Abstract
AIM: To study the effects of ferulic acid on gastrointe-stinal motility both in vitro and in vivo.
METHODS: Ferulic acid induced concentration-dependent stimulation of the basal tone of isolated guinea pig ileum (2-20 μmol/L) and isolated rat fundus (0.05-0.4 mmol/L).
RESULTS: Ferulic acid significantly accelerated the gastrointestinal transit and gastric emptying in rats in a dose-dependent manner (50-200 mg/kg, po). Cisplatin (2.5-20 mg/kg, ip) induced a dose-dependent delay in gastric emptying in rats. Pretreatment with ferulic acid dose-dependently, significantly reversed the cisplatin-induced delay in gastric emptying.
CONCLUSION: The endogenous prostaglandins (PGs) are involved in mediating the stimulant effects of ferulic acid. This effect of dietary ferulic acid may help improve other accompanying gastrointestinal symptoms such as abdominal discomfort and also may protect against emesis induced by cytotoxic drugs.
Keywords: Ferulic acid, Cisplatin, Gastric emptying, Rats
INTRODUCTION
Phenolic acids are plant components ubiquitously present in many fruits, vegetables and grains. They constitute a major portion of the human daily intake of non-nutrients[1]. There is increasing evidence of positive health benefits of the high dietary intake of such compounds. These dietary phenolic acids have demonstrated antioxidant[2], anti-inflammatory[3], cardioprotectant[4] and anticancer properties[5].
Ferulic acid, a hydroxycinnamic acid, is found in rice, wheat, barley, roasted coffee, tomatoes, asparagus olives, berries, vegetables, citrus fruits and leaves, and many other plants. Ferulic acid exhibits strong antioxidant and anti-inflammatory activities[6]. Moreover, it has been claimed to protect against chemotherapy-induced side effects by enhancing the natural immune defense[7] .
There are previous reports that indicate beneficial effects of phenolic acids on the gastrointestinal tract[8] and caffeic acid may enhance intestinal motility. Green tea polyphenolics improved bowel movement regularly[9] and softened stool consistency[10]. Also black tea polyphenols have been shown to enhance gastrointestinal motility both in vitro and in vivo[11].
Cancer chemotherapy causes severe nausea, vomiting and abdominal discomfort which limits its adminis-tration[12]. Most anticancer agents slow down gastric emptying[13-15]. Cisplatin is extensively used for management of oncological disorders, particularly of the ovary, testis, bladder, head and neck[16]. Although effective, cisplatin is associated with many adverse drug reactions, such as renal damage, gastrointestinal dysfunction, auditory toxicity and peripheral nerve toxicity[17]. Cisplatin produced dose-related inhibition in gastric emptying reflects an accumulation of food in the stomach[18]. Cisplatin-induced side effects in the gastrointestinal tract may be altered by metoclopramide[19], 5HT3 receptor antagonists[14,15] and antioxidants[20].
The aim of the present study was to investigate the effect of ferulic acid on gastrointestinal tract both in vitro and in vivo. The effect of ferulic acid, in selected doses, on cisplatin-induced delay in gastric emptying in rats was also investigated. Specific 5HT3 receptor antagonists are effective against drug-induced emesis[21] and ondansetron has been shown to reverse the delay in gastric emptying in rats[14], therefore ondansetron was used for comparison.
MATERIALS AND METHODS
Chemicals
All chemicals, unless specified otherwise, were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Animals
Experiments were performed using Egyptian Giza strain guinea pigs (300-500 g) of either sex and adult male Wistar albino rats (175-210 g), both bred in the animal facility of Faculty of Pharmacy, Al-Azhar University. The animals were housed under conditions of 24 ± 2°C, 50 ± 10 relative humidity and 12 h light and dark cycles. The animals were fed with a laboratory diet manufactured by Al-Nasr Co., Cairo, Egypt, and were fasted overnight before drug administration.
In vitro experiments
Isolated rat fundus: The experiment was made according to Vane[22]. Adult male Wistar albino rats were fasted overnight with free access to water, and then they were sacrificed. The abdomen of each animal was opened, and the entire stomach was dissected free from the abdomen and placed into a dish containing aerated Krebs solution at 37°C. The grey fundal part was separated from the pink pyloric part of each stomach. Each part was cut longitudinally to form a sheet strip of gastric fundus approximately 4 cm long and 3 mm wide (the cuts were parallel to the longitudinal muscle fibers by making alternate transverse cuts on opposite sides of the muscle). A thread was attached to each end of the preparation, and then it was suspended in a 20 mL organ bath filled with Krebs solution at 37°C and aerated with a mixture of oxygen (95%) and carbon dioxide (5%). One thread was attached to a fixed pin in the bath and the other to isotonic transducer (Washington 400 MD2C Bioscience, Sheerness, Kent, UK). The gastric fundus preparation was allowed to equilibrate for 30 min under tension of 1 g before starting the experiment. Ferulic acid at different concentrations was allowed to contact with the preparation for 6 min. Serotonin (14 μmol/L) was used at the beginning of each experiment to test the sensitivity of the tissue used. To determine the site of action of ferulic acid, a submaximal concentration (0.05 mmol/L) was tested before and after complete blockade of H1 receptors (mepyramine 10 μmol/L), muscarinic receptors (atropine sulfate 10 μmol/L), 5HT3 receptors (partially blocked by ondansetron 1 mmol/L) and PGE2 effect (blocked by indomethacin 0.08 mmol/L).
Isolated guinea pig ileum: Experiments were done according to Perry[23]. Guinea pigs were killed by exsanguination. The guinea pig ileum preparation was set up in a 20 mL organ bath containing Tyrode solution at 37°C gassed with 95% O2 and 5% CO2. The normal tone and changes in force of contraction after different concentrations of ferulic acid (2-20 μmol/L) were measured (at a speed of recording 0.25 mm/sec) using isotonic transducer (Washington 400 MD2C Bioscience, Sheerness, Kent, UK). Histamine (2-25 μmol/L) was used to test the sensitivity of the tissue. To determine the site of action of ferulic acid, mepyramine (0.8 μmol/L), atropine sulfate (1 μmol/L), ondansetron (5 μmol/L) and indomethacin (0.14 mmol/L) were used to mediate blockade of H1 receptors, muscarinic receptors, 5HT3 receptors and the PGE2 effect, respectively.
In vivo experiments
Intestinal transit rate in rats: Gastrointestinal transit was measured in the charcoal propulsion test[24]. The test compound (50, 100 or 200 mg/kg) and saline (control) were administered orally. Rats were distributed into groups ( 8 each) : group 1 received 1 mL saline (control); groups 2-4 were treated with ferulic acid in 3 doses (50, 100 or 200 mg/kg, oral); group 5 was given indomethacin (0.75 mg/kg, ip) [11]; and Group 6 received indomethacin (0.75 mg/kg, ip) 50 min after oral administration of ferulic acid (100 mg/kg). One hour after ferulic acid administration, each rat was orally administered 1 mL charcoal meal (5% activated charcoal suspended in 10% aqueous tragacanth). Rats were killed 30 min later by cervical dislocation. The extent of charcoal propulsion in the small intestine was measured (distance traveled by the charcoal head from the pylorus as well as total length of the small intestine). Intestinal transit rate% = distance traveled by charcoal head/length of the small intestine × 100.
Gastrointestinal emptying in rats: The rate of gastric emptying of the non-nutrient semisolid meal was determined according to the method described previously[25]. Male Wistar rats were fasted overnight with water ad libitum. Briefly, a test meal (0.05% phenol red in a 1.5% aqueous methylcellulose solution) was given (1.5 mL/rat) by gastric tube. Thirty minutes after the meal was given, the animals were sacrificed. The cardias and the pylori were clamped, the stomach was removed out, homogenized with its contents in 100 mL of 0.1 mol/L NaOH. Proteins (in 5 mL homogenate) were precipitated with 0.5 mL of trichloroacetic acid (20% w/v) and centrifuged. The supernatant was mixed with 4 mL of 0.5 mol/L NaOH and absorbance of the sample was read at a wavelength of 560 nm. Phenol red was recovered from the stomach of rats killed immediately after administration of the methylcellulose meal served as the standard stomach. The percentage of gastric emptying was calculated from the following formula: Gastric emptying (%) = 1-(amount of phenol red recovered from the test stomach/(average amount of phenol red recovered from the stomach) × 100.
Activity is expressed as the percent change of gastric emptying in treated rats versus controls.
Tested compounds
Effect of ferulic acid: Ferulic acid at three different doses (50, 100 or 200 mg/kg) was orally administered 30 min before the test meal.
Effect of cisplatin: To assess the dose dependency of cisplatin-induced delay in gastric emptying and to standardize the dose that would be more suitable to study the effect of ferulic acid pretreatment, cisplatin was administered at different doses of 2.5, 5, 10 and 20 mg/kg, ip, 30 min before the test meal administration.
Effect of ferulic acid on cisplatin-induced delay in gastric emptying: Effect of ferulic acid (100 mg/kg) was investigated on the delay of gastric emptying induced by cisplatin 10 mg/kg, ip Ferulic acid was given 20 min before cisplatin. The effect of ferulic acid was compared with the 5HT3 receptor antagonist ondansetron administered at a dose of 3 mg/kg, po, 30 min before cisplatin administration.
Statistical analysis
The results are expressed as the mean ± SE. One way analysis of variance (ANOVA) followed by Bonferrioni test for multiple comparisons was used for statistical comparison. P values less than 0.05 were considered statistically significant.
RESULTS
Isolated rat fundus
Ferulic acid (0.05-0.4 mmol/L) produced concentration-dependent contractions of isolated rat fundus (Figure 1). These contractions were not affected by blockade of muscarinic receptors by atropine sulfate (10 μmol/L) or by the histaminiergic receptor with mepyramine (10 mmol/L). On the other hand, ondansetron (1 mmol/L) did not affect the contraction. Complete blockade of PGE2 by indomethacin partially decreased the contractile response due to ferulic acid.
Figure 1.
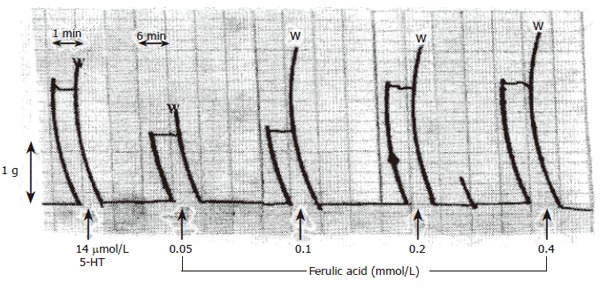
Effect of ferulic acid on isolated rat fundus. Fundic strip was suspended in oxygenated Krebs solution at 37°C. The 1 min and 6 min scales represent the speed of recording and the 1-g scale represents the calibration tension and W is a wash.
Guinea pig ileum
Ferulic acid (2-20 μmol/L) induced a significant (P < 0.05) increase in the height of contraction of isolated guinea pig ileum with a maximum concentration of 20 μmol/L (Figure 2). A submaximal concentration (4 μmol/L) of ferulic acid was used to investigate the site of its stimulant effect. Complete blockade of either H1 or muscarinic receptors by mepyramine or atropine did not affect the response of ileum to ferulic acid. Also, partial blockade of 5HT3 receptors by ondansetron failed to prevent the stimulant effect. The stimulant effect of ferulic acid was partially inhibited by indomethacin (0.14 mmol/L). This stimulant effect may be mediated at least partly via stimulation of PGE2 receptors.
Figure 2.
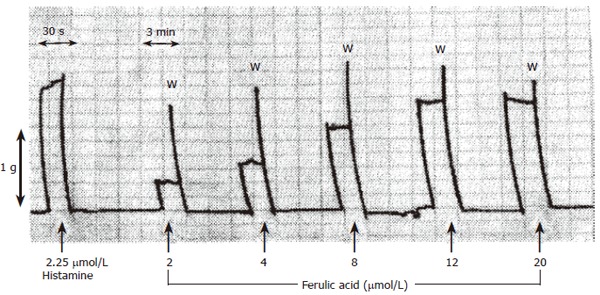
Effect of ferulic acid on isolated guinea pig ileum. Ileum was suspended in oxygenated Tyrode solution at 37°C. The 30 s and 3 min scales represent the speed of recording and the 1-g scale represents the calibration tension and W is a wash.
Intestinal transit rate in rats
Ferulic acid significantly induced a dose-dependent increase in the distance traveled by charcoal meal in the gut of rats at 50, 100 or 200 mg/kg. The facilitatory effect of ferulic acid (100 mg/kg) was significantly reduced after pretreatment with indomethacin. Indomethacin alone significantly reduced the intestinal transit rate as compared with the control group (a decrease of 12.1%). Ferulic acid (100 mg/kg) significantly increased the intestinal transit rate of the indomethacin-treated group (by 27.5% compared with the indomethacin group and 12.1% compared with the control group) (Table 1).
Table 1.
Effect of ferulic acid on gastrointestinal transit rate of a semisolid charcoal meal in rats
| Treatment | Dose | Transit rate (% ± SE) | % change of control |
| Saline (control) | 1 mL, oral | 58 ± 1.5 | 0.0 |
| Ferulic acid | 50 mg/kg, oral | 73 ± 3.1 | 25.9 |
| 100 mg/kg, oral | 85 ± 2.1 | 46.6 | |
| 200 mg/kg, oral | 91 ± 3.2 | 56.9 | |
| Indomethacin | 0.75 mg/kg, ip | 51 ± 2.0 | - 12.1 |
| Indomethacin + ferulic acid | 0.75 mg/kg, ip + 100 mg/kg, po | 65 ± 3.3 | 12.1 |
Compounds were orally administered 60 min before the test meal (10 mL/kg body weight of 5% charcoal in 5% gum tragacanth). P < 0.05 vs control group or indomethacin group, using ANOVA followed by Bonferroni test for multiple comparisons.
Gastric emptying rate
Effect of cisplatin: Intraperitoneal administration of cisplatin dose-dependently inhibited the gastric emptying rate of the phenol red methylcellulose meal in rats. After administration of cisplatin 2.5, 5, 10, and 20 mg/kg, the gastric emptying was 41% ± 3.1%, 25% ± 2.2%, 15% ± 1.2% and 6% ± 0.4%, respectively as compared with the control 61% ± 3.0% .
Effect of ferulic acid: Ferulic acid induced a dose-dependent (50-200 mg/kg, po) significant increase on the gastric emptying rate. The percentage increases at 50, 100 and 200 mg/kg were 22.4%, 48.3% and 70.7%, respectively.
Effect of 5HT3 receptor antagonist ondansetron: Ondansetron 3 mg/kg, po when administered 30 min before cisplatin significantly increased the gastric emptying rate to 49% ± 2.5% compared with cisplatin (10 mg/kg, ip) group 15 ± 1.2 (Figure 3).
Figure 3.
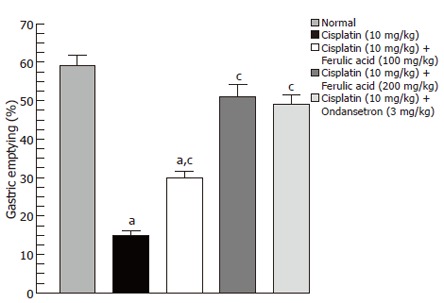
Effect of ferulic acid and ondansetron on cisplatin-induced delay in gastric emptying in rats (mean ± SE, n = 8, a,cP < 0.05, vs control or cisplatin group, respectively).
Effect of ferulic acid on cisplatin-induced delay in gastric emptying: Pretreatment with oral ferulic acid at doses of 100 and 200 mg/kg, increased gastric emptying to 30% ± 1.7% and 51% ± 3.1%, respectively as compared to cisplatin (10 mg/kg) alone. The reversal of delayed gastric emptying was statistically significant at both doses (P < 0.05) (Figure 3).
DISCUSSION
The present data revealed the prokinetic effect of ferulic acid in vitro and in vivo. Ferulic acid showed a significant dose-dependent stimulation of the basal tone of isolated rat fundus and isolated guinea pig ileum. The findings have provided evidence for the involvement of endogenous PGs in mediating the stimulant effect of ferulic acid. Both the gastric and intestinal mucosa synthesize large amounts of PGs[26]. PGE2 was shown to contract longitudinal smooth muscles[27] and relax the circular smooth muscle of the intestine[28], while indomethacin could relax the longitudinal intestinal smooth muscles[29] and contract the circular muscles due to inhibition mostly of PGE2 and PGI2[30]. The antagonistic effect of ferulic acid on gastrointestinal transit time by indomethacin indicates a role of PGs in the mechanism of action of ferulic acid on gastrointestinal motility.
The effect of ferulic acid on peristalsis and gastric emptying further supports the gastrokinetic effect of ferulic acid. The observation that indomethacin could reduce the facilitatory effect of ferulic acid further indicates the partial PG involvement in the mechanism of action of ferulic acid on gastrointestinal motility.
Phenolic acids were reported to inhibit lipoxygenase activity[31], leukotrienes[32] and thromboxan[33] biosynthesis, so ferulic acid may shunt the arachidonic acid metabolism to the cyclooxygenase pathway especially towards PGs biosynthesis direction.
There is a possibility, although not investigated in the current study, that the gastrokinetic activity of ferulic acid may be partially mediated via interference with nitric oxide (NO) production, and NO plays a key role in regulation of gastrointestinal motility by its smooth muscle relaxing and vasodilating activity[34]. Based on this assumption, Soliman and Mazzio[35] and Son et al[36] reported that caffeic acid significantly inhibited NO production probably via inhibition of NO synthease gene expression.
Further studies on the mechanism of action of ferulic acid on gastrointestinal motility may be helpful in determining the therapeutic values of ferulic acid in gastrointestinal motor disorders.
Cisplatin plays an important role in the treatment of malignant diseases[14]. It causes severe nausea and vomiting, accompanying gastrointestinal symptoms such as abdominal discomfort in the patients[37]. Cisplatin when given intravenously in dogs causes abnormal myoelectric activity in the antrum and small intestines[38], abdominal distension and delayed gastric emptying in rats[14,20].
In the present study in rats, ip administration of cisplatin dose-dependently inhibited the gastric emptying rate after a non-nutrient meal. These results are consistent with the finding that cisplatin causes a delay in gastric emptying in rats[14,15,20]. This effect may be due to the release of 5HT from the mucosal enterochromaffin cells in the gastrointestinal tract. The 5HT releases the peripheral 5HT3 receptors on the vagal afferent fibers and causes relaxation of the stomach possibly leading to delay in gastric emptying[37]. Also, cisplatin-induced free radical generation in the intestine[39] may lead to subsequent release of 5HT[40] which may act on peripheral 5HT3 to the relax the stomach and lead to the delay in gastric emptying.
The inhibitory action of cisplatin on gastric emptying was significantly reversed by pretreatment with ferulic acid. The effect of ferulic acid was dose-dependent. The beneficial effect of ferulic acid could be attributed at least partly to its stimulant effect on gastrointestinal tract and its antioxidant effect. Ferulic acid, as one of the phenolic acids, has demonstrated strong antioxidant properties as its structure dependent hydrogen-donating abilities and its propensity for nitrate make these compounds powerful scavengers of reactive oxygen species and reactive nitrogen species[2]. Ferulic acid has been shown to protect gastrointestinal organs against carcinogenesis[41] and antioxidants may prevent cisplatin-induced delay in gastric emptying in rats. In conclusion, ferulic acid inhibits the cisplatin-induced delay in gastric emptying in rats. This agent may be useful in reducing cisplatin-induced emesis and improve gastrointestinal symptoms such as abdominal discomfort induced by cytotoxic agents.
Footnotes
S- Editor Pan BR L- Editor Ma JY E- Editor Liu WF
References
- 1.King A, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc. 1999;99:213–218. doi: 10.1016/S0002-8223(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 2.Kono Y, Kobayashi K, Tagawa S, Adachi K, Ueda A, Sawa Y, Shibata H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim Biophys Acta. 1997;1335:335–342. doi: 10.1016/s0304-4165(96)00151-1. [DOI] [PubMed] [Google Scholar]
- 3.Fernández MA, Sáenz MT, García MD. Anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J Pharm Pharmacol. 1998;50:1183–1186. doi: 10.1111/j.2042-7158.1998.tb03332.x. [DOI] [PubMed] [Google Scholar]
- 4.Ursini F, Tubaro F, Rong J, Sevanian A. Optimization of nutrition: polyphenols and vascular protection. Nutr Rev. 1999;57:241–249. doi: 10.1111/j.1753-4887.1999.tb06951.x. [DOI] [PubMed] [Google Scholar]
- 5.Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2000;9:1163–1170. [PubMed] [Google Scholar]
- 6.Rice-Evans CA, Miller NJ, Paganaga G. Antioxidant properties of phenolic compounds. Trends Plant Sci Rev. 1997;2:152–159. [Google Scholar]
- 7.Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13:435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- 8.Czok G, Midani W, Finke RI. Effect of some phenols and hydroxyphenyl carboxy acid on biliary secretion and intestine motility. Colloq Int Chim Cafes. 1971;5:408–416. [Google Scholar]
- 9.Hara Y. Influence of tea catechins on the digestive tract. J Cell Biochem Suppl. 1997;27:52–58. [PubMed] [Google Scholar]
- 10.Bingham SA, Vorster H, Jerling JC, Magee E, Mulligan A, Runswick SA, Cummings JH. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br J Nutr. 1997;78:41–55. doi: 10.1079/bjn19970117. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri L, Basu S, Seth P, Chaudhuri T, Besra SE, Vedasiromoni JR, Ganguly DK. Prokinetic effect of black tea on gastrointestinal motility. Life Sci. 2000;66:847–854. doi: 10.1016/s0024-3205(99)00657-8. [DOI] [PubMed] [Google Scholar]
- 12.Eglen RM, Sharif NA, To ZP. Muscarinic M3 receptors mediate total inositol phosphates accumulation in murine HSDM1C1 fibrosarcoma cells. Eur J Pharmacol. 1993;244:49–55. doi: 10.1016/0922-4106(93)90058-h. [DOI] [PubMed] [Google Scholar]
- 13.Visnovský P. [The effect of cyclophosphamide and methotrexate on gastric emptying and secretion in rats] Bratisl Lek Listy. 1992;93:90–92. [PubMed] [Google Scholar]
- 14.Yoshida N, Omoya H, Ito T. DAT-582, a novel serotonin3 receptor antagonist, is a potent and long-lasting antiemetic agent in the ferret and dog. J Pharmacol Exp Ther. 1992;260:1159–1165. [PubMed] [Google Scholar]
- 15.Kishibayashi N, Ichikawa S, Yokoyama T, Ishii A, Karasawa A. Pharmacological properties of KF18259, a novel 5-HT3-receptor antagonist, in rats: inhibition of the distal colonic function. Jpn J Pharmacol. 1993;63:495–502. doi: 10.1254/jjp.63.495. [DOI] [PubMed] [Google Scholar]
- 16.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34:1522–1534. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 17.Sassi G, Striano B, Merlo UA. A reporting system for the assessment of chemotherapy toxicity. J Oncol Pharm Pract. 2005;11:63–67. doi: 10.1191/1078155205jp154oa. [DOI] [PubMed] [Google Scholar]
- 18.Bradner WT, Schurig JE. Toxicology screening in small animals. Cancer Treat Rev. 1981;8:93–102. doi: 10.1016/s0305-7372(81)80029-1. [DOI] [PubMed] [Google Scholar]
- 19.Roos IA, Fairlie DP, Whitehouse MW. A peculiar toxicity manifested by platinum(II)amines in rats: gastric distension after intraperitoneal administration. Chem Biol Interact. 1981;35:111–117. doi: 10.1016/0009-2797(81)90066-1. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SS, Gupta YK. Reversal of cisplatin-induced delay in gastric emptying in rats by ginger (Zingiber officinale) J Ethnopharmacol. 1998;62:49–55. doi: 10.1016/s0378-8741(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari P, Gupta YK, Seth SD. BRL 43694, a new 5HT3 receptor antagonist, prevents cisplatin-induced emesis in dogs. Methods Find Exp Clin Pharmacol. 1989;11:361–363. [PubMed] [Google Scholar]
- 22.VANE JR. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957;12:344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry WLM. Pharmacological experiments on isolated preparations. 2nd ed. Churchill Livingstone: Edinburgh London and new York; 1970. pp. 1–11. [Google Scholar]
- 24.Capasso F, De Ruggiero G, Di Rosa M, Sorrentino L. [Pharmacological research on a deethylate metabolite of 4-amino-5-chloro-N-(2-diethylaminoethyl)-2-methoxybenzamide (metoclopramide)] Boll Chim Farm. 1976;115:649–657. [PubMed] [Google Scholar]
- 25.Scarpignato C, Capovilla T, Bertaccini G. Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 1980;246:286–294. [PubMed] [Google Scholar]
- 26.Hawkey CJ, Rampton DS. Prostaglandins and the gastrointestinal mucosa: are they important in its function, disease, or treatment. Gastroenterology. 1985;89:1162–1188. doi: 10.1016/0016-5085(85)90225-2. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi K, Nishiwaki H, Hara N, Okabe S. Effects of gastric distension and prostaglandin on acid ethanol-induced mucosal lesions in the rat. Dig Dis Sci. 1988;33:1569–1577. doi: 10.1007/BF01535948. [DOI] [PubMed] [Google Scholar]
- 28.Cubeddu LX. Mechanisms by which cancer chemotherapeutic drugs induce emesis. Semin Oncol. 1992;19:2–13. [PubMed] [Google Scholar]
- 29.Kadlec O, Masek K, Seferna I. A modulating role of prostaglandins in contractions of the guinea-pig ileum. Br J Pharmacol. 1974;51:565–570. doi: 10.1111/j.1476-5381.1974.tb09675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders KM. Evidence that prostaglandins are local regulatory agents in canine ileal circular muscle. Am J Physiol. 1984;246:G361–G371. doi: 10.1152/ajpgi.1984.246.4.G361. [DOI] [PubMed] [Google Scholar]
- 31.Nardini M, D'Aquino M, Tomassi G, Gentili V, Di Felice M, Scaccini C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic Biol Med. 1995;19:541–552. doi: 10.1016/0891-5849(95)00052-y. [DOI] [PubMed] [Google Scholar]
- 32.de la Puerta R, Ruiz Gutierrez V, Hoult JR. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem Pharmacol. 1999;57:445–449. doi: 10.1016/s0006-2952(98)00320-7. [DOI] [PubMed] [Google Scholar]
- 33.Kayahara H, Miao Z, Fujiwara G. Synthesis and biological activities of ferulic acid derivatives. Anticancer Res. 1999;19:3763–3768. [PubMed] [Google Scholar]
- 34.Guslandi M. Nitric oxide: an ubiquitous actor in the gastrointestinal tract. Dig Dis. 1994;12:28–36. doi: 10.1159/000171434. [DOI] [PubMed] [Google Scholar]
- 35.Soliman KF, Mazzio EA. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc Soc Exp Biol Med. 1998;218:390–397. doi: 10.3181/00379727-218-44309. [DOI] [PubMed] [Google Scholar]
- 36.Son S, Lewis BA. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J Agric Food Chem. 2002;50:468–472. doi: 10.1021/jf010830b. [DOI] [PubMed] [Google Scholar]
- 37.Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- 38.Chey RD, Lee KY, Asbury R, Chey WY. Effect of cisplatin on myoelectric activity of the stomach and small intestine in dogs. Dig Dis Sci. 1988;33:338–344. doi: 10.1007/BF01535760. [DOI] [PubMed] [Google Scholar]
- 39.Sangeetha P, Das UN, Koratkar R, Suryaprabha P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Radic Biol Med. 1990;8:15–19. doi: 10.1016/0891-5849(90)90139-a. [DOI] [PubMed] [Google Scholar]
- 40.Matsuki N, Torii Y, Kaji T, Nishiyama N, Ishiyama J, Chida N, Saito H. Emetic responses of Sorex unguiculatus. Jikken Dobutsu. 1993;42:225–228. [PubMed] [Google Scholar]
- 41.Mori H, Kawabata K, Yoshimi N, Tanaka T, Murakami T, Okada T, Murai H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999;19:3775–3778. [PubMed] [Google Scholar]


