal tumors.
In recent studies, mutations affecting c-kit that cause constitutive tyrosine kinase activation have been shown to be important for the pathogenesis of GIST [26,27]. Joensuu et al[28] reported a patient in whom Imatinib(STI-571, Gleevec), a tyrosine kinase inhibitor, was effective against a GIST. And Imatinib has been proven to be remarkably efficacious in heavily pretreated GISTs patients with advanced disease in phase III clinical trials[29]. The expression of the Hh pathway is upregulated by the activation of tyrosine kinase through the epidermal growth factor pathway[30], and may be upregulated by the c-kit/tyrosine kinase pathway.
ACKNOWLEDGMENTS
We are grateful to Mr. Toshiyuki Kawada (Nagasaki University Graduate School of Biomedical Sciences, Molecular Pathology) for his excellent immunohisto-chemical assistance.
Footnotes
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
References
- 1.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24:211–222. doi: 10.1097/00000478-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol. 2000;24:1339–1352. doi: 10.1097/00000478-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Ueyama T, Guo KJ, Hashimoto H, Daimaru Y, Enjoji M. A clinicopathologic and immunohistochemical study of gastrointestinal stromal tumors. Cancer. 1992;69:947–955. doi: 10.1002/1097-0142(19920215)69:4<947::aid-cncr2820690419>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81–89. doi: 10.1177/106689690201000201. [DOI] [PubMed] [Google Scholar]
- 6.Appelman HD. Mesenchymal tumors of the gut: historical perspectives, new approaches, new results, and does it make any difference. Monogr Pathol. 1990;(31):220–246. [PubMed] [Google Scholar]
- 7.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 8.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377–389. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Virolainen M. Gastrointestinal stromal tumors--value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol. 1995;19:207–216. doi: 10.1097/00000478-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Rosai J. Stromal tumors. Rosai and Ackerman's surgical pathology. 9th ed. London: Mosby Press; 2004. pp. 674–680. [Google Scholar]
- 11.Kalderon D. Transducing the hedgehog signal. Cell. 2000;103:371–374. doi: 10.1016/s0092-8674(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 12.McMahon AP. More surprises in the Hedgehog signaling pathway. Cell. 2000;100:185–188. doi: 10.1016/s0092-8674(00)81555-x. [DOI] [PubMed] [Google Scholar]
- 13.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 14.Hardcastle Z, Hui CC, Sharpe PT. The Shh signalling pathway in early tooth development. Cell Mol Biol (Noisy-le-grand) 1999;45:567–578. [PubMed] [Google Scholar]
- 15.Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- 16.van den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628–633. doi: 10.1136/gut.51.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 18.Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371:609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- 19.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;125:2203–2212. doi: 10.1242/dev.125.12.2203. [DOI] [PubMed] [Google Scholar]
- 22.Saldanha G. The Hedgehog signalling pathway and cancer. J Pathol. 2001;193:427–432. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH815>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 25.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 26.Plaat BE, Hollema H, Molenaar WM, Torn Broers GH, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper RJ, van der Graaf WT. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol. 2000;18:3211–3220. doi: 10.1200/JCO.2000.18.18.3211. [DOI] [PubMed] [Google Scholar]
- 27.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, Verweij J, Santoro A, Buesa J, Tursz T. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 28.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 29.Maki RG. Gastrointestinal Stromal Tumors Respond to Tyrosine Kinase-targeted Therapy. Curr Treat Options Gastroenterol. 2004;7:13–17. doi: 10.1007/s11938-004-0021-5. [DOI] [PubMed] [Google Scholar]
- 30.Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–15708. doi: 10.1074/jbc.M413037200. [DOI] [PubMed] [Google Scholar]


