Abstract
Hepatobiliary cystadenomas (HBC) and cystadenocarcinomas are rare cystic lesions. Most patients with these lesions are asymptomatic, but presentation with obstructive jaundice may occur. The first patient presented with intermittent colicky pain and recurrent obstructive jaundice. Imaging studies revealed a polypoid lesion in the left hepatic duct. The second patient had recurrent jaundice and cholangitis. Endoscopic retrograde cholangiopancreatography (ERCP) showed a cystic lesion at the confluence of the hepatic duct. In the third patient with intermittent jaundice and cholangitis, cholangioscopy revealed a papillomatous structure protruding into the left bile duct system. In the fourth patient with obstructive jaundice, CT-scan showed slight dilatation of the intrahepatic bile ducts and dilatation of the common bile duct of 3 cm. ERCP showed filling of a cystic lesion. All patients underwent partial liver resection, revealing HBC in the specimen. In the fifth patient presenting with obstructive jaundice, ultrasound examination showed a hyperechogenic cystic lesion centrally in the liver. The resection specimen revealed a hepatobiliary cystadenocarcinoma. HBC and cystadenocarcinoma may give rise to obstructive jaundice. Evaluation with cross-sectional imaging techniques is useful. ERCP is a useful tool to differentiate extraductal from intraductal obstruction.
Keywords: Liver, Hepatobiliary cystadenoma, Cystadenocarcinoma, Obstructive jaundice, Endoscopic retrograde cholangiopancreatography
INTRODUCTION
Hepatobiliary cystadenomas (HBC) are rare neoplasms of the liver or extrahepatic bile ducts, accounting for less than 5% of all the cystdenomas found in the liver. These lesions are mainly seen in middle-aged females[1,2] and can show malignant degeneration to hepatobiliary cystadenocarcinoma. Most of these patients are asymptomatic, because these lesions usually are incidental findings on abdominal diagnostic imaging for evaluation of other complaints. If there are presenting symptoms, these are right upper quadrant pain or discomfort. Obstructive jaundice only rarely occurs as a presenting symptom[3]. We describe here the diagnostic evaluation, surgical management and pathological characteristics of four patients with HBC and one patient with hepatobiliary cystadenocarcinoma who presented with obstructive jaundice.
CASE REPORT
Case 1
A 50-year old woman underwent laparoscopic cholecystectomy because of symptomatic cholecystolithiasis 3 years prior to presentation. She was admitted for intermittent colicky pain and recurrent biliary obstruction. Physical examination showed no abnormalities. Routine liver function tests revealed elevated plasma levels of alkaline phospatase (AP) of 118 U/L (normal range, 40-120 U/L) and gamma-glutamyltransferase (GGT) of 260 U/L (normal, < 40 U/L). Serum bilirubin level was normal. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a polypoid lesion inside the left hepatic duct. Helical computed tomography (CT) of the liver revealed a cystic lesion measuring 10 cm in diameter located in the left lobe of the liver with protrusion into the left hepatic duct. Calcifications were present in the wall of the lesion and septations were visible inside the cyst (Figure 1A). ERCP showed the image of an extrinsic mass proximal to the polypoid lesion, creating a compression on the intrahepatic ducts of the left biliary system (Figure 1B). These findings suggested that the episodes of colicky pain and cholestasis were caused by a cystic lesion with a polypoid protrusion into the left bile duct, giving rise to intermittent obstruction. Distinction between hepatobiliary cystadenoma and cystadenocarcinoma could not be made on the basis of imaging studies. The patient underwent left hemihepatectomy and the histopathological diagnosis of hepatobiliary mucinous cystadenoma was made. The postoperative course was uneventful and the complaints resolved after operation. The patient died 56 mo after operation because of other reasons not related to the initial disease.
Figure 1.
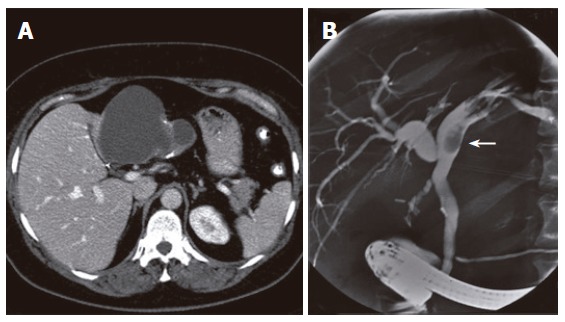
Abdominal CT-scan showing a large cystic mass in the left liver lobe with internal septations and calcifications in the cyst wall (A) and ERCP showing a polypoid lesion in the left hepatic duct (arrow) in case 1 (B).
Case 2
A 46-year old woman underwent cholecystectomy with common bile duct exploration because of gallstones 6 years prior to presentation. During cholecystectomy, fenestration of a presumed simple intraluminal cyst at the confluence of the hepatic duct was performed. Microscopic examination of the cyst wall showed biliary epithelium without mesenchymal stroma. She had recurrent jaundice and cholangitis. Initial investigations revealed elevated levels of serum bilirubin level of 79 μmol/L (normal, < 17 μmol/L), AP of 311 U/L (normal range, 40-120 U/L), GGT of 448 U/L (normal, < 40 U/L), AST of 96 U/L and ALT of 142 U/L (normal, < 45 U/L). ERCP showed filling of a cystic lesion at the confluence of the hepatic duct. Brush cytology revealed no malignancy. A plastic stent was placed for decompression of the biliary system. Abdominal ultrasonography (US) and CT-scan revealed multiple cystic lesions involving segment 4 of the liver with dilatation of the left central and peripheral intrahepatic bile ducts and the right central intrahepatic bile ducts. At laparotomy, multiple cysts in segment 4 of the liver were found with a dominant cystic lesion of 2.5 cm located in the area of hepatic hilum. Hilar resection in combination with left hemihepatectomy was performed. Microscopically, the cyst wall was composed of cylindrical epithelium. The features of the subepithelial stroma were typical for the diagnosis hepatobiliary mucinous cystadenoma. The complaints resolved after operation. The patient was still alive 103 mo after operation.
Case 3
A 40-year old woman underwent laparoscopic cholecystectomy for symptomatic cholecystolithiasis 4 mo ago. She had intermittent complaints of jaundice and cholangitis. Liver function tests showed slightly elevated AP levels of 103 U/L and GGT level of 44 U/L. The serum bilirubin and alanine transaminase levels were normal. ERCP revealed a filling defect in the left hepatic duct (Figure 2A). A stent was placed for decompression of the biliary system. The second ERCP at our hospital suggested that the filling defect was caused by an intraductal tissue mass. Subsequent cholangioscopy showed a papillomatous structure protruding into the left bile duct system from the bile duct wall. CT scan showed a cystic lesion with septations located in segment 4 of the liver. It was unclear if there was a connection between the lesion and the left hepatic duct. The lesion was 3.6 cm in diameter (Figure 2B). The patient underwent a left hemihepatectomy. Macroscopically, a tumour with smooth surface was seen protruding inside the bile duct and filling up the entire lumen (Figure 2C). Cut sections revealed a multicystic lesion encapsulated by a thick fibrous capsule arising from the left hepatic duct (Figure 2D). The cystic lesion was surrounded by stroma containing spindle-shaped cells, resembling ovarian stroma (Figure 2E). The diagnosis of hepatobiliary mucinous cystadenoma was made. No signs of malignancy were seen. The patient remained alive 19 mo after operation.
Figure 2.
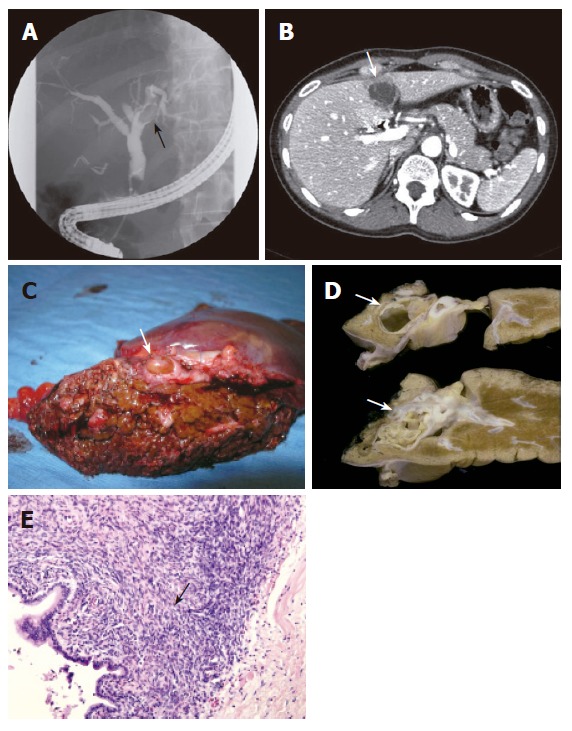
ERCP showing a filling defect due to an intraluminal lesion (arrow) in the left hepatic duct (A), CT-scan showing a cystic lesion with internal septations measuring 3.6 cm located in segment 4 (arrow) (B), specimen after left hemihepatectomy showing macroscopic features of a large lesion (arrow) inside the left bile duct filling up the entire lumen (C), macroscopical cut sections of a multicystic lesion (arrows) encapsulated by a thick fibrous capsule arising from the left hepatic duct (D), microscopical features showing columnar mucinous epithelium with underlying dense-cellular stroma resembling ovarian stroma (arrow) (HE X 200) (E) in case 3.
Case 4
A 43-year old woman with an unremarkable previous medical history presented with progressive obstructive jaundice three weeks ago. Abdominal US showed dilatation of intra- and extra-hepatic bile ducts and a collapsed gallbladder without a clear cause. ERCP showed a normal pancreatic duct but the common bile duct could not be visualized. CT showed slight dilatation of the intrahepatic bile ducts and dilatation of part of the common bile duct with a diameter of 3 cm (Figure 3A). Subsequent MRCP showed the same images as obtained with CT. ERCP showed filling of a cystic lesion connected to the common bile duct (Figure 3B). A diagnosis of a duplicate gallbladder or choledochal cyst at the level of the common hepatic duct was considered. A cystic lesion was detected during laparotomy and a decision was made to resect the common bile duct and the gallbladder. Continuity of the bile duct was restored using a hepaticojejunostomy. Histopathological examination of the resection specimen revealed a multilocular hepatobiliary cystadenoma with necrotizing inflammation. The patient was still alive 46 mo after operation.
Figure 3.

Abdominal coronal CT-scan showing dilated intrahepatic bile ducts and common bile duct (arrow) (A), ERCP showing filling of a cystic lesion (arrow) connected to the common bile duct, initially diagnosed as a duplicate gallbladder or choledochal cyst (B) in case 4.
Case 5
A 39-year old woman presented with obstructive jaundice, nausea and weight loss which began three months before presentation. Liver function tests showed elevated bilirubin levels of 128 μmol/L, AP of 1053 U/L, GGT of 500 U/L, AST of 172 U/L and ALT of 142 U/L. The alpha-fetoprotein level was normal. Because of failure of ERCP, percutaneous transhepatic drainage (PTD) was preformed for biliary decompression via the right intrahepatic bile ducts (Figure 4A). Complete obstruction at the level of the proximal bile duct was seen. US showed a well defined hyperechogenic cystic lesion of 4.4 cm, centrally in the liver. A solid calicification of 1 cm was situated inside the lesion. CT showed a cystic lesion with irregularly thickened wall, in conjunction with dilatated intrahepatic bile ducts (Figure 4B). Preoperative differential diagnosis of hepatobiliary cystadenoma, cystadenocarcinoma or choledochal cyst was made. The patient underwent hilar resection combined with extended right hemihepatectomy and biliary reconstruction using a hepaticojejunostomy. The diagnosis of hepatobiliary cystadenocarcinoma was made microscopically. The lesion was completely resected with microscopically free margins. The patient was still alive 59 mo after operation. Follow-up imaging showed no signs of local or distant tumor recurrence.
Figure 4.
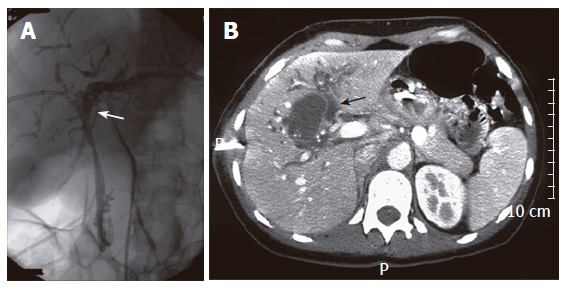
Percutaneous transhepatic drainage (PTD) showing complete obstruction at level of the proximal bile duct (A), abdominal CT showing a cystic lesion with irregularly thickened wall in conjunction with dilatated intrahepatic bile ducts (arrow) (B) in case 5.
DISCUSSION
HBC are rare cystic neoplasms that may occur in the liver or in the extrahepatic biliary system. It is estimated that these cystic liver neoplasms comprise 5% of all cystic liver lesions[1]. Only a minority of patients with HBC develop symptoms, the most commonly reported symptoms are right abdominal pain, abdominal swelling, anorexia or nausea. Rarely, patients present with colicky pain or jaundice caused by obstruction of the biliary system. The differential diagnosis of HBC without symptoms includes simple cysts, choledochal cysts, hepatobiliary cystadenocarcinoma, hydatid cysts, abscess or haematoma. Obstructive jaundice is usually a presenting symptom of choledocholithiasis or malignant cholangiocarcinoma.
In the past 14 years, 13 patients have been diagnosed with HBC or cystadenocarcinoma in our institution. Five of these patients (38.5%) presented with biliary obstruction, which is usually caused by external biliary compression or by internal obstruction due to a mass inside the bile duct. Mucus hypersecretion in case of HBC or cystadenocarcinoma communicating with the bile ducts may also give rise to obstruction symptoms[4]. Three patients in this series presented with intermittent obstruction jaundice and two patients had continuous jaundice. In contrast, continuous biliary obstruction usually is the presenting symptom of patients with hilar cholangiocarcinoma.
Typically elevated cholestatic parameters in the blood are secondary to obstruction or compression of the biliary system. Some authors have suggested that serum CA-19-9 levels can be used for diagnosis or as a parameter of tumor activity during follow-up after resection[5,6]. CA-19-9, a serum marker normally synthesized by normal pancreatic and biliary ductal epithelium, is elevated both in benign biliary lesions and in malignant pancreatic carcinomas[7]. These serum markers, however, may be elevated in the presence of cholestasis and are therefore less reliable in the diagnosis of bile duct lesions. On imaging studies, distinction between HBC and simple cysts can be made by the presence of septations and irregularly thickened cyst walls with or without calcifications[8]. The most accurate imaging methods for detecting cystadenomas are abdominal ultrasound and CT-scan[9].
ERCP is useful, apart from establishing the diagnosis, in stenting the bile duct and decompressing the biliary system as was done in our patients. It can also help to differentiate between extraductal and intraductal obstructions. However, it still remains difficult to differentiate between cystadenoma and cystadenocarcinoma by imaging methods[10]. In patients with cystic lesion at the hepatic hilum, diagnosis of choledochal cyst should be considered as well[11,12]. Liver hydatid disease, caused by Echinococcus granulosis, is usually asymptomatic and should also be considered as rupture of a hydatid cyst into the biliary tract, a well know complication, which also may give rise to colicky pain, cholangitis and obstructive jaundice. In these patients, ERCP is useful in detecting communication between the cystic lesion and the biliary system[13].
The use of percutaneous biopsy for preoperative diagnosis has no additional value considering the fact that it rarely produces a definitive diagnosis. There is also the additional risk of peritoneal dissemination in case of malignancy. Histopathological examination is required for definitive diagnosis. Microscopically, the linings of HBC are composed of a biliary type, mucus-secreting cuboidal or columnar epithelium. The underlying stroma shows presence of ovarian stroma in 85%-90% of HBC[14]. Distinction with simple liver cysts is made among other features, on the basis of the presence of subepithelial ovarian stroma in HBC. Simple liver cysts are composed of an outer layer of fibrous tissue and an inner lining of single columnar or cuboidal epithelium.
In the past, different treatment strategies such as partial resection, percutaneous aspiration or application of sclerosant agents inside the lesions have been applied to HBC. Patients treated with these techniques have shown high recurrence rates when compared to those who have undergone radical partial liver resection[15]. Another reason for resection is the possibility of malignant degeneration, although the precise risk remains unknown. In our series, cystadenocarcinoma was only seen in 2 of the 13 patients (15.4%) with HBC, which is comparable with the incidence in literature ranging from 5% to 25%[3,16]. The mean age of patients with cystadenocarcinomas has been reported to be approximately 17 years which is older than that of those without malignant degeneration[14]. The risk of malignant degeneration is also supported by the presence of benign epithelium in the wall of most cystadenocarcinomas[1]. Therefore, the treatment of choice should be radical surgical resection. The 5-year survival rate for cystadenocarcinomas after surgical resection ranges from 25% to 100%[17,18]. We observed no recurrence of cystadenocarcinomas during a mean follow-up of 56 mo (range 19-103 mo) by abdominal ultrasound or CT.
In conclusion, hepatobiliary cystadenoma or cystadenocarcinoma should be considered in patients with obstructive jaundice in the presence of a cystic liver lesion. ERCP and cross-sectional imaging techniques have a great value for establishing the diagnosis.
Footnotes
S- Editor Liu Y L- Editor Wang XL E- Editor Bi L
References
- 1.Ishak KG, Willis GW, Cummins SD, Bullock AA. Biliary cystadenoma and cystadenocarcinoma: report of 14 cases and review of the literature. Cancer. 1977;39:322–338. doi: 10.1002/1097-0142(197701)39:1<322::aid-cncr2820390149>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–1091. [PubMed] [Google Scholar]
- 3.den Hoed PT, Lameris H, Klooswijk B, IJzermans JN. Biliary cystadenoma: an uncommon cause of cholestatic jaundice. Eur J Surg Oncol. 1999;25:335–336. [PubMed] [Google Scholar]
- 4.Wang YJ, Lee SD, Lai KH, Wang SS, Lo KJ. Primary biliary cystic tumors of the liver. Am J Gastroenterol. 1993;88:599–603. [PubMed] [Google Scholar]
- 5.Thomas JA, Scriven MW, Puntis MC, Jasani B, Williams GT. Elevated serum CA 19-9 levels in hepatobiliary cystadenoma with mesenchymal stroma. Two case reports with immunohistochemical confirmation. Cancer. 1992;70:1841–1846. doi: 10.1002/1097-0142(19921001)70:7<1841::aid-cncr2820700706>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Caturelli E, Bisceglia M, Villani MR, de Maio G, Siena DA. CA 19-9 production by a cystadenoma with mesenchymal stroma of the common hepatic duct: a case report. Liver. 1998;18:221–224. doi: 10.1111/j.1600-0676.1998.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–355. [PubMed] [Google Scholar]
- 8.Federle MP, Filly RA, Moss AA. Cystic hepatic neoplasms: complementary roles of CT and sonography. AJR Am J Roentgenol. 1981;136:345–348. doi: 10.2214/ajr.136.2.345. [DOI] [PubMed] [Google Scholar]
- 9.Palacios E, Shannon M, Solomon C, Guzman M. Biliary cystadenoma: ultrasound, CT, and MRI. Gastrointest Radiol. 1990;15:313–316. doi: 10.1007/BF01888807. [DOI] [PubMed] [Google Scholar]
- 10.Hai S, Hirohashi K, Uenishi T, Yamamoto T, Shuto T, Tanaka H, Kubo S, Tanaka S, Kinoshita H. Surgical management of cystic hepatic neoplasms. J Gastroenterol. 2003;38:759–764. doi: 10.1007/s00535-003-1142-7. [DOI] [PubMed] [Google Scholar]
- 11.de Vries JS, de Vries S, Aronson DC, Bosman DK, Rauws EA, Bosma A, Heij HA, Gouma DJ, van Gulik TM. Choledochal cysts: age of presentation, symptoms, and late complications related to Todani's classification. J Pediatr Surg. 2002;37:1568–1573. doi: 10.1053/jpsu.2002.36186. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Lee DH, Kim HJ, Ko YT, Lim JW, Yang MH. Unilocular extrahepatic biliary cystadenoma mimicking choledochal cyst: a case report. Korean J Radiol. 2004;5:287–290. doi: 10.3348/kjr.2004.5.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atli M, Kama NA, Yuksek YN, Doganay M, Gozalan U, Kologlu M, Daglar G. Intrabiliary rupture of a hepatic hydatid cyst: associated clinical factors and proper management. Arch Surg. 2001;136:1249–1255. doi: 10.1001/archsurg.136.11.1249. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts. A clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56:1434–1445. doi: 10.1002/1097-0142(19850915)56:6<1434::aid-cncr2820560635>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Organ B, Petrek J. Biliary cystadenoma. South Med J. 1984;77:262–265. doi: 10.1097/00007611-198402000-00037. [DOI] [PubMed] [Google Scholar]
- 16.Thomas KT, Welch D, Trueblood A, Sulur P, Wise P, Gorden DL, Chari RS, Wright JK Jr, Washington K, Pinson CW. Effective treatment of biliary cystadenoma. Ann Surg. 2005;241:769–773; discussion 773-775. doi: 10.1097/01.sla.0000161982.57360.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota E, Katsumi K, Iida M, Kishimoto A, Ban Y, Nakata K, Takahashi N, Kobayashi K, Andoh K, Takamatsu S, et al. Biliary cystadenocarcinoma followed up as benign cystadenoma for 10 years. J Gastroenterol. 2003;38:278–282. doi: 10.1007/s005350300048. [DOI] [PubMed] [Google Scholar]
- 18.Lewis WD, Jenkins RL, Rossi RL, Munson L, ReMine SG, Cady B, Braasch JW, McDermott WV. Surgical treatment of biliary cystadenoma. A report of 15 cases. Arch Surg. 1988;123:563–568. doi: 10.1001/archsurg.1988.01400290045007. [DOI] [PubMed] [Google Scholar]


