Abstract
AIM: To simultaneously evaluate the presence of defects in gallbladder and gastric emptying, as well as in intestinal transit in gallstone patients (GS) and the effect of chronic ursodeoxycholic acid (UDCA) administration on these parameters and on serum bile acids and clinical outcome in GS and controls (CTR).
METHODS: After a standard liquid test meal, gallbla-dder and gastric emptying (by ultrasound), oroileal transit time (OITT) (by an immunoenzymatic technique) and serum bile acids (by HPLC) were evaluated before and after 3 mo of UDCA (12 mg/kg bw/d) or placebo administration in 10 symptomatic GS and 10 matched healthy CTR.
RESULTS: OITT was longer in GS than in CTR (P < 0.0001); UDCA significantly reduced OITT in GS (P < 0.0001), but not in CTR. GS had longer gastric half-emptying time (t1/2) than CTR (P < 0.0044) at baseline; after UDCA, t1/2 significantly decreased (P < 0.006) in GS but not in CTR. Placebo administration had no effect on gastric emptying and intestinal transit in both GS and CTR.
CONCLUSION: The gallstone patient has simultaneous multiple impairments of gallbladder and gastric emptying, as well as of intestinal transit. UDCA administration restores these defects in GS, without any effect in CTR. These results confirm the pathogenetic role of gastrointestinal motility in gallstone disease and suggest an additional mechanism of action for UDCA in reducing bile cholesterol supersaturation.
Keywords: Bile acids, Biliary cholesterol, Deoxycholic acid, Gallbladder emptying, Gastric emptying, Intestinal transit, Tauroursodeoxycholic acid
INTRODUCTION
Cholesterol gallstone disease is frequent, in fact its prevalence ranges from 10% to 20% in Western countries[1]. Although most gallstone patients (GS) are asymptomatic[1,2], a progressively increased rate of cholecystectomies has been reported[3], thus confirming that gallstone disease is one of the major gastrointestinal problems throughout the Western world. Cholesterol gallstone pathogenesis is complex and multifactorial, involving both genetic defects and environmental factors[4,5], all of which contribute to cholesterol-supersaturated bile, rapid nucleation time of cholesterol microcrystals (as a result of excess promoters and/or a deficiency of crystallization inhibitors), and impaired gallbladder motility. However in GS not only is gallbladder motility defective[6], but it has been recently documented[7-9] that also intestinal transit time and gastric emptying are delayed. However, no study has simultaneously evaluated gallbladder and gastric emptying and intestinal transit in GS.
Ursodeoxycholic acid (UDCA) is a drug known to be able to reduce bile cholesterol supersaturation and to dissolve cholesterol gallstones[10], without significant side effects[11]. Reduction in bile cholesterol supersaturation is reached, according to some[12,13] but not to others[14,15], mainly through a reduction in intestinal cholesterol absorption and by formation of a liquid crystalline phase[16]. Furthermore, UDCA has been proposed in gallstone prevention in subset populations, i.e. obese patients during weight loss[17] and more recently, it has been shown that chronic, long-term UDCA administration reduces biliary pain in GS[18]. Conflicting results exist regarding the effect of UDCA on gallbladder motility, since fasting and residual gallbladder volumes were found to be increased while gallbladder emptying has been found to be either decreased or unmodified[6,19]. Moreover, no definitive data is available on the effect of chronic UDCA administration on gastrointestinal (GI) motility in man.
In a preliminary study aimed at simultaneously evaluating gallbladder and gastric emptying and intestinal transit before and after UDCA treatment in GS and healthy subjects, we found[20] that GS presented impaired gallbladder and gastric emptying and delayed intestinal transit and that UDCA administration improved these defects in GS without inducing any significant effect in healthy subjects.
Since these observed effects of UDCA administration could have been biased by the lack of a placebo arm in the study protocol, we performed a new placebo-controlled study aimed at evaluating the effect of chronic administration of UDCA on gastric and gallbladder emptying and intestinal transit in GS and controls.
MATERIALS AND METHODS
Study design
This single blinded, placebo controlled study was designed to evaluate the effects of UDCA, or placebo, on meal stimulated gallbladder and gastric emptying, small intestinal transit and serum bile acid pattern in GS and healthy controls (CTR). All individuals were assigned to UDCA for the first treatment period of the study lasting 3 mo, then after an interim 15 d washout period, placebo was administered for additional 3 mo. Patients and controls were unaware of the study design, while ultrasonographers were unaware of the results of biochemical analyses (serum bile acid determination, intestinal transit time), and biochemical investigators were unaware of the results of ultrasonographic investigations.
The study design was in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the Local Ethical Committee. Written informed consent was obtained from all subjects.
Study population
The studied population was comprised of 10 consecutive GS (4 males, 6 females, mean age 49.5 ± 2.2 years, range 30-63; mean body mass index (BMI) 23.0 ± 0.8 kg/m2) and 10 matched CTR (4 males, 6 females, mean age 50.5 ± 1.4 years, range 28-61; mean BMI 22.8 ± 0.4 kg/m2). CTR were recruited from local staff members of our department. Characteristics of patients and controls are shown in Table 1, while in Table 2 the biochemical parameters are reported. All enrolled GS had cholesterol gallstones, as evaluated by oral cholecystography. They had a history of biliary pain, defined according to the previously identified criteria[2], as a pain at the epigastrium or right hypochondrium, unrelated to meal ingestion, which does not disappear with bowel movements. No GS had past or present signs of complicated gallstone disease (i.e. acute cholecystitis, biliary obstruction or biliary pancreatitis). Furthermore, no GS had other GI and/or liver diseases or was taking drugs potentially affecting gastrointestinal function. All GS were suitable to bile acid dissolution therapy according to the indications of a recent Working Team Report[21], i.e. radiolucent gallstones less than 1.5 cm in diameter, in functional gallbladder. All GS patients and controls had daily bowel evacuation and fertile female subjects were studied within the follicular phase to prevent any influence of hormones on GI motility behaviour.
Table 1.
Characteristics of gallstone patients (GS) and healthy controls
| Characteristics | GS (n = 10) | Controls (n = 10) | P |
| Gender | |||
| Male | 4 | 4 | |
| Female | 6 | 6 | |
| Age (yr) | |||
| Mean ± SE | 49.5 ± 2.3 | 50.5 ± 1.4 | NS |
| Range | 30-63 | 28-61 | |
| BMI (kg/m2 ) | |||
| Mean ± SE | 23.0 ± 0.8 | 22.8 ± 0.4 | NS |
| Range | 21-28 | 20-29 | |
| Biliary colic | 10/10 | 0/10 | |
| Gallstones | |||
| n (single/multiple) | 3/7 | ||
| Size (< 1.5 cm) | 10/10 |
NS: Not significant.
Table 2.
Baseline biochemical parameters in gallstone patients (GS) and controls (mean ± SD)
| Biochemical parameter | GS (n = 10) | Controls (n = 10) | P |
| Serum creatinine (mg/dL) | 0.98 ± 0.25 | 0.96 ± 0.21 | NS |
| Blood nitrogen (mg/dL) | 39.4 ± 13.0 | 37.7 ± 10.6 | NS |
| AST (IU/I) | 23.2 ± 15.0 | 21.3 ± 12.7 | NS |
| ALT (IU/I) | 23.9 ± 14.6 | 21.8 ± 11.6 | NS |
| AP (IU/I) | 147.3 ± 63.2 | 150 ± 60.5 | NS |
| γGT (IU/I) | 28.0 ± 18.0 | 30.2 ± 15.8 | NS |
| Total proteins (g/dL) | 6.8 ± 0.57 | 6.9 ± 0.58 | NS |
| Total cholesterol (mg/dL) | 185.0 ± 38.5 | 178.4 ± 35 | NS |
| Fasting triglycerides (mg/dL) | 141.7 ± 30.8 | 138.5 ± 27.2 | NS |
| Fasting glucose (mg/dL) | 96.8 ± 12.0 | 95.3 ± 11.4 | NS |
NS: Not significant.
Study protocol
Gallbladder and gastric emptying, oro-ileal transit time (OITT) and serum bile acids were assessed in all subjects at enrollment, after 3 mo administration of UDCA (Deursil® Sanofi-Synthelabo, Paris-Cedex, France) (12 mg/kg bw/d), after the wash-out period and at the end of placebo administration. Furthermore, the presence of biliary pain and/or dyspeptic symptoms (i.e. heartburn, vomiting, nausea, belching, bloating feeling after meals, intolerance to fatty or fried foods, epigastric discomfort, uncomfortable feeling at the right hypocondrium), were evaluated before and during treatment.
Gallbladder emptying
Gallbladder emptying was evaluated by a previously described ultrasonographic technique using the ellipsoid method[6]. Both GS and control subjects were studied after an overnight fast. Gallbladder volume variations were measured in response to a standard liquid test meal (200 mL containing 375 kcal, 17 g fats, 10.4 g proteins, 10 g carbohydrates); measurements were performed before and every 10 min after meal ingestion until 80% gallbladder refilling was reached. Results were expressed as fasting gallbladder volume (FV, in mL), given as the mean of four measurements obtained before meal intake; residual gallbladder volumes (RV, mL), given by the minimal gallbladder volume after postprandial gallbladder emptying, and percent gallbladder emptying (%E), given by: (FV-RV/FV) × 100. All measurements were performed by two sonographers (AC, LS) and inter- and intra-observer variations less than 8% were documented.
Oro-ileal-transit time
OITT was evaluated as previously described[8], using the tauroursodeoxycholic acid (TUDCA) load test and evaluating serum TUDCA appearance time. TUDCA was used since this bile acid is selectively and rapidly absorbed in the terminal ileum[22]. After 12 h of fasting, each individual was given the standard liquid test meal to which 1.5 g of TUDCA was added. Blood samples were collected by peripheral venous catheter every 30 min for 7 h and then stored at -20°C until analyzed. OITT was evaluated measuring serum concentrations of TUDCA by means of an enzymatic immunoassay using a specific antibody against this bile acid[23]. The assay fulfils all the requirements of precision and accuracy; the intra- and inter- assay precision, evaluated on six replicates of human serum samples spiked with three levels of TUDCA (0.001, 0.01, 0.1 μmol/L), presents a CV % below 8%. The accuracy of the method has been evaluated by comparison with the results obtained using a high-pressure-liquid chromatography -ES- mass spectrometry (HPLC-ES-MS) reference method[24].
OITT was defined, as previously described[8], as the time-interval between test meal administration and peak serum concentration of TUDCA.
Serum bile acid levels
Fasting serum bile acids were measured by HPLC-MS[24]. In brief, serum samples (0.1 mL) were diluted with 3.5 mL of 0.1 mmol/L NaOH, incubated at 64°C for 30 min and submitted to a clean-up procedure by means of a conventional C18 reverse-phase extraction. The apparatus consisted of an analytical HPLC system (Alliance 2695 Waters, Milford, MA, USA) connected with a triple quadrupole mass spectrometer (Quattro LC, Micromass, Wythenshawe, UK), with electrospray interface. Individual bile acids were separated on a C18 analytical column (Ultrasphere XL, 3 μm, 70 mm × 4.6 mm I.D., Beckman Instruments, Berkeley CA, USA) and analysed by electrospray mass spectrometry in the negative-ion acquisition mode following the procedure previously described[24].
Gastric emptying
Gastric emptying was evaluated simultaneously with gallbladder motility, monitoring changes in the gastric antral area by an ultrasonographic technique[9,25]. Gastric antral area variations were evaluated before and after administration of the same standard liquid test meal used to assess gallbladder motility. Results were expressed as half-emptying time (t 1/2): the time, in minutes, to observe a 50% decrease in maximal antral area measured by linear regression analysis.
Statistical analyses
All results were analysed using the Statistical Package for Social Science (SPSS version 11.5). Unless otherwise stated, all results were expressed as mean ± SE. Student’s t test for paired and unpaired data was used to evaluate significant intra- or inter-group differences. A repeated measure ANOVA model was individually fitted for each of the following variables: gallbladder basal and residual volume, gallbladder emptying, intestinal transit time, gastric emptying, serum levels of cholic (CA), deoxycholic (DCA), lithocholic (LCA), ursodeoxycholic (UDCA) and chenodeoxycholic (CDCA) acids. Treatment (basal, UDCA and placebo) was considered as the within-subject factor and controls versus GS was considered as the between-subject factor.
Univariate and multivariate linear regression analyses were performed to evaluate the relationship between serum bile acid levels and gastric t1/2, OITT, fasting gallbladder volume and gallbladder emptying. Each statistical test was conducted at the significance level of 0.05 (two-sided).
RESULTS
Clinical outcome
All enrolled individuals completed UDCA or placebo treatment and no adverse treatment-related events, and in particular stool frequency change, were recorded. At enrolment, all GS were symptomatic and dyspeptic symptoms were present in 4 out of 10 GS (40%). No significant difference was present between controls and GS in terms of frequency and severity of dyspeptic symptoms. Biliary pain disappeared during UDCA treatment in all GS, while during placebo administration 20% of patients had symptom recurrence. No significant change (reduction or increase) in the number or size of the gallstones was observed during the study period.
Serum bile acids
Serum bile acid profiles were different in GS and CTR (Figure 1) groups. Baseline serum DCA was significantly higher in GS than controls (DCA%: 25.7 ± 0.5 vs 16.7 ± 0.4, P < 0.05); LCA was higher in GS than controls (LCA% = 2.6 ± 0.5 vs 1.1 ± 0.2), but not significantly, while CA and CDCA were significantly lower in GS than in CTR (CA%: 33.2 ± 1.1 vs 37.2 ± 0.6; P < 0.02; CDCA %: 37.8 ± 0.7 vs 43.7 ± 0.6; P < 0.05).
Figure 1.
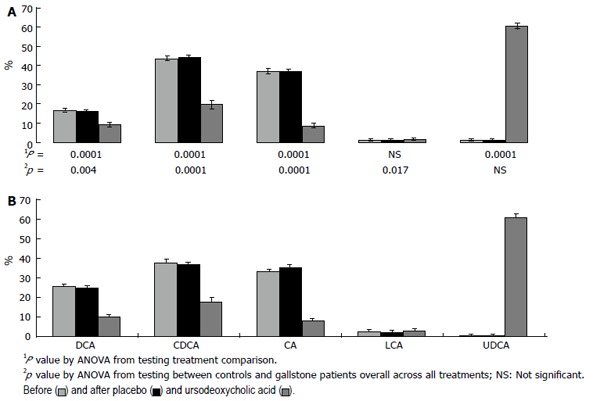
Serum bile acids concentrations (percent values) in gallstone patients (down) and controls (up), before and after placebo and ursodeoxycholic acid treatment. Each bile acid concentration is expressed as percentages of total serum bile acids. DCA: Deoxycholic acid; CDCA: Chenodeoxycholic acid; CA: Cholic acid; LCA: Lithocholic acid; UDCA: Ursodeoxycholic acid.
After UDCA administration, CA, DCA and CDCA significantly decreased both in GS and CTR (P < 0.05); UDCA significantly increased (P < 0.05), reaching up to 60% of total serum bile acids in both groups (Figure 1). After placebo administration, in both GS and CTR, serum bile acid proportion did not significantly change compared to baseline values.
Oro-Ileal-transit time
At baseline, GS showed longer OITT compared to controls (GS: 320.0 ± 6.9 min vs CTR: 204.0 ± 7.2 min.; P < 0.05) (Figure 2). UDCA treatment significantly reduced OITT in GS patients (P < 0.05) but not in controls. At the end of treatment, OITT was no longer significantly different in GS and controls (219.5 ± 8.9 min and 198.0 ± 8.8 min, respectively). Placebo administration did not significantly modify OITT in both GS and controls.
Figure 2.
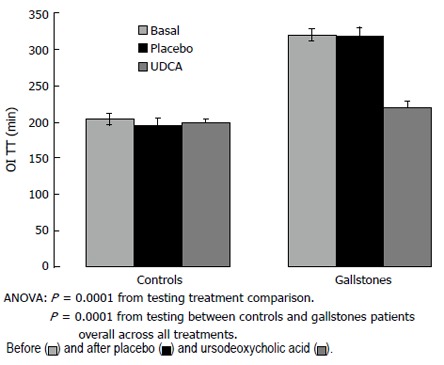
Oro-ileal transit time (OITT) in gallstone patients and healthy controls before and after placebo and ursodeoxycholic acid (UDCA) administration.
Gallbladder emptying
At baseline, with respect to controls, GS showed significantly larger fasting gallbladder volume ( GS: 23.5 ± 0.9 mL vs CTR: 18.8 ± 0.9 mL, P < 0.05) and residual gallbladder volume (GS: 8.5 ± 0.3 mL vs CTR: 4.7 ± 0.8 mL, P < 0.05) than controls; percent gallbladder emptying was significantly smaller in GS than in controls (63.8 ± 2.4 and 76.0 ± 2.3, respectively, P < 0.05) (Table 3).
Table 3.
Fasting and residual gallbladder volumes, and percent gallbladder emptying before and after ursodeoxycholic acid (UDCA) and placebo administration in gallstone patients (n = 10) and healthy controls (n = 10)
| Basal | UDCA | Placebo | P2 | |
| Fasting Volume (mL): | ||||
| Controls | 18.8 (0.9) | 22.9 (0.6) | 19.1 (0.9) | 0.001 |
| Gallstones | 23.5 (0.9) | 26.2 (0.5) | 24.1 (0.9) | |
| P1 | 0.0001 | |||
| Residual Volume (mL): | ||||
| Controls | 4.7 (0.8) | 6.4 (0.7) | 4.9 (0.7) | 0.003 |
| Gallstones | 8.5 (0.3) | 11.0 (1.0) | 8.5 (0.2) | |
| P1 | 0.0001 | |||
| Gallbladder emptying (%) | ||||
| Controls | 76 (2.3) | 71.9 (3.2) | 74.5 (2.2) | 0.066 |
| Gallstones | 63.8 (2.4) | 59.5 (3.3) | 61.6 (2.2) | |
| P1 | 0.001 |
Repeated measures Analysis of Variance.
From testing between controls and gallstone patients overall across all treatment; 2 For testing the treatment effect (basal, UDCA, placebo).
No significant effect was observed after placebo treatment in both GS and controls; after UDCA treatment, fasting and residual gallbladder volumes significantly increased both in GS and controls, while the percent of gallbladder emptying did not significantly change in both groups, as shown in Table 3. No difference was observed in fasting and gallbladder emptying between basal UDCA and basal placebo treatment.
Gastric emptying
At baseline half emptying time (t1/2) was significantly longer in GS than in controls (34.3 ± 1.8 min and 26.4 ± 1.7 min, respectively, P < 0.05) (Figure 3).
Figure 3.
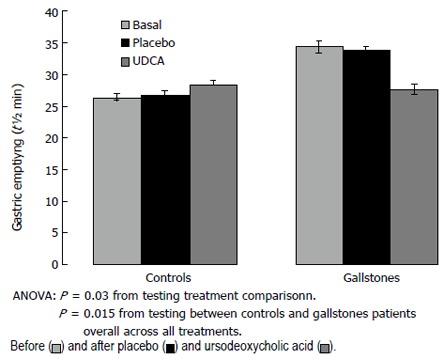
Gastric half emptying-time (t1/2, min) in gallstone patients and healthy controls before and after placebo and ursodeoxycholic acid (UDCA) administration.
Following UDCA, t1/2 was significantly reduced only in GS, while no significant change was observed in controls; as a consequence, half-emptying time was similar in the two groups (GS: 27.6 ± 1.6 min, controls: 28.3 ± 1.6 min). Gastric t 1/2 did not change significantly after placebo in either GS or controls (Figure 3).
Relationship between serum bile acid, gallbladder and gastric emptying, and intestinal transit
At baseline, a direct linear correlation between serum DCA and OITT (P = 0.0001) and serum DCA and gastric t 1/2 (P = 0.024) was observed, while an inverse relationship was present for CA and OITT (P = 0.001) as well as for CDCA vs OITT (P = 0.0001) (Table 4). Furthermore, serum DCA percent concentrations directly related with fasting gallbladder volume (P = 0.0007) and inversely with percent gallbladder emptying (P = 0.004). At multivariate analysis, only DCA significantly related to OITT (b = 12.1, 95% CI = 6.2-17.9, P = 0.0001). The relationships found to be present at univariate analysis remained after placebo administration, but they disappeared at the end of UDCA treatment.
Table 4.
Relationship between serum bile acids and intestinal transit time (OITT), gastric half emptying time (t½ min ) and gallbladder emptying (%) in gallstone patients (n = 10) and healthy controls (n = 10) (univariate analysis)
| OITT (min) | Gastric emptying (t½, min) | Gallbladder emptying (%) | ||||
| r | P | r | P | r | P | |
| CA | -0.696 | 0.001 | -0.118 | 0.62 | 0.44 | 0.05 |
| DCA | 0.887 | 0.0001 | 0.503 | 0.024 | -0.617 | 0.004 |
| CDCA | -0.728 | 0.0001 | -0.547 | 0.012 | 0.594 | 0.006 |
CA: Cholic acid; DCA: Deoxycholic acid; CDCA: Chenodeoxycholic acid.
DISCUSSION
Data emerging from the present study, simultaneously evaluating the motor behaviour of the various GI segments in cholesterol gallstone patients and in healthy subjects, documented in GS the presence of multiple defects involving the gallbladder, the stomach and the small intestine. However, the most important aspect of this paper is that only chronic UDCA administration, and not placebo, significantly influenced gallbladder and gastric emptying, and intestinal transit. Furthermore, this effect is achieved only in GS, being UDCA ineffective in controls. In fact, only in GS did oro-ileal transit time and gastric emptying become faster, normal values being achieved at the end of the UDCA treatment period. All of these effects occurred with a significant improvement in clinical manifestations of the gallstone disease; in fact, most patients no longer experienced episodes of biliary pain during treatment with UDCA.
It is accepted that among the different events contributing to gallstone formation, motility defects could play an important role[5]; however only gallbladder dysfunction has been extensively studied and confirmed evaluating both gallbladder volume variations by US and gallbladder contractility[26]. In the present study, GS showed, when compared to controls, greater fasting and residual gallbladder volumes, and a decrease in percent gallbladder emptying, confirming previous observations from our experience[6] and that of other groups[19,27].
In GS a prolonged time of the migrating motor complex and an altered motilin release pattern have been demonstrated during the interprandial period, leading to an increased fasting gallbladder volume[28]. The pathogenetic mechanism responsible for gallbladder hypocontractility has been related to an excessive accumulation of cholesterol molecules within the sarcolemma membrane of smooth muscle cells leading to stiffening of the cell membrane and to impairment in the agonist-receptor signal transduction and defective relaxation[29,30]. Moreover, a role of mucosal inflammation and of inflammatory mediators induced by cholesterol accumulation has also been suggested to be present in the pathway of events leading to defective gallbladder contractility[31].
In the present study GS patients showed longer OITT than controls, confirming previous observations from our[8] and other laboratories[7,27]. The pathogenetic mechanisms underlying this intestinal abnormality are not well understood, even if some hypotheses have been advanced.
The sluggish intestinal transit, together with gallbladder stasis, could contribute to the increase of DCA formation, allowing longer exposure of CA to intestinal bacteria[32]. In fact we observed higher serum DCA concentrations in GS than in controls. However, this observation is in agreement with some authors[7,8,27], but in contrast with others[33,34]. Furthermore, we found in GS a direct linear correlation between serum DCA levels and OITT, and an inverse correlation between serum CA and OITT. As far as concerns the DCA mechanism of action on intestinal motility, it has been demonstrated in experimental animals that DCA directly delays OITT inducing a defective contractility of intestinal smooth muscle cells in response to cholecystokinin (CCK)[35] or inhibits the pace-maker currents of interstitial cells of Cajal by activating ATP-sensitive K+ channels through the production of PGE2[36]. However a cause-effect relationship is not proven.
In the present study, a direct correlation was demo-nstrated in GS between fasting gallbladder volume and serum DCA levels and an inverse correlation between percent gallbladder emptying and DCA serum levels. It is important to note that DCA also has an effect on gallbladder motility, since it has been demonstrated that it is able to induce both relaxation and defective contraction of gallbladder smooth muscle, probably involving intramural neurons[37].
As far as gastric motility is concerned, we found a delay in half emptying-time in GS compared to controls. Although this result was obtained using a liquid meal, and not a solid or semi-solid meal, which is considered as the gold standard, it was obtained in the same subject before and after treatments. However, the liquid meal has just been previously used in evaluating gastric emptying by others[9]. Even for this defect the pathogenetic mechanism is not clear, although a detrimental effect of DCA on smooth muscle cells could be proposed[38] and it has been speculated that this impaired motility could be related with hormonal alterations, in particular of neurotensin, as suggested for gallbladder[39] and gastric motility[40].
Apart from the speculation based on a pathogenetic role for DCA, other hypotheses could be formulated to explain the mechanisms underlying the diffuse motility defects present in GS. Bile cholesterol supersaturation (e.g. the main and indispensable pathogenetic factor in GS formation)[10], in itself, could play a role. In experimental studies, a cholesterol-rich diet was associated with impaired small intestinal smooth muscle contractility and prolonged small intestinal transit time[41]. Cholesterol accumulation in the intestinal smooth muscle cells could modify membrane fluidity, which, in turn, affects the activity of membrane regulatory proteins, such as receptors and ion channels[42,43]. These proteins control electrical excitability and influence the periodicity of events, such as migrating motor complex[28]. Alternatively it has been recently speculated in an experimental study[44] that a key event underlying cholesterol gallstone pathogenesis could be a CCK-r deficiency, that could provoke intestinal and gallbladder motility dysfunction, thus allowing an excessive cholesterol absorption by both organs and inducing a vicious cycle.
The most important result emerging from the present study concerns the effect of UDCA on gastric and gallbladder emptying and intestinal transit. UDCA significantly improved gastric emptying and OITT, but only in GS since no significant change occurred in controls, while placebo administration had no effect in both GS and controls.
This result could be partially biased by the study design, which was a single blinded, placebo controlled one. However, since both patients and controls were unaware of the study design, we believe to have correctly detected the different effects of UDCA and placebo.
This result confirms those of our previous, uncontrolled study[20], stresses the effects of UDCA on gallbladder and gastric emptying and intestinal transit. These effects could be due to a protective action of UDCA[35,45] towards the damaging action of DCA (the relative proportion of which we found to be significantly diminished) and/or to an ability to reduce cholesterol bile supersaturation by influencing cholesterol bile secretion[10] and/or intestinal cholesterol absorption[13]. The reduction of cholesterol bile saturation could improve intestinal contractility by a mechanism similar to that observed at the level of gallbladder smooth muscle cells[46].
As far as gallbladder motility in GS is concerned, while there is a general agreement regarding the effect of UDCA in increasing fasting and residual gallbladder volumes, that on gallbladder emptying is controversial. In fact either a decreased or unmodified emptying was observed in in vivo studies[6,19].
As far as the different effects of UDCA on gastric emptying and intestinal transit time and on gallbladder emptying is concerned, we can only suggest some hypotheses. The damaging effect of lithogenic bile on gallbladder could be more pronounced with respect to other organs and, consequently, more time is probably needed to obtain a positive effect.
All these documented effects of UDCA on gastrointestinal motility suggest for this bile acid an additional mechanism of action[10] in inducing bile cholesterol under saturation. In particular, the reduction in intestinal transit time could influence intestinal cholesterol absorption, as suggested by the experimental study performed by Wang et al[44].
A further effect of UDCA administration was the reduction in biliary pain rate. In fact, all our GS patients were symptomatic at enrollment, but none of them reported further episodes of biliary pain during UDCA treatment. On the contrary, during placebo treatment, a 20% recurrence of biliary pain rate was observed. Although these results were obtained in a small population and after a relatively short period, it seems important from a clinical point of view. Furthermore, a positive effect of UDCA on symptom frequency has been reported by us in a randomized study comparing UDCA and CDCA as gallstone dissolving agents[47] and more recently by others on a larger population and for a longer period of time[18]. Different hypotheses have been advanced to explain the effect of UDCA on biliary pain frequency: dissolution of microlithiasis or crystals[18], increased bile wash-out within the gallbladder[48] and reduction of bile viscosity and sedimentable fractions[49].
In conclusion, this study documents that gallstone patients do not have a single defect (defective gallbladder emptying), but simultaneous, multiple defects (delayed gastric emptying and intestinal transit). Chronic UDCA administration influences these parameters only in gallstone patients, but not in controls; thus restoring impaired OITT and gastric emptying, and suggesting an additional mechanism of action for UDCA in reducing bile cholesterol saturation. Furthermore UDCA improves clinical outcomes in gallstone patients. Further experimental and clinical studies are needed to better elucidate the mechanisms of action of UDCA on gastrointestinal motility. Furthermore, it will be important to evaluate the presence of gastrointestinal motility defects also in patients at high risk of gallstone development(obese subjects, patients with insulin-resistance syndrome, pregnant women, etc), to confirm their pathogenetic role in GS formation and to identify a possible relationship with genetic and/or environmental factors.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Bi L
References
- 1.Attili AF, Carulli N, Roda E, Barbara B, Capocaccia L, Menotti A, Okoliksanyi L, Ricci G, Capocaccia R, Festi D. Epidemiology of gallstone disease in Italy: prevalence data of the Multicenter Italian Study on Cholelithiasis (M.I.COL.) Am J Epidemiol. 1995;141:158–165. doi: 10.1093/oxfordjournals.aje.a117403. [DOI] [PubMed] [Google Scholar]
- 2.Festi D, Sottili S, Colecchia A, Attili A, Mazzella G, Roda E, Romano F. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL) Hepatology. 1999;30:839–846. doi: 10.1002/hep.510300401. [DOI] [PubMed] [Google Scholar]
- 3.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 4.Khanuja B, Cheah YC, Hunt M, Nishina PM, Wang DQ, Chen HW, Billheimer JT, Carey MC, Paigen B. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc Natl Acad Sci USA. 1995;92:7729–7733. doi: 10.1073/pnas.92.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apstein MD, Carey MC. Pathogenesis of cholesterol gallstones: a parsimonious hypothesis. Eur J Clin Invest. 1996;26:343–352. doi: 10.1046/j.1365-2362.1996.148287.x. [DOI] [PubMed] [Google Scholar]
- 6.Festi D, Frabboni R, Bazzoli F, Sangermano A, Ronchi M, Rossi L, Parini P, Orsini M, Primerano AM, Mazzella G. Gallbladder motility in cholesterol gallstone disease. Effect of ursodeoxycholic acid administration and gallstone dissolution. Gastroenterology. 1990;99:1779–1785. doi: 10.1016/0016-5085(90)90487-l. [DOI] [PubMed] [Google Scholar]
- 7.Thomas LA, Veysey MJ, Bathgate T, King A, French G, Smeeton NC, Murphy GM, Dowling RH. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000;119:806–815. doi: 10.1053/gast.2000.16495. [DOI] [PubMed] [Google Scholar]
- 8.Azzaroli F, Mazzella G, Mazzeo C, Simoni P, Festi D, Colecchia A, Montagnani M, Martino C, Villanova N, Roda A, et al. Sluggish small bowel motility is involved in determining increased biliary deoxycholic acid in cholesterol gallstone patients. Am J Gastroenterol. 1999;94:2453–2459. doi: 10.1111/j.1572-0241.1999.01375.x. [DOI] [PubMed] [Google Scholar]
- 9.Portincasa P, Di Ciaula A, Palmieri V, Velardi A, VanBerge-Henegouwen GP, Palasciano G. Impaired gallbladder and gastric motility and pathological gastro-oesophageal reflux in gallstone patients. Eur J Clin Invest. 1997;27:653–661. doi: 10.1046/j.1365-2362.1997.1600709.x. [DOI] [PubMed] [Google Scholar]
- 10.Roda E, Aldini R, Bazzoli F, Festi D, Mazzella G, Roda A. Pathophysiology and pharmacotherapy of cholelithiasis. Pharmacol Ther. 1992;53:167–185. doi: 10.1016/0163-7258(92)90007-m. [DOI] [PubMed] [Google Scholar]
- 11.Hempfling W, Dilger K, Beuers U. Systematic review: ursodeoxycholic acid--adverse effects and drug interactions. Aliment Pharmacol Ther. 2003;18:963–972. doi: 10.1046/j.1365-2036.2003.01792.x. [DOI] [PubMed] [Google Scholar]
- 12.Ponz de Leon M, Carulli N, Loria P, Iori R, Zironi F. Cholesterol absorption during bile acid feeding. Effect of ursodeoxycholic acid (UDCA) administration. Gastroenterology. 1980;78:214–219. [PubMed] [Google Scholar]
- 13.Wang DQ, Tazuma S, Cohen DE, Carey MC. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol. 2003;285:G494–G502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- 14.LaRusso NF, Thistle JL. Effect of litholytic bile acids on cholesterol absorption in gallstone patients. Gastroenterology. 1983;84:265–271. [PubMed] [Google Scholar]
- 15.Woollett LA, Buckley DD, Yao L, Jones PJ, Granholm NA, Tolley EA, Heubi JE. Effect of ursodeoxycholic acid on cholesterol absorption and metabolism in humans. J Lipid Res. 2003;44:935–942. doi: 10.1194/jlr.M200478-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Su CC, Park JY, Higuchi WI, Alkan MH, Corrigan OI, Hofmann AF, Danzinger RG. Mesophase formation during in vitro cholesterol gallstone dissolution: a specific effect of ursodeoxycholic acid. J Pharm Sci. 1981;70:713–715. doi: 10.1002/jps.2600700644. [DOI] [PubMed] [Google Scholar]
- 17.Broomfield PH, Chopra R, Sheinbaum RC, Bonorris GG, Silverman A, Schoenfield LJ, Marks JW. Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med. 1988;319:1567–1572. doi: 10.1056/NEJM198812153192403. [DOI] [PubMed] [Google Scholar]
- 18.Tomida S, Abei M, Yamaguchi T, Matsuzaki Y, Shoda J, Tanaka N, Osuga T. Long-term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: a cohort analysis. Hepatology. 1999;30:6–13. doi: 10.1002/hep.510300108. [DOI] [PubMed] [Google Scholar]
- 19.van Erpecum KJ, van Berge Henegouwen GP, Stolk MF, Hopman WP, Jansen JB, Lamers CB. Effects of ursodeoxycholic acid on gallbladder contraction and cholecystokinin release in gallstone patients and normal subjects. Gastroenterology. 1990;99:836–842. doi: 10.1016/0016-5085(90)90977-9. [DOI] [PubMed] [Google Scholar]
- 20.Colecchia A, Sandri L, Simoni P. Effect of chronic admini-stration of ursodeoxycholic acid on gallbladder and gastrointestinal motility in gallstone patients and healthy controls. Gastroenterology. 2004;126:A234. [Google Scholar]
- 21.Roda E, Festi D, Lezoche E, Leuschner U, Paumgartnet G, Sauerbruch T. Strategies in the treatment of biliary stones. Gastroenterol Int. 2000;13:7–15. [Google Scholar]
- 22.Hofmann AF. The enterohepatic circulation of bile acids in health and diseases. In: Gastrointestinal disease, Pathophysiology, Diagnosis and Management, Sleisenger MH, Fordtran S, Eds, Philadelphia, Saunders; 1993. pp. 127–150. [Google Scholar]
- 23.Simoni P, Sabatini L, Baraldini M, Mirasoli M, Roda A, Roda E. Pharmacokinetics and bioavailability of four modified-release ursodeoxycholic acid preparations for once-a-day administration. Int J Clin Pharmacol Res. 2002;22:37–45. [PubMed] [Google Scholar]
- 24.Roda A, Gioacchini AM, Cerrè C, Baraldini M. High-performance liquid chromatographic-electrospray mass spectrometric analysis of bile acids in biological fluids. J Chromatogr B Biomed Appl. 1995;665:281–294. doi: 10.1016/0378-4347(94)00544-f. [DOI] [PubMed] [Google Scholar]
- 25.Portincasa P, Colecchia A, Di Ciaula A, Larocca A, Muraca M, Palasciano G, Roda E, Festi D. Standards for diagnosis of gastrointestinal motility disorders. Section: ultrasonography. A position statement from the Gruppo Italiano di Studio Motilità Apparato Digerente. Dig Liver Dis. 2000;32:160–172. doi: 10.1016/s1590-8658(00)80404-1. [DOI] [PubMed] [Google Scholar]
- 26.Pauletzki J, Paumgartner G. Review article: defects in gall-bladder motor function--role in gallstone formation and recurrence. Aliment Pharmacol Ther. 2000;14 Suppl 2:32–34. doi: 10.1046/j.1365-2036.2000.014s2032.x. [DOI] [PubMed] [Google Scholar]
- 27.Shoda J, He BF, Tanaka N, Matsuzaki Y, Osuga T, Yamamori S, Miyazaki H, Sjövall J. Increase of deoxycholate in supersaturated bile of patients with cholesterol gallstone disease and its correlation with de novo syntheses of cholesterol and bile acids in liver, gallbladder emptying, and small intestinal transit. Hepatology. 1995;21:1291–1302. [PubMed] [Google Scholar]
- 28.Xu QW, Scott RB, Tan DT, Shaffer EA. Altered migrating myoelectrical complex in an animal model of cholesterol gallstone disease: the effect of erythromycin. Gut. 1998;43:817–822. doi: 10.1136/gut.43.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Amaral J, Biancani P, Behar J. Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology. 1999;116:678–685. doi: 10.1016/s0016-5085(99)70190-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Amaral J, Oh S, Biancani P, Behar J. Gallbladder relaxation in patients with pigment and cholesterol stones. Gastroenterology. 1997;113:930–937. doi: 10.1016/s0016-5085(97)70189-6. [DOI] [PubMed] [Google Scholar]
- 31.Kano M, Shoda J, Satoh S, Kobayashi M, Matsuzaki Y, Abei M, Tanaka N. Increased expression of gallbladder cholecystokinin: a receptor in prairie dogs fed a high-cholesterol diet and its dissociation with decreased contractility in response to cholecystokinin. J Lab Clin Med. 2002;139:285–294. doi: 10.1067/mlc.2002.122863. [DOI] [PubMed] [Google Scholar]
- 32.Marcus SN, Heaton KW. Deoxycholic acid and the pathogenesis of gall stones. Gut. 1988;29:522–533. doi: 10.1136/gut.29.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noshiro H, Chijiiwa K, Makino I, Nakano K, Hirota I. Deoxycholic acid in gall bladder bile does not account for the shortened nucleation time in patients with cholesterol gall stones. Gut. 1995;36:121–125. doi: 10.1136/gut.36.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustafsson U, Sahlin S, Einarsson C. Biliary lipid composition in patients with cholesterol and pigment gallstones and gallstone-free subjects: deoxycholic acid does not contribute to formation of cholesterol gallstones. Eur J Clin Invest. 2000;30:1099–1106. doi: 10.1046/j.1365-2362.2000.00740.x. [DOI] [PubMed] [Google Scholar]
- 35.Xu Q, Shaffer EA. The influence of bile salts on small intestinal motility in the guinea pig in vitro. Gastroenterology. 1992;103:29–35. doi: 10.1016/0016-5085(92)91091-h. [DOI] [PubMed] [Google Scholar]
- 36.Jun JY, Choi S, Chang IY, Yoon CK, Jeong HG, Kong ID, So I, Kim KW, You HJ. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2005;144:242–251. doi: 10.1038/sj.bjp.0706074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu QW, Freedman SM, Shaffer EA. Inhibitory effect of bile salts on gallbladder smooth muscle contractility in the guinea pig in vitro. Gastroenterology. 1997;112:1699–1706. doi: 10.1016/s0016-5085(97)70053-2. [DOI] [PubMed] [Google Scholar]
- 38.Feldman S, Gibaldi M. Effect of bile salts on gastric emptying and intestinal transit in the rat. Gastroenterology. 1968;54:918–921. [PubMed] [Google Scholar]
- 39.Walker JP, Khalil T, Wiener I, Fagan CJ, Townsend CM Jr, Greeley GH Jr, Thompson JC. The role of neurotensin in human gallbladder motility. Ann Surg. 1985;201:678–683. doi: 10.1097/00000658-198506000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn AM, Fletcher DR, Bloom SR, Christofides ND, Long RG, Fitzpatrick ML, Baron JH. Effect of neurotensin on gastric function in man. Lancet. 1980;1:987–989. doi: 10.1016/s0140-6736(80)91434-8. [DOI] [PubMed] [Google Scholar]
- 41.Xu QW, Scott RB, Tan DT, Shaffer EA. Slow intestinal transit: a motor disorder contributing to cholesterol gallstone formation in the ground squirrel. Hepatology. 1996;23:1664–1672. doi: 10.1002/hep.510230650. [DOI] [PubMed] [Google Scholar]
- 42.Xiao ZL, Chen Q, Amaral J, Biancani P, Jensen RT, Behar J. CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am J Physiol. 1999;276:G1401–G1407. doi: 10.1152/ajpgi.1999.276.6.G1401. [DOI] [PubMed] [Google Scholar]
- 43.Bialecki RA, Tulenko TN. Excess membrane cholesterol alters calcium channels in arterial smooth muscle. Am J Physiol. 1989;257:C306–C314. doi: 10.1152/ajpcell.1989.257.2.C306. [DOI] [PubMed] [Google Scholar]
- 44.Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest. 2004;114:521–528. doi: 10.1172/JCI16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao ZL, Rho AK, Biancani P, Behar J. Effects of bile acids on the muscle functions of guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol. 2002;283:G87–G94. doi: 10.1152/ajpgi.00536.2001. [DOI] [PubMed] [Google Scholar]
- 46.van de Heijning BJ, van de Meeberg PC, Portincasa P, Doornewaard H, Hoebers FJ, van Erpecum KJ, Vanberge-Henegouwen GP. Effects of ursodeoxycholic acid therapy on in vitro gallbladder contractility in patients with cholesterol gallstones. Dig Dis Sci. 1999;44:190–196. doi: 10.1023/a:1026635124115. [DOI] [PubMed] [Google Scholar]
- 47.Roda E, Bazzoli F, Labate AM, Mazzella G, Roda A, Sama C, Festi D, Aldini R, Taroni F, Barbara L. Ursodeoxycholic acid vs. chenodeoxycholic acid as cholesterol gallstone-dissolving agents: a comparative randomized study. Hepatology. 1982;2:804–810. doi: 10.1002/hep.1840020611. [DOI] [PubMed] [Google Scholar]
- 48.Jazrawi RP. Normal gallbladder motor function. In: Afdhal NH, ed. Gallbladder and Biliary Tract Diseases. New York Marcel Dekker Inc; 2000. pp. 251–274. [Google Scholar]
- 49.Fischer S, Müller I, Zündt BZ, Jüngst C, Meyer G, Jüngst D. Ursodeoxycholic acid decreases viscosity and sedimentable fractions of gallbladder bile in patients with cholesterol gallstones. Eur J Gastroenterol Hepatol. 2004;16:305–311. doi: 10.1097/00042737-200403000-00010. [DOI] [PubMed] [Google Scholar]


