Abstract
AIM: To report the endoscopic treatment of large hyperplastic polyps of the esophagus and esophago-gastric junction (EGJ) associated with Barrett’s esophagus (BE) with low-grade dysplasia (LGD), by endoscopic mucosal resection (EMR).
METHODS: Cap fitted EMR (EMR-C) was performed in 3 patients with hyperplastic-inflammatory polyps (HIPs) and BE.
RESULTS: The polyps were successfully removed in the 3 patients. In two patients, with short segment BE (SSBE) (≤ 3 cm), the metaplastic tissue was completely excised. A 2 cm circumferential EMR was performed in one patient with a polyp involving the whole EGJ. A simultaneous EMR-C of a BE-associated polypoid dysplastic lesion measuring 1 cm x 10 cm, was also carried out. In the two patients, histologic assessment detected LGD in BE. No complications occurred. Complete neosquamous re-epithelialization occurred in the two patients with SSBE. An esophageal recurrence occurred in the remaining one and was successfully retreated by EMR.
CONCLUSION: EMR-C appears to be a safe and effective method for treating benign esophageal mucosal lesions, allowing also the complete removal of SSBE.
Keywords: Hyperplastic polyps, Endoscopic mucosal resection, Barrett’s esophagus
INTRODUCTION
Benign esophageal tumors are rare, representing less than 1% of all esophageal tumors[1]. They are usually asymptomatic and often discovered incidentally[2]. Many classifications have been proposed for benign esophageal tumors: by histological cell types, which are divided into epithelial and non-epithelial tumors, or by the location in the esophageal wall, categorized as intramural and extramural tumors[3,4].
Epithelial polypoid lesions of the esophagus and esophagogastric junction (EGJ) are uncommon[5-8]. Among them, hyperplastic-inflammatory polyps (HIPs) often occur in combination with gastroesophageal reflux disease (GERD)[9-13]. Endoscopic mucosal resection (EMR) is a promising therapeutic option for the removal of superficial carcinomas throughout the gastrointestinal tract. This technique permits resection of the mucosa and submucosa, exposing the muscularis propria[14]. The authors report the outcome of EMR in three patients with large hyperplastic polyps of the EGJ associated with Barrett’s esophagus (BE).
MATERIALS AND METHODS
Endoscopy
Upper gastrointestinal endoscopy (UGIE) was performed with standard forward-viewing videoendoscope (GIF-Q145, Olympus Optical Co. Ltd., Tokyo, Japan).
Before EMR, endoscopic ultrasonography (EUS) (GF-UMQ 130, 7.5-20 MHz, Olympus Optical Co. Ltd., Tokyo, Japan) was performed to assess lesion depth and lymph node status.
Endoscopic resection technique
EMR was performed with the “cap” technique (EMR-C). A 13 mm diameter transparent plastic cap (MH-594, Olympus Co. Ltd., Tokyo, Japan) was preloaded on the tip of a standard diagnostic forward-viewing endoscope (GIF-Q145, Olympus Optical Co. Ltd., Tokyo, Japan). Inside the distal end of the cap is a gutter that positions the opened monofilament polypectomy snare (SD-221U-25, Olympus Co. Ltd., Tokyo, Japan). After submucosal injection of diluted epinephrine (1:200 000), with methylene blue, the cap was pressed against the mucosa and suction applied. The polypectomy snare was closed around the tissue and resection performed by endocut mode only, using the ERBE-ICC 200 cautery device (ERBE Elektromedizin Gmgh, Tubingen, Germany).
After each resection the endoscope was withdrawn to collect the tissue specimen.
Deep sedation with propofol was used to perform the endoscopic procedures.
RESULTS
All resected fragments were fixed in 40 g/L neutral formaldehyde, embedded in paraffin and serially sectioned. The sections were stained with hematoxylin and eosin; in addition all specimens were stained with periodic acid-Schiff/Alcian blue stain at pH 2.5 for evaluation of intestinal metaplasia (IM).
Immunohistochemical staining for p53 was performed in all cases (clone DO-7, BioGenex, San Ramon, CA; final dilution 1:20 000).
Case 1
A 72-year old man complained of gastroesophageal reflux symptoms since the age of 25, which became worse in the last five years, occurring also during the night. He was taking over-the-counter drugs (ranitidine) for his reflux symptoms, with poor control. He never complained of dysphagia. The past medical history of the patient was unremarkable. Endoscopy showed a 3 cm hiatal hernia, and a circumferential BE 10 cm long. A sessile villous-like polyp, 10 mm wide, extending for the whole length of BE, was observed (Figure 1). The colour of the lesion was similar to BE, and at the EGJ became wider, involving the entire circumference of the junction for a length of 15 mm.
Figure 1.
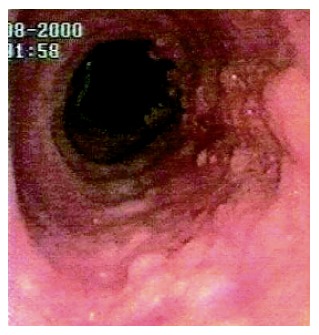
A sessile villous-like polyp extending for the whole length of BE.
EUS demonstrated a lesion confined to the mucosa. No pathologic lymph nodes were detected. Multiple biopsies from BE showed incomplete IM with low-grade epithelial dysplasia (LGD), rare pancreatic pits and positivity for p53; biopsies from the EGJ polyp showed proliferation of hyperplastic cardiac-type epithelium with some cystic dilatation of gastric pits, rare goblet cells, rare parietal cells (Figure 2) and some groups of cells with LGD. Additional features included oedema and inflammation in the lamina propria.
Figure 2.
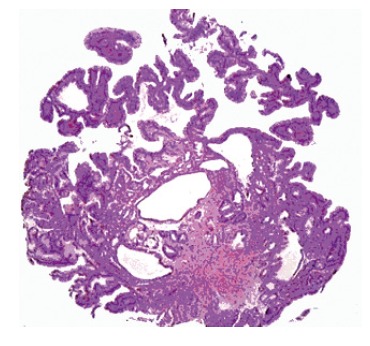
Hyperplastic polyp from the gastroesophageal junction was comprised of cardiac type mucosa with foveolar hyperplasia and cystic dilatation of gastric pits (HE x 100).
Biopsies from the sessile lesion in BE showed fragments of adenomatous villous-type with incomplete IM, epithelial LGD (Figure 3) and over expression of p53.
Figure 3.
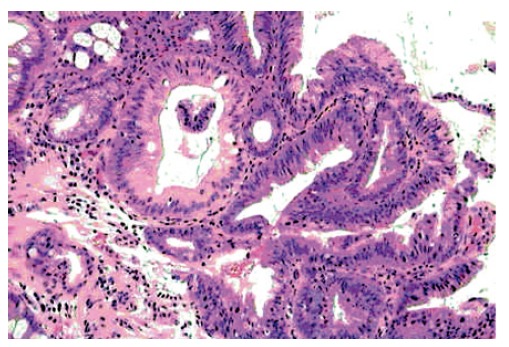
Sessile lesion arising in the Barrett’s esophagus: the adenomatous villous-type polyp is composed of low grade dysplastic epithelium (HE x 250).
The patient was hospitalised and EMR-C performed. Both lesions (the long esophageal one, 1 cm × 10 cm, and its 2 cm of circumferential expansion at the EGJ), were completely removed in one endoscopic session. No intraprocedural or delayed complications occurred. On histology, the EGJ lesion was a hyperplastic polyp, and the lesion arising in BE was a villous adenomatous polyp with LGD (also named BE-associated polypoid dysplastic lesion).
Intravenous omeprazole was administered during the following 24 h. Then 40 mg of rabeprazole were given. The patient was kept fasting for 24 h. Then a liquid diet was started, and solid foods were reintroduced five days later. He was dismissed after four days on long term rabeprazole 40 mg.
UGIE was not repeated until 18 mo later, as the patient refused earlier endoscopic examinations. He was asymptomatic, and still taking 40 mg of rabeprazole. Endoscopy showed a 10 cm long area of neosquamous re-epithelialization where EMR had been performed. Three areas of mucosal abnormality (average size: 12 mm) were detected in the lower esophagus (Figure 4), and EMR was repeated, with resection of larger areas of metaplastic epithelium to favour neosquamous re-epithelialization. No complications occurred and the patient left the hospital three days later. Histology showed both hyperplastic cardiac-type and cystic hyperplastic gastric fundic polyps. The resected associated BE showed incomplete IM without dysplasia.
Figure 4.
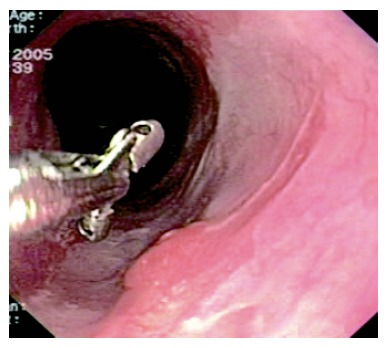
Areas of neosquamous re-epithelialization coexisting with tiny mucosal abnormalities.
A further endoscopy performed 3 mo later, revealed large areas of re-epithelialization and no mucosal abnormalities in the residual BE. Multiple biopsies were taken from the residual BE, that showed IM without dysplasia.
Case 2
A 66-year old woman underwent endoscopy for recurrent mild epigastric pain during the previous three months, without gastroesophageal reflux symptoms. The past medical history was unremarkable. Endoscopy showed a 3 cm hiatal hernia, a short segment BE (SSBE-20 mm), and a 25 mm sessile polyp at the EGJ. Multiple biopsies showed hyperplastic squamous epithelium. EUS showed a lesion confined to the mucosa, without lymph node involvement. Biopsies of the BE showed incomplete IM with LGD, and p53 negativity.
The patient was hospitalised and EMR-C performed. The polyp and BE were both excised. No early or delayed complications occurred. The patient left the hospital three days later, and rabeprazole 40 mg was advised. The first repeat endoscopy was scheduled after 3 mo, when histology revealed proliferative hyperplastic squamous epithelium and chronic inflammatory cells in the lamina propria. Endoscopy 3 and 6 mo later showed complete neosquamous re-epithelialization of the lower esophagus, without stricture.
Case 3
A 72-year old man underwent endoscopy for recurrent epigastric pain occurring over the previous five years, without reflux symptoms. Endoscopy showed a 30 mm tongue of metaplastic epithelium in the distal esophagus, a 3 cm hiatal hernia, and a 30 mm sessile polyp at the EGJ. Biopsies from the BE and polyp showed incomplete IM and squamous epithelial hyperplasia. EUS showed a lesion confined to the mucosa. No pathologic lymph nodes were detected.
Both polyp and BE were completely removed by EMR-C. No complications occurred and the patient left the hospital three days later. He was advised to assume 40 mg of rabeprazole indefinitely. Histology confirmed that the polyp consisted of hyperplastic squamous epithelium and inflammatory infiltrate. The resected BE showed incomplete IM without dysplasia. Immunohistochemical stain for p53 was negative. Endoscopy 3 and 6 mo later showed complete neosquamous re-epithelialization of the lower esophagus, with minimal scarring.
DISCUSSION
We were able to remove large polyps of the esophagus and EGJ by EMR in 3 patients with BE, without complications. In the two patients with a SSBE, the metaplastic tissue was completely removed, allowing complete neosquamous re-epithelialization.
At histology, inflammatory polyps are characterized by hyperplastic epithelium with variable amounts of inflamed stroma[5]. Occasionally, esophageal polyps containing stroma and granulation tissue, without an epithelial component, have been reported[5-7]. Other types of polyps are the inflammatory pseudotumors, also known as inflammatory polyps with pseudomalignant erosion. They usually occur in the distal esophagus, arise from the mucosal layer and contain inflamed granulation tissue with bizarre stromal cells. Pseudomalignant erosion has been found in 14.3% of inflammatory polyps of the EGJ[15]. Inflammatory esophagogastric polyps are thought to be a complication of gastroesophageal reflux[6,16]. They have also been reported in patients with hiatal hernia (88%) and/or reflux esophagitis (91%)[6,17]. The association of hyperplastic polyps and BE is infrequent, and to our knowledge, only Abraham et al[5] has reported it previously. In fact, adenoma is more frequently associated with BE. It has been suggested that the use of the term “adenoma” in BE may be misleading, because it carries a “benign” connotation[18]. The appropriate term for these lesions should be “BE-associated polypoid dysplastic lesion”, because they share similar clinical, pathological and molecular features as those of flat dysplasia[19].
In a series of 27 patients with a total of 30 hyperplastic esophageal and EGJ polyps, 80% of them were composed of cardiac-type mucosa, 17% of squamous mucosa, and 3% contained both cardiac and squamous mucosa. IM was present in only two polyps (7%)[5]. The location of HIPs was the EGJ region (67%), distal esophagus (30%) and mid-esophagus (3%), in accordance with other data reported in the literature[16,17].
Only four cases of hyperplastic polyps in the cervical esophagus have been reported[20-23], all arising from ectopic gastric mucosa[24,25].
Less often, polyps are located at the end of a prominent inflamed gastric fold very close to the squamocolumnar junction, also known as “the sentinel fold”, seen in reflux esophagitis[26].
Histologically similar polyps have been reported in von Recklinghausen’s[27], and Crohn’s disease[28,29], protracted vomiting[5,30], and infectious esophagitis (Candida, Cytomegalovirus, Herpes simplex virus)[5]. Other esophageal injuries, due to drugs (K-dur, alendronate sodium and ibuprofen)[5], sclerotherapy for esophageal varices[12], polypectomy, and photodynamic therapy[5], can result in hyperplastic polyp occurrence. Their pathogenesis is similar to that of gastric and colonic hyperplastic polyps that frequently seem to occur in response to mucosal injury[31,32]. However, there are cases in which the etiology cannot be defined[5,33]. Malignant transformation has been occasionally reported in gastric and colonic hyperplastic polyps[32,34], but not in those arising in the esophagus or EGJ.
In the study of Abraham et al[5], 4 of 27 patients with esophageal hyperplastic polyps also had BE. IM within cardiac-type mucosa was present in two polyps (6.7%): one contained goblet cells, and the other one was surrounded by Barrett’s mucosa. Only the latter polyp (3%) showed LGD. Three out of 4 patients with BE had previous, concomitant, or subsequent development of dysplasia in the non-polypoid esophagus. Furthermore, the frequency of dysplasia within esophageal hyperplastic polyps is low and it has been reported only in those cases associated with dysplastic Barrett’s esophagus[5].
In our patients, histological evaluation showed hyperplastic epithelium and inflamed stroma in the polyps located at EGJ. Histological assessment of the 10 cm long polyp arising in BE showed a “BE-associated polypoid dysplastic lesion”. The treatment of HIPs of the esophagus and EGJ has changed over the years. In the past open surgical resection was considered standard care in the management of all significant esophageal lesions[2,33]. Transabdominal Nissen fundoplication operation to prevent reflux has also been used in cases where the correlation between HIPs and GERD was documented[11].
More recently thoracoscopic approaches have been performed for mucosal or submucosal lesions > 30 mm[2]. Endoscopic polypectomy for HIPs is the current treatment for lesions ≤ 30 mm[15,16,27]. Oguma et al[21] reported the successful removal of a pedunculated hyperplastic polyp (size: 9.0 mm × 4.5 mm × 4.0 mm) arising in the ectopic gastric mucosa in the cervical esophagus[23]. Two of our patients had LGD in the BE. In patients with BE, cancer can develop through several steps encompassing LGD, high-grade dysplasia (HGD) and invasive adenocarcinoma[35]. However, adenocarcinomas have also been detected in patients whose previous biopsies revealed only low-grade or even no dysplasia[36-39]. The information on the natural history of LGD is limited. Moreover, the diagnosis of LGD is highly subjective and associated with interobserver variability[40]. In three articles, the reported rate of progression from LGD to HGD or adenocarcinoma was 10%[41], 12%[42], and 28%[37]. In these studies the agreement among pathologists in diagnosing LGD was associated with an increased risk of progression to HGD or cancer.
At present, patients with HGD are advised to undergo surgical treatment due to the reported frequency of undetected synchronous cancers[43,44]. However, surgery carries substantial morbidity and mortality[45]. EMR could become a therapeutic alternative to esophagectomy in selected patients[46-50].
Some authors suggest EMR in patients with BE and LGD to improve the histologic accuracy[46,51]. However, complications can occur during this procedure. Bleeding and perforation have been reported in a median 10% of patients and less than 1% of cases, respectively[50]. Esophageal stenosis is a late complication, and may occur in 0%-30% of cases[50]. Larger EMR resections may increase the risk of stenosis. In a study encompassing 137 patients, stenosis occurred only when EMR involved more than two-thirds of the esophageal circumference[52]. However, in one report of circumferential EMR, only two of 12 patients developed stenosis[53]. Overall, complications seem fewer for EMR than for surgical resection[54].
We were able to remove the large sessile lesions with EMR-C, in one session, without complications. In two of our patients, with non-circumferential SSBE, the metaplastic epithelium was completely removed. The patient with a 10 cm long BE showed a large neosquamous re-epithelialization, involving half of the BE surface. In the same patient the 20 mm circumferential EMR at the EGJ level did not cause any stricture.
In conclusion, our experience indicates that esophageal and EGJ hyperplastic polyps could represent an exuberant epithelial regeneration following mucosal injury, particularly GERD. The presence of BE in all of them seems to confirm the hypothesis of GERD in causing inflammatory reaction. The malignant transformation of these polyps is rare, but possible[5]. Also, the presence of dysplasia within a segment of BE is patchy, and random biopsies may fail to detect it[55].
We performed EMR to remove the large hyperplastic polyps and the surrounding areas of BE, as EMR provides greater diagnostic precision than endoscopic biopsy. In the study of Conio et al[50] reclassification of the histology after EMR occurred in 26% in a series of 39 patients with BE. Other authors have reported reclassification of the histologic diagnosis after EMR in 0% to 75% of cases[53,56]. We can conclude that EMR is minimally invasive and a low risk method in treating large benign esophageal mucosal lesions.
ACKNOWLEDGMENTS
Alan J Cameron, MD, for his support in revising the manuscript.
Footnotes
S- Editor Pan BR L- Editor Lutze M E- Editor Liu WF
References
- 1.Watson RR, O'Connor TM, Weisel W. Solid benign tumors of the esophagus. Ann Thorac Surg. 1967;4:80–91. doi: 10.1016/s0003-4975(10)66482-x. [DOI] [PubMed] [Google Scholar]
- 2.Kinney T, Waxman I. Treatment of benign esophageal tumors by endoscopic techniques. Semin Thorac Cardiovasc Surg. 2003;15:27–34. doi: 10.1016/s1043-0679(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 3.Kessler B, Stegemann B, Langhans P, Pircher W. [Benign tumors of the esophagus (author's transl)] Leber Magen Darm. 1980;10:28–31. [PubMed] [Google Scholar]
- 4.Choong CK, Meyers BF. Benign esophageal tumors: introduction, incidence, classification, and clinical features. Semin Thorac Cardiovasc Surg. 2003;15:3–8. doi: 10.1016/s1043-0679(03)70035-5. [DOI] [PubMed] [Google Scholar]
- 5.Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the esophagus and esophagogastric junction: histologic and clinicopathologic findings. Am J Surg Pathol. 2001;25:1180–1187. doi: 10.1097/00000478-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bleshman MH, Banner MP, Johnson RC, DeFord JW. The inflammatory esophagogastric polyp and fold. Radiology. 1978;128:589–593. doi: 10.1148/128.3.589. [DOI] [PubMed] [Google Scholar]
- 7.Ghahremani GG, Fisher MR, Rushovich AM. Prolapsing inflammatory pseudopolyp-fold complex of the oesophagogastric region. Eur J Radiol. 1984;4:47–51. [PubMed] [Google Scholar]
- 8.Kato S, Ozawa A, Shibuya H, Nakagawa H, Naganuma H. Inflammatory esophagogastric polyp and fold in an adolescent. Acta Paediatr Jpn. 1993;35:53–56. doi: 10.1111/j.1442-200x.1993.tb03006.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu C, Levine MS, Laufer I. Solitary ulcers in reflux esophagitis: radiographic findings. Abdom Imaging. 1997;22:5–7. doi: 10.1007/s002619900128. [DOI] [PubMed] [Google Scholar]
- 10.Eller JL, Ziter FM Jr, Zuck TF, Brott W. Inflammatory polyp: a complication in esophagus lined by columnar epithelium. Radiology. 1971;98:145–146. doi: 10.1148/98.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Rabin MS, Bremner CG, Botha JR. The reflux gastroesophageal polyp. Am J Gastroenterol. 1980;73:451–452. [PubMed] [Google Scholar]
- 12.Van der Veer LD, Kramer K, Relkin R, Clearfield H. The esophagogastric polyp-fold complex. Am J Gastroenterol. 1984;79:918–920. [PubMed] [Google Scholar]
- 13.Zitsman JL, Schullinger JN, Berdon WE. Inflammatory esophagogastric polyps: resolution following antireflux surgery. J Pediatr Surg. 1988;23:1016–1017. doi: 10.1016/s0022-3468(88)80011-3. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H. Endoscopic mucosal resection for the entire gastrointestinal mucosal lesions. Gastrointest Endosc Clin N Am. 2001;11:459–478. [PubMed] [Google Scholar]
- 15.Moriyama T, Matsumoto T, Jo Y, Iwai K, Yao T, Iida M. Pseudomalignant erosion in an inflammatory polyp at esophagocardial junction. Gastrointest Endosc. 2003;57:987–989. doi: 10.1016/s0016-5107(03)70043-1. [DOI] [PubMed] [Google Scholar]
- 16.Glassman M, Bostwick HE, Godine L, Newman LJ. Endoscopic removal of inflammatory esophagogastric polyps in children. J Pediatr Gastroenterol Nutr. 1991;13:110–114. doi: 10.1097/00005176-199107000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Styles RA, Gibb SP, Tarshis A, Silverman ML, Scholz FJ. Esophagogastric polyps: radiographic and endoscopic findings. Radiology. 1985;154:307–311. doi: 10.1148/radiology.154.2.3966116. [DOI] [PubMed] [Google Scholar]
- 18.Thurberg BL, Duray PH, Odze RD. Polypoid dysplasia in Barrett's esophagus: a clinicopathologic, immunohistochemical, and molecular study of five cases. Hum Pathol. 1999;30:745–752. doi: 10.1016/s0046-8177(99)90134-x. [DOI] [PubMed] [Google Scholar]
- 19.Arnold GL, Mardini HE. Barrett's esophagus-associated polypoid dysplasia: a case report and review of the literature. Dig Dis Sci. 2002;47:1897–1900. doi: 10.1023/a:1016481620175. [DOI] [PubMed] [Google Scholar]
- 20.Shah KK, DeRidder PH, Shah KK. Ectopic gastric mucosa in proximal esophagus. Its clinical significance and hormonal profile. J Clin Gastroenterol. 1986;8:509–513. doi: 10.1097/00004836-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Raine CH. Ectopic gastric mucosa in the upper esophagus as a cause of dysphagia. Ann Otol Rhinol Laryngol. 1983;92:65–66. doi: 10.1177/000348948309200115. [DOI] [PubMed] [Google Scholar]
- 22.Chatelain D, Fléjou JF. [Hyperplastic polyp in heterotopic gastric mucosa. A rare lesion of the cervical esophagus] Ann Pathol. 1998;18:415–417. [PubMed] [Google Scholar]
- 23.Oguma J, Ozawa S, Omori T, Kitagawa Y, Saikawa Y, Mikami S, Kitajima M. EMR of a hyperplastic polyp arising in ectopic gastric mucosa in the cervical esophagus: case report. Gastrointest Endosc. 2005;61:335–338. doi: 10.1016/s0016-5107(04)02469-1. [DOI] [PubMed] [Google Scholar]
- 24.Takagi A, Ema Y, Horii S, Morishita M, Miyaishi O, Kino I. Early adenocarcinoma arising from ectopic gastric mucosa in the cervical esophagus. Gastrointest Endosc. 1995;41:167–170. doi: 10.1016/s0016-5107(05)80605-4. [DOI] [PubMed] [Google Scholar]
- 25.Mion F, Lambert R, Partensky C, Cherkaoui M, Berger F. High-grade dysplasia in an adenoma of the upper esophagus developing on heterotopic gastric mucosa. Endoscopy. 1996;28:633–635. doi: 10.1055/s-2007-1005561. [DOI] [PubMed] [Google Scholar]
- 26.Bach KK, Postma GN, Koufman JA. Esophagitis with an inflammatory polyp. Ear Nose Throat J. 2002;81:824. [PubMed] [Google Scholar]
- 27.De Giacomo C, Gullotta R, Perotti P, Bawa P, Cornaggia M, Fiocca R. Hyperplastic esophagogastric polyps in two children with neurofibromatosis type 1. J Pediatr Gastroenterol Nutr. 1994;18:107–110. doi: 10.1097/00005176-199401000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Shim KS, Suh JM, Baeg NJ, Yang YS, Kim BS. Post-inflammatory polyps of esophagus: a rare sequela of endoscopic injection sclerotherapy for esophageal varix. Gastrointest Endosc. 1993;39:861–862. doi: 10.1016/s0016-5107(93)70298-9. [DOI] [PubMed] [Google Scholar]
- 29.Cockey BM, Jones B, Bayless TM, Shauer AB. Filiform polyps of the esophagus with inflammatory bowel disease. AJR Am J Roentgenol. 1985;144:1207–1208. doi: 10.2214/ajr.144.6.1207. [DOI] [PubMed] [Google Scholar]
- 30.Walker RS, Breuer RI, Victor T, Gore RM. Crohn's esophagitis: a unique cause of esophageal polyposis. Gastrointest Endosc. 1996;43:511–515. doi: 10.1016/s0016-5107(96)70298-5. [DOI] [PubMed] [Google Scholar]
- 31.Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001;25:500–507. doi: 10.1097/00000478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Jørgensen H, Mogensen AM, Svendsen LB. Hyperplastic polyposis of the large bowel. Three cases and a review of the literature. Scand J Gastroenterol. 1996;31:825–830. doi: 10.3109/00365529609010360. [DOI] [PubMed] [Google Scholar]
- 33.Croyle P, Nikaidoh H, Currarino G. Inflammatory esophagogastric junction polyp. Am J Gastroenterol. 1981;76:438–440. [PubMed] [Google Scholar]
- 34.Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential. Cancer Epidemiol Biomarkers Prev. 2002;11:1012–1018. [PubMed] [Google Scholar]
- 35.van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut. 1998;43:216–222. doi: 10.1136/gut.43.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spechler SJ. Dysplasia in Barrett's esophagus: limitations of current management strategies. Am J Gastroenterol. 2005;100:927–935. doi: 10.1111/j.1572-0241.2005.41201.x. [DOI] [PubMed] [Google Scholar]
- 37.Skacel M, Petras RE, Gramlich TL, Sigel JE, Richter JE, Goldblum JR. The diagnosis of low-grade dysplasia in Barrett's esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383–3387. doi: 10.1111/j.1572-0241.2000.03348.x. [DOI] [PubMed] [Google Scholar]
- 38.Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O'Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 40.Sharma P. Low-grade dysplasia in Barrett's esophagus. Gastroenterology. 2004;127:1233–1238. doi: 10.1053/j.gastro.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 41.Weston AP, Banerjee SK, Sharma P, Tran TM, Richards R, Cherian R. p53 protein overexpression in low grade dysplasia (LGD) in Barrett's esophagus: immunohistochemical marker predictive of progression. Am J Gastroenterol. 2001;96:1355–1362. doi: 10.1111/j.1572-0241.2001.03851.x. [DOI] [PubMed] [Google Scholar]
- 42.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collard JM. High-grade dysplasia in Barrett's esophagus. The case for esophagectomy. Chest Surg Clin N Am. 2002;12:77–92. doi: 10.1016/s1052-3359(03)00067-x. [DOI] [PubMed] [Google Scholar]
- 44.Heitmiller RF, Redmond M, Hamilton SR. Barrett's esophagus with high-grade dysplasia. An indication for prophylactic esophagectomy. Ann Surg. 1996;224:66–71. doi: 10.1097/00000658-199607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice TW, Falk GW, Achkar E, Petras RE. Surgical management of high-grade dysplasia in Barrett's esophagus. Am J Gastroenterol. 1993;88:1832–1836. [PubMed] [Google Scholar]
- 46.Conio M, Cameron AJ, Chak A, Blanchi S, Filiberti R. Endoscopic treatment of high-grade dysplasia and early cancer in Barrett's oesophagus. Lancet Oncol. 2005;6:311–321. doi: 10.1016/S1470-2045(05)70167-4. [DOI] [PubMed] [Google Scholar]
- 47.Ell C, May A, Gossner L, Pech O, Günter E, Mayer G, Henrich R, Vieth M, Müller H, Seitz G, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 48.May A, Gossner L, Pech O, Fritz A, Günter E, Mayer G, Müller H, Seitz G, Vieth M, Stolte M, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol. 2002;14:1085–1091. doi: 10.1097/00042737-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Hull MJ, Mino-Kenudson M, Nishioka NS, Ban S, Sepehr A, Puricelli W, Nakatsuka L, Ota S, Shimizu M, Brugge WR, et al. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol. 2006;30:114–118. doi: 10.1097/01.pas.0000180438.56528.a0. [DOI] [PubMed] [Google Scholar]
- 50.Conio M, Repici A, Cestari R, Blanchi S, Lapertosa G, Missale G, Della Casa D, Villanacci V, Calandri PG, Filiberti R. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma in Barrett's esophagus: an Italian experience. World J Gastroenterol. 2005;11:6650–6655. doi: 10.3748/wjg.v11.i42.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conio M, Ponchon T, Blanchi S, Filiberti R. Endoscopic mucosal resection. Am J Gastroenterol. 2006;101:653–663. doi: 10.1111/j.1572-0241.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 52.Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165–169. doi: 10.1067/mge.2003.73. [DOI] [PubMed] [Google Scholar]
- 53.Seewald S, Akaraviputh T, Seitz U, Brand B, Groth S, Mendoza G, He X, Thonke F, Stolte M, Schroeder S, et al. Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854–859. doi: 10.1016/s0016-5107(03)70020-0. [DOI] [PubMed] [Google Scholar]
- 54.Pacifico RJ, Wang KK, Wongkeesong LM, Buttar NS, Lutzke LS. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett's esophagus. Clin Gastroenterol Hepatol. 2003;1:252–257. [PubMed] [Google Scholar]
- 55.Cameron AJ, Carpenter HA. Barrett's esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92:586–591. [PubMed] [Google Scholar]
- 56.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]


