Abstract
AIM: To review clinical and pathologic features of Gas-trointestinal stromal tumors (GISTs) occurring synchro-nously with other primary gastrointestinal neoplasms.
METHODS: Twenty-eight patients with primary GIST were treated at our institution between 1989 and 2005. Clinical and pathologic records were reviewed.
RESULTS: The gastrointestinal stromal tumor occurred simultaneously with other primary GI malignancies in 14% of all patients with GIST. The synchronous stromal tumors were located in the stomach and were incidentally found during the operation. The coexistent neoplasms were colon adenocarcinoma, gastric cancer (2 cases) and gastric lymphoma.
CONCLUSION: The synchronous occurrence of GISTs and other gastrointestinal malignancies is more common than it has been considered. The development of gastrointestinal stromal tumors and other neoplasms may involve the same carcinogenic agents.
Keywords: Gastrointestinal stromal tumors, Synchronous neoplasms
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are an un-common mesenchymal neoplasm affecting the GI tract. The synchronous occurrence of mesenchymal tumors and other primary gastrointestinal malignancies has been rarely reported in the literature[1,2]. Most of these publications describe single case reports. We present a series of four patients, from a single institution, with GIST and a second primary neoplasm occurring synchronously. The aim of this study was to evaluate clinical and pathologic features of GISTs concomitant with other gastrointestinal malignancies.
MATERIALS AND METHODS
Patients
Between 1989 and 2005, 28 patients with primary gastrointestinal stromal tumor were treated operatively at the department of General and Gastroenterological Surgery and Nutrition, Warsaw University of Medicine. Most of the patients were women (62%). Median age of the patients at the time of presentation was 63.5 years. The primary tumor was located in the stomach (57%), small intestine (32%), large intestine (7%) and mesentery (4%). GIST was incidental in 29% of the patients. Hospital charts, operative and pathological reports were reviewed for each patient.
Methods
The histological diagnosis of all GISTs was confirmed at the department of pathology of the Warsaw University of Medicine. Specimens were fixed in 10% formaldehyde and processed routinely for paraffin embedding. 5-μm-thick sections were stained with HE. Mitoses were counted in 50 high-power fields. Malignant potential of the GISTs was stratified according to the risk categories proposed by Fletcher et al[3].
Immunohistochemistry was performed using commercially available antibodies against CD117 (polyclonal, Dako, Glostrup, Denmark) and against CD34 (monoclonal, Dako, Glostrup, Denmark).
RESULTS
GIST occurred synchronously with an other gastrointestinal malignancy in four patients (14%) out of all the patients with primary gastrointestinal stromal tumor treated in our department. Twenty-five percent of the gastric stromal tumors and 50% of the incidental GISTs were synchronous with the second gastrointestinal malignancy. The concomitant neoplasms were also primaries. All these GISTs were located in the stomach. In 75% of the cases, GIST was synchronous with other gastric malignancies, and one patient had a coexistent gastric stromal tumor and colorectal cancer. The patients presented clinically with abdominal pain, weight loss or partial intestinal obstruction. All the patients required operation due to the symptoms as a result of the tumor and GIST synchrony. The stromal tumor was always an incidental finding during the operation. The clinical characteristics of the patient population are shown in Table 1.
Table 1.
Characteristics of the patient population
| No. | Age | Sex | Synchronous malignancy | Clinical presentation |
| 1 | 63 | F | Colon cancer | Intestinal obstruction |
| 2 | 77 | F | Gastric lymphoma | Abdominal pain |
| 3 | 64 | F | Gastric cancer | Pyloric stenosis |
| 4 | 66 | M | Gastric cancer | Abdominal pain, weight loss |
The synchronous gastric tumors were located in the different parts of the stomach. The average size of GIST was 1.5 cm. Morphologically, the 1-cm GISTs were a whitish, smooth and firm intramural nodule that was slightly elevated above the stomach wall. Larger GISTs were irregular, soft and a lobulated mass attached to the stomach (Figure 1). In one of the patients, the GIST was initially considered intraoperatively as a metastatic lymph node (Case 3). However, the intraoperative frozen section revealed GIST tissue. The pathologic features of the synchronous tumors are summarized in Table 2.
Figure 1.
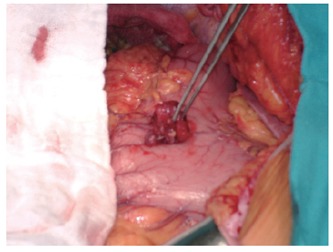
Intraoperative view of the gastric stromal tumor that was synchronous with colon adenocarcinoma (Case 1).
Table 2.
Pathologic features of the synchronous GISTs and other primary gastrointestinal neoplasms
| No. |
GIST |
Synchronous gastrointestinal malignancy |
|||
| Location | Size (cm) | Type | Location | Size (cm) | |
| 1 | Anterior gastric wall (corpus) | 2 | Adenocarcinoma (pT4, N2, M0) | Cecum | 10 |
| 2 | Anterior gastric wall (fundus) | 1 | Lymphoma (diffuse large B cell) | Lesser curvature at the gastric angle | 2 |
| 3 | Anterior gastric wall (corpus) | 2 | Adenocarcinoma -Lauren diffuse type (pT4, N0, M0) | Antrum | 5 |
| 4 | Anterior gastric wall (corpus) | 1 | Adenocarcinoma -Lauren intestinal type (pT1, N0, M0) | Posterior wall at the gastric angle | 1 |
Total (n = 1) or subtotal gastrectomies (n = 2) were performed in the patients with gastric malignancy. In these cases, the concomitant GIST was located within the resection margins. The patient with both colon cancer and gastric stromal tumor had a right hemicolectomy and additionally local resection of the stromal tumor.
The synchronous gastrointestinal stromal tumors were uniformly CD117 and CD34 positive (Figure 2) and could be classified as low and very low risk tumors for malignant potential. The histopathologic features of the synchronous GISTs are shown in Table 3.
Figure 2.
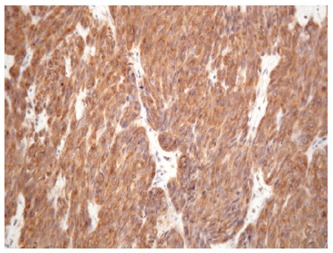
Strongly positive CD117 immunostaining in the synchronous gastric stromal tumor (Case 2).
Table 3.
Histopathologic features of the synchronous GISTs
| No. | CD117 reactivity | CD34 reactivity | Tumor size(cm) | Mitotic index | Risk category for malignant behavior[3] |
| 1 | + | + | 2 | 2/50 hpf1 | Low |
| 2 | + | + | 1 | 0/50 hpf | Very low |
| 3 | + | + | 2 | 2/50 hpf | Low |
| 4 | + | + | 1 | 0/50 hpf | Very low |
High-power fields.
DISCUSSION
Gastrointestinal stromal tumors are uncommon mesenchymal neoplasms occurring within the abdominal cavity. Most GISTs are located in the stomach and small intestine[4]. GISTs usually develop in a sporadic fashion. However, familial occurrence has also been reported[5]. In patients with Carney’s triad, GISTs may develop together with pulmonary chondroma and extra-adrenal paraganglioma[6]. Although 10% of the patients enrolled in the Polish GIST Clinical Registry had a second neoplasm[7], these were usually metachronous and occurred earlier than the GIST. Slightly above 30 cases of the synchronous occurrence of mesenchymal tumors (including GIST) and other gastrointestinal malignancy have been reported in the literature[1]. Most of these publications describe gastric stromal tumors synchronous with another gastric malignancy. We also observed such gastric tumor association in our group. In one of our patients, GIST occurred simultaneously with colon cancer. To our knowledge, only a few reports of such tumor co-occurrence have been published in the literature[8].
GISTs have been reported to occur synchronously with adenocarcinoma, lymphoma and carcinoid[1,9]. Similar to other authors, GIST was most frequently synchronous with adenocarcinoma also in our series (75%). High percentage of synchronous GISTs and other gastrointestinal tumors in our series is both surprising and difficult to explain. 14% of all the GISTs and 25% of the gastric stromal tumors developed synchronously with a second gastrointestinal malignancy. This rate of neoplasm co-occurrence is greater than twice that observed in the largest group of synchronous GISTs published by Maiorana et al[1]. In their series, 11.5% of gastric GISTs (6 cases) were associated with other gastrointestinal malignancies.
Although the synchronous occurrence of GIST and other abdominal malignancy seems to be just a coincidence, the development of these tumors may involve common carcinogenic agents. For example Sugimura et al[10] revealed that enteral nitrosoguanidine produces adenocarcinoma in rats. In contrast, simultaneous exposure to both nitrosoguanidine and acetylsalicylic acid causes synchronous development of both gastric cancer and leiomyosarcoma[11]. In conclusion the synchronous occurrence of GISTs and other gastrointestinal malignanies is more common than it has been considered. The concomitant GIST is usually discovered incidentally during the operation performed because of the other malignancy. The development of GIST and other neoplasms may involve the same carcinogenic agents.
Footnotes
S- Editor Pan BR L- Editor Alpini GD E- Editor Bi L
References
- 1.Maiorana A, Fante R, Maria Cesinaro A, Adriana Fano R. Synchronous occurrence of epithelial and stromal tumors in the stomach: a report of 6 cases. Arch Pathol Lab Med. 2000;124:682–686. doi: 10.5858/2000-124-0682-SOOEAS. [DOI] [PubMed] [Google Scholar]
- 2.Lin YL, Tzeng JE, Wei CK, Lin CW. Small gastrointestinal stromal tumor concomitant with early gastric cancer: a case report. World J Gastroenterol. 2006;12:815–817. doi: 10.3748/wjg.v12.i5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 4.Rutkowski P, Nowecki ZI, Nowak-Dement A, Nasierowska-Guttmejer A, Tuziak T, Remiszewski P. Nowotwory zrebu przewodu pokarmowego-obraz kliniczno-morfologiczny. Polski Przegl Chir. 2003;75:374–384. [Google Scholar]
- 5.Nishida T, Hirota S, Taniguchi M, Hashimoto K, Isozaki K, Nakamura H, Kanakura Y, Tanaka T, Takabayashi A, Matsuda H, et al. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet. 1998;19:323–324. doi: 10.1038/1209. [DOI] [PubMed] [Google Scholar]
- 6.Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543–552. doi: 10.4065/74.6.543. [DOI] [PubMed] [Google Scholar]
- 7.Ruka W, Rutkowski P, Nowecki Z, Nasierowska-Guttmejer A, Debiec-Rychter M. Other malignant neoplasms in patients with gastrointestinal stromal tumors (GIST) Med Sci Monit. 2004;10:LE13–LE14. [PubMed] [Google Scholar]
- 8.Urbańczyk K, Limon J, Korobowicz E, Chosia M, Sygut J, Karcz D, Iwanik K, Osuch C, Lasota J, Stachura J. Gastrointestinal stromal tumors. A multicenter experience. Pol J Pathol. 2005;56:51–61. [PubMed] [Google Scholar]
- 9.Kaffes A, Hughes L, Hollinshead J, Katelaris P. Synchronous primary adenocarcinoma, mucosa-associated lymphoid tissue lymphoma and a stromal tumor in a Helicobacter pylori-infected stomach. J Gastroenterol Hepatol. 2002;17:1033–1036. doi: 10.1046/j.1440-1746.2002.02649.x. [DOI] [PubMed] [Google Scholar]
- 10.Sugimura T, Fujimura S, Baba T. Tumor production in the glandular stomach and alimentary tract of the rat by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1970;30:455–465. [PubMed] [Google Scholar]
- 11.Cohen A, Geller SA, Horowitz I, Toth LS, Werther JL. Experimental models for gastric leiomyosarcoma. The effects of N-methyl-N'-nitro-N-nitrosoguanidine in combination with stress, aspirin, or sodium taurocholate. Cancer. 1984;53:1088–1092. doi: 10.1002/1097-0142(19840301)53:5<1088::aid-cncr2820530512>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]


