Abstract
AIM: To investigate H pylori expression in gastric cancer patients in relation to primary tumor angiogenic markers, such as microvessel density (MVD), thymidine phosphorylase (TP), vascular endothelial growth factor receptor-1 (VEGF-R1), p53 and circulating VEGF levels.
METHODS: Angiogenic markers were analyzed immunohistochemically in 56 primary gastric cancers. H pylori cytotoxin (vacA) and the cytotoxin-associated gene (cagA) amplification were evaluated using PCR assay. Serum H pylori IgG antibodies and serum/plasma circulating VEGF levels were detected in 39 and 38 patients by ELISA, respectively.
RESULTS: A total of 69% of patients were positive for circulating IgG antibodies against H pylori. cagA-positive H pylori strains were found in 41% of gastric patients. vacA was found in 50% of patients; s1 strains were more highly expressed among vacA-positive patients. The presence of the s1 strain was significantly associated with cagA (P = 0.0001). MVD was significantly correlated with both tumor VEGF expression (r = 0.361, P = 0.009) and serum VEGF levels (r = -0.347, P = 0.041). Conversely, neither VEGF-R1 expression nor MVD was related to p53 expression. However, H pylori was not related to any angiogenic markers except for the plasma VEGF level (P = 0.026).
CONCLUSION: H pylori antigen is related to higher plasma VEGF levels, but not to angiogenic characteristics. It can be hypothesized that the toxic effects of H pylori on angiogenesis occurs in early preclinical disease phase or in long-lasting aggressive infections, but only when high H pylori IgG levels are persistent.
Keywords: H pylori, Gastric carcinoma, Angiogenesis
INTRODUCTION
H pylori infection is a well-known risk factor for the development of pre-neoplastic and neoplastic gastric mucosal alterations[1,2]. An increase in proliferative activity of gastric epithelial cells without a corresponding increase in apoptosis has been implicated in H pylori gastric carcinogenesis[3,4]. In addition, specific virulence determinants of H pylori strains can influence the outcome of the infection. Urease, vacuolating cytotoxin vacA, and the pathogenicity island (cag PAI) gene products are the main virulence factors of this organism involved in the development of gastric carcinoma. Thus, individuals infected with strains that express these virulence factors are prone to develop severe local inflammation which may induce the development of peptic ulcers and gastric cancers. Also, H pylori activity may be associated with virulence; in fact, urease activity may be an important colonization factor and exert a direct toxic effect upon intercellular junctions, resulting in alteration of gastric mucosal permeability[5]. The subsequent passage toward cancer is probably prompted by other factors, such as the onset of infection or other agents independent of H pylori.
Several studies have suggested that angiogenesis might also contribute to gastric tumorigenesis[6-8]. Angiogenesis is a complex multistep cascade modulated by positive soluble factors, such as the vascular endothelial growth factor (VEGF). The tumor neo-angiogenesis has been demonstrated in almost all solid tumors using various morphological techniques. The current method of angiogenesis quantification is the evaluation of CD34 antigen expression, a cell surface glycoprotein also present in the vascular endothelium permitting the study of intratumor endothelial cells[9]. The cellular receptor for VEGF, VEGF-R1 or Flt-1, is highly expressed in gastric carcinoma cells, suggesting that this pathway could influence tumor growth and metastasis through paracrine and autocrine mechanisms[10]. An additional tissue factor is thymidine phosphorylase (TP), an enzyme involved in pyrimidine nucleoside metabolism, which is identical to the platelet-derived endothelial cell growth factor and is endowed with angiogenic activities in various solid tumors[11] Furthermore, the p53 oncosuppressor gene has been reported to be involved in inhibition of tumor vascularization by fostering unopposed angiopoietin-2 activity[12] .
Recent publications have suggested that H pylori infection may regulate the angiogenesis and invasion of gastric carcinoma. In fact, H pylori influences in vitro angiogenesis-related gene expression; in particular, it has been demonstrated to up-regulate VEGF expression in gastric epithelial cells, an effect which appears to be related to vacA-expression[13,14]. Moreover, H pylori has been shown to up-regulate the expression of epidermal growth factor (EGF)-related growth factors and COX-2 in in vitro human gastric epithelial cells as well as in human gastric mucosa in vivo[15,16]. Lastly, its relationship with p53, which has been described as an angiogenesis-related factor, has been documented[17-20]. In spite of these evidences originating from in vitro studies, suggesting a relationship between pathophysiological roles for H pylori in the induction of tumor neo-angiogenesis, to our best of knowledge, no data are available in literature in patient series. Our hypothesis was that H pylori-related gastric cancer could involve different neo-angiogenic characteristics with respect to tumors without bacterial infection.
To verify the association between H pylori infection and different angiogenesis-related characteristics, 56 gastric cancer patients were studied for microvessel density (MVD), thymidine phosphorylase (TP), vascular endothelial growth factor-receptor (VEGF-R1) and p53 expressions in addition to circulating serum and plasma VEGF levels. H pylori was investigated at the molecular and at circulating blood levels.
MATERIALS AND METHODS
Patients
Fifty-six patients (37 men and 19 women; median age 64 years, range 42-83 years) with T1-4 N0-1 M0-1 gastric carcinoma were enrolled in this study. All patients had primary surgery for gastric cancer at National Cancer Institute of Bari. Primary tumor tissues were utilized for the immunohistochemical analysis of MVD, p53, VEGF-R1 and TP expressions.
Formalin-fixed and paraffin-embedded specimen of the primary tumor was selected by the pathologist for each patient on the basis of the quality of morphological preservation and neoplastic cellularity. In accordance with standardized sampling protocols, the sample was comprehensive both at the deeper portions of tumor, as well as the edges of the lesions. Five-micrometer thick sections were cut for immunohistochemical assay and for determination of H pylori status by means of polymerase chain reaction (PCR). A section contiguous to those selected for immunohistochemistry and DNA extraction was always stained with haematoxylin and eosin and confirmed by the pathologist as rich in neoplastic cellularity. Enzyme-linked immuno-sorbent assay (ELISA) for IgG antibodies against H pylori was performed on blood samples from 39 patients. Circulating VEGF levels were detected by ELISA in serum and plasma of 38 patients. The patients characteristics are shown in Table 1.
Table 1.
Clinicopathological features and distribution of cagA, vacA and IgG anti-H pylori in a series of 56 gastric cancer patients
| Clinicopathological features | n |
| Sex | |
| Male | 37 |
| Female | 19 |
| Tumour category | |
| pT1-2-3 | 28 |
| pT4 | 28 |
| Location | |
| Antrum | 23 |
| Other | 33 |
| IgG anti H pylori (ELISA) | 39 |
| IgG - (≤ 7 KU/L) | 12 |
| IgG + (> 7 KU/L) | 27 |
| cagA (PCR) | 56 |
| cagA - | 32 |
| cagA + | 23 |
| NE | 1 |
| vacA (PCR) | 56 |
| NEG | 28 |
| s1m1 | 10 |
| s2m2 | 5 |
| s1m2 | 9 |
| s1m1/s1m2a | 1 |
| NE | 3 |
NEG: Negative; NE: Not evaluable;
Multiple genome.
DNA extraction and PCR analysis
DNA extraction from paraffin-embedded specimens was performed using the method described by Lin et al[21]. Briefly, samples were incubated with a lysis buffer and proteinase K for 3 h at 55°C. Total DNA was extracted with phenol/chloroform, precipitated with acidic ethanol, and dissolved in sterile water.
Amplification of cagA and vacA
The extracted DNA was subjected to PCR for detection of H pylori genes, cagA and vacA. The cagA gene was amplified using the primers described elsewhere[22,23]. The vacA gene was amplified using primers described by Atherton et al[24] which evaluate the mid region (m) and the region encoding for the signal peptide (s) of the gene. Four different PCR products were obtained: s1 or s2 from the s region, and m1 and m2 from the m region. PCR products were analyzed by electrophoresis on 20 g/L agarose gel. Positive and negative controls were examined with each batch of PCR.
Detection of anti-H pylori IgG
An enzyme-linked immuno-sorbent assay was used to detect H pylori-specific IgG serum antibodies (Anti-H pylori EIA Quant- COBAS2- Roche Diagnostics). The anti-H pylori IgG EIA is a second-generation two-step EIA for the detection of IgG antibodies to H pylori in human serum, based on a set of fast protein liquid chromatography-purified cell surface antigens, including the native urease enzyme[25]. According to the manufacturer, patients were considered positive for IgG against H pylori when IgG value was higher than 7 U/mL.
Immunohistochemistry
Serial sections of paraffin-embedded gastric tissue were deparaffinized and rehydrated. For antigen retrieval, the sections were microwaved at 500 W for 10 min in citrate buffer (pH 6) and endogenous peroxidase activity was blocked with 30 mL/L hydrogen peroxide solution. Adjacent slides were incubated with different monoclonal antibodies. The bound antibody was visualized using a biotinylated secondary antibody, avidin-biotin peroxidase complex, and 3-amino-9-ethylcarbazole (Ultra Vision Detection System anti-Polyvalent, HRP/DAB, Lab Vision Corporation). For negative control sections, primary antibody was replaced with phosphate-buffered saline and processed in the same manner. Gastric carcinomas known to express high levels of CD34, p53, TP and VEGF-R1 proteins were used as positive controls.
Anti-CD34 (QB–END/10, Novocastra Laboratories Ltd) was diluted at 1:50 for 1 h at room temperature as a pan-endothelial marker for MVD analysis. The modified Weidner’s method was utilized for the evaluation of MVD according to CD34 endothelial cell immunostaining[26]. For the microvessel counting, positive stainings for MVD, in five most highly vascularized areas (‘hot spots’) in each slide, were counted in 400 × fields with an image analysis system (Quantimet 500 Leica; 0.19 mm2/field) and MVD was expressed as the average of the microvessel count in these areas[27]. Any EC or endothelial cluster positive for CD34 (brown yellow staining) was considered to be a single countable microvessel. Sclerotic areas, both hypocellular and necrotic, within the tumor were not considered for vessel evaluation.
Anti-p53 monoclonal antibodies (PAb 1801, Neo-Markers), grown against human p53 and recognizing wild-type and mutant forms of the p53 protein, were diluted at 1:150 for 1 h at room temperature. Tumor cells expressing p53 immunoreactivity were quantified by evaluating a total of 1000 neoplastic cells in random fields from representative areas. Exclusive nuclear staining was scored as positive. The immunoreactive cells were expressed as percentages[28]. Anti-TP monoclonal antibodies (P-GF.44C Neo-Markers), recognizing full length human TP protein, were diluted at 1:100 for 1 h at room temperature. TP positivity was determined at 400 × fields with the image analysis system and was evaluated on the basis of percentage of stained epithelial tumor cells. Tumor cells with moderate or strong staining intensity were counted. TP expression in macrophages was considered an internal positive control. The polyclonal antibody anti-VEGF-R1 (Flt-1 polyclonal rabbit antibody, Santa Cruz Biotechnology Inc.), recognizing the carboxyl terminus of the receptor for VEGF, VEGF-R1 or the Flt-1 protein of human origin, was used at a dilution of 1:100 for 1 h at room temperature. VEGF-R1 positivity was scored as cytoplasmic immunostaining using an image analysis system (Quantimet 500 Leica). Immunoreactivity was expressed as the percentage ratio between the area of immuno-positive tumor cells and the entire area of invasive neoplastic tissue.
The laboratory, where the immunohistochemical analyses were performed, participated to Quality Control programs managed by INQAT[29].
Circulating VEGF detection
Blood samples were collected before surgery. Venous blood was dispensed into a serum separator tube (Becton Dickinson Vacutainer Systems) for serum obtainment, and into sodium citrate, theophylline, adenosine, dipyridamole (CTAD) tubes for plasma (Becton Dickinson Hemogard Vacutainer Systems).
Circulating VEGF levels were examined in plasma and serum using the Quantikine Human VEGF-enzyme-linked immuno-sorbent assay (ELISA, R&D System Inc.) which recognizes VEGF165. According to the manufacturer, the minimum detectable dose of VEGF is typically less than 9.0 ng/L. Values below 9.0 ng/L were equal to zero. VEGF levels in plasma and serum were analyzed, as we previously demonstrated that the two determinations provide alternative and additional information on circulating VEGF, also in relation to the role played by the activation and quantity of platelets in VEGF release[30].
Statistical analysis
The associations between MVD, TP, VEGF-R1 and p53 expression, markers of H pylori status and histological diagnosis were evaluated using the Chi-square test. A correlation analysis among the aforementioned biomarkers, considered as continuous variables, was performed by Pearson’s correlation coefficient (r). In the statistical analysis, vacA genotypes were classified into two subgroups: “cytotoxic strains” which included s1m1, s1m2 and s1m1/s1m2 strains and “others” which included negative and s2m2. Patients with s1m1/s1m2 strains were infected by multiple genotypes. Backwards stepwise logistic regression analysis was used to estimate the independent association of any biological markers with H pylori characteristics. Statistical analysis was carried out using the software SPSS for windows, release 9.0.
RESULTS
H pylori status
Cytotoxin-associated gene (cagA)-positive H pylori strains were found in 41% of gastric patients. vacA was found in 50% of patients; s1 strains were more highly expressed among vacA-positive patients. Moreover, a single patient was found to be infected with different H pylori strains (multiple genomes). The results are summarized in Table 1. The presence of the s1 strain was significantly associated with cagA (P = 0.0001). Only one patient infected with the s2 strain showed cagA-positivity, while 26 (81%) patients were negative for both cagA and vacA. A total of 69% of patients were positive for circulating IgG antibodies against H pylori, with a mean and median value of 77 U/mL (range 1-476 U/mL) and 15 U/mL, respectively.
Immunohistochemical analysis
TP immunoreactivity was observed in normal epithelial cells, malignant epithelial cells, macrophages and endothelial cells. The usual pattern of positive staining was both cytoplasmic and nuclear. A mean of 5% (range 0%-80%) of cells showed TP positivity.
p53 expression was generally confined to neoplastic tissues, while the normal mucosa was rarely stained. A mean of 3% (range 0%-50%) of cells showed p53 positivity.
CD34 immunostaining was detected in the endothelial cells, especially in the area surrounding the tumor (Figure 1A). In the ‘hot spot’ tumor area, a mean of 39 vessels (range 0-100 vessels) was found with only 2% of cases not demonstrating any microvessels. VEGF-R1 immunostaining was mainly localized at the membrane and cytoplasm of epithelial and endothelial cells (Figure 1B). By counting only the epithelial component, a mean of 24% (range 0%-100%) of VEGF-R1-immunostained cells was found. About 80% of tumors demonstrated VEGF-R1 expression.
Figure 1.
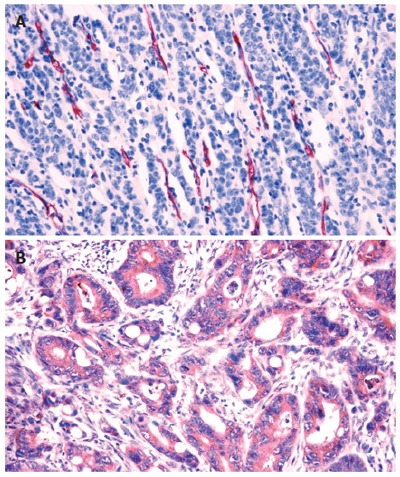
Immunohistochemical assay for detection of microvessel density (A) and VEGF-R1 protein expression (B) in gastric cancer. VEGF-R1 is stained in the cytoplasm of cancer cells.
Regarding the relationship among the different angiogenic characteristics, only MVD was significantly correlated with both tumor VEGF expression (r = 0.361, P = 0.009; Figure 2) and serum VEGF levels (r = -0.347, P = 0.041; Figure 3). Conversely, neither VEGF-R1 expression nor MVD was related to p53 expression.
Figure 2.
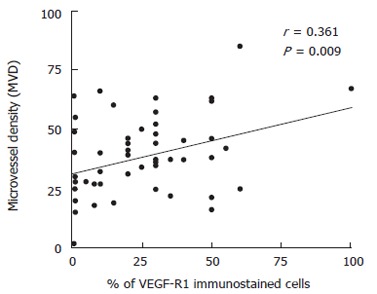
Correlation between percentage of VEGF-R1-immunoreactive cells and microvessel density (MVD) within each tumor.
Figure 3.
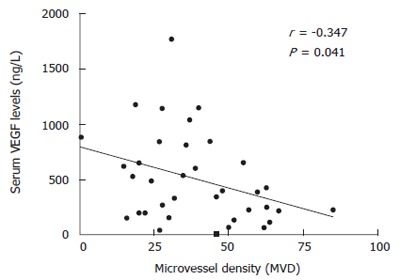
Correlation between serum VEGF levels and microvessel density (MVD) within each tumor.
Table 2 shows the association between the markers of H pylori status and angiogenic factors. There was no significant association between markers of H pylori status, cagA, vacA and angiogenic biomarker expression. When the correlation between angiogenesis related-markers and IgG status was analysed a significant correlation between IgG status and plasma VEGF levels was observed (P = 0.026). The lack of association between H pylori characteristics and biomarkers was also confirmed with multivariate logistic regression analysis.
Table 2.
cag A, vac A, IgG anti-H pylori and angiogenic factors in gastric cancer patients
|
IgG anti H pylori |
cag A |
vac A |
||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| With IgG- (≤ 7 U/mL) | With IgG+ (> 7 U/mL) | Negative for cagA | Positive for cagA | Cytotoxic strains (s1; m1/2) | Others (absent; s2m2) | |
| TP expression1 | ||||||
| 02 | 9 (31) | 20 (69) | 24 (57) | 18 (43) | 24 (60) | 16 (40) |
| > 0 | 2 (25) | 6 (75) | 8 (80) | 2 (10) | 8 (80) | 2 (20) |
| p53 expression1 | ||||||
| 02 | 8 (25) | 24 (75) | 27 (60) | 18 (40) | 28 (65) | 15 (35) |
| > 0 | 3 (60) | 2 (40) | 5 (63) | 3 (37) | 4 (50) | 4 (50) |
| MVD (CD34)1 | ||||||
| ≤ 372 | 7 (32) | 15 (68) | 16 (59) | 11 (41) | 15 (58) | 11 (42) |
| > 37 | 3 (21) | 11 (79) | 16 (64) | 9 (36) | 17 (71) | 7 (29) |
| VEGF-R1 expression1 | ||||||
| ≤ 202 | 6 (29) | 15 (71) | 16 (59) | 11 (41) | 14 (54) | 12 (46) |
| > 20 | 5 (31) | 11 (69) | 15 (58) | 11 (42) | 17 (68) | 8 (32) |
| pVEGF levels | ||||||
| ≤ 262 | 9 (50)a | 9 (50)a | 10 (53) | 9 (47) | 11 (61) | 7 (39) |
| > 26 | 2 (13)a | 13 (87)a | 9 (60) | 6 (40) | 9 (60) | 6 (40) |
| sVEGF levels | ||||||
| ≤ 4322 | 5 (29) | 12 (71) | 11 (61) | 7 (49) | 12 (71) | 5 (29) |
| > 432 | 6 (32) | 13 (68) | 9 (47) | 10 (53) | 9 (47) | 10 (53) |
sVEGF: Serum VEGF; pVEGF: Plasma VEGF;
% of immunostained cells;
Cut-off median value of the series;
P = 0.026.
Angiogenic marker expression and markers of H pylori status were analyzed with respect to clinico-pathological features. Plasma VEGF levels and tumor TP expression were both significantly associated with tumor size (P = 0.030 and P = 0.035, respectively; Table 3). Regarding plasma VEGF levels in particular, T4 tumors showed a significantly smaller percentage of low IgG cases as compared with T1-3 tumors (31% vs 74%; P < 0.03).
Table 3.
Association between angiogenic characteristics, markers of H pylori status and clinicopathological features
| Biomarkers |
Tumor stage |
M status |
||||
| T1-2-3 (n = 28) | T4 (n = 28) | P | M0 (n = 38) | M1 (n = 18) | P | |
| cagA negative (n = 32) | 54 | 63 | NS | 58 | 59 | NS |
| vacA no cytotoxic strains (n = 33) | 62 | 63 | NS | 58 | 71 | NS |
| TP negative (n = 42) | 93 | 65 | 0.035 | 83 | 71 | NS |
| p53 negative (n = 46) | 78 | 93 | NS | 78 | 100 | 0.038 |
| low MVD values (n = 27) | 56 | 46 | NS | 54 | 44 | NS |
| Low VEGF-R1 expression (n = 27) | 56 | 44 | NS | 51 | 47 | NS |
| High IgG levels (n = 27) | 65 | 74 | NS | 77 | 54 | NS |
| Low pVEGF levels (n = 19) | 74 | 31 | 0.03 | 52 | 58 | NS |
| Low sVEGF levels (n = 19) | 35 | 67 | NS | 50 | 50 | NS |
NS: Non-significant.
Lastly, p53 expression was significantly associated with metastatic status (P = 0.038), as 100% of patients with metastatic disease did not express p53. No association was found between cytohistological tumor grading or H pylori infection site and angiogenesis-related markers and H pylori characteristics.
DISCUSSION
The role of H pylori in gastric cancerogenesis has been extensively investigated; conversely, information is lacking regarding the biological impact of H pylori on the progression of gastric cancer. Several factors emphasize the importance that various H pylori components can have their roles on the development of pre-neoplastic and neoplastic alterations of the gastric mucosa. Specific virulence factors produced by the bacterium, such as the vacuolating cytotoxin (vacA) or the cytotoxin-associated protein (cagA), contribute to gastroduodenal mucosal injury and impair the healing process of the damaged mucosa[31,32]. In addition, the host response to the infection and the presence of environmental factors are thought to be involved in the pathogenesis of H pylori-related gastroduodenal disease[33,34]. The vacuolating toxin (vacA) is believed to be a major determinant of H pylori-associated gastric disease[35,36]. The vacuolating cytotoxin gene A (vacA), which encodes the vacA protein, is present in all H pylori strains, but its encoded products are associated both with and without in vitro vacuolating activity[37]. It has been suggested that the vacA s1a genotype is closely associated with high cytotoxin production, while the vacA s2 allele can demonstrate a negative in vitro association with cytotoxin activity. The presence of these virulence factors can be used to identify patients at risk to develop gastric cancer; in fact, patients with neoplastic transformation of the gastric mucosa are more likely to be infected by the cagA+ strain[19,38,39]. However, conflicting results regarding the association between these virulence factors and clinical outcome of gastric cancer are found in the literature[36,40-43].
p53 mutations and the genotypic characterization of H pylori have also been thoroughly studied to identify possible links between H pylori infection and p53 alterations without reaching definitive conclusions. Alterations of the p53 gene and/or its abnormal protein accumulation have both been described during the later stage of gastric carcinogenesis[44,45] and in precancerous gastric lesions[46]. As cagA+ H pylori strains induce particularly severe inflammation in the gastric mucosa[41,47]. it has been hypothesized that gastric tumors from subjects infected with cagA+ H pylori might have a higher prevalence of p53 mutation than tumors from non-infected subjects.
H pylori infection may also regulate the angiogenesis and invasion of gastric carcinomas[13,14], but whether H pylori exerts its effects to induce neovascularization early in the development of gastric pre-neoplastic lesions or late in clinical phases of the disease is still unknown. However, it is clear that H pylori infection can increase the expression of the platelet-derived endothelial cell growth factor by infiltrating interstitial cells in pre-malignant lesions, such as intestinal metaplasia, thereby assisting in creating a favourable environment for tumor development[48]. Furthermore, it has been demonstrated that H pylori is able to up-regulate VEGF expression in gastric epithelial cells determining effects related to vacA-expression.
Recently, for the first time, Caputo et al[13] showed that H pylori up-regulated VEGF expression in gastric mucosa cells in vitro and that this effect was strictly vacA-dependent; and, interestingly, this result was not observed when using an isogenic mutant specifically lacking vacA. Moreover, in vitro and in vivo up-regulation of a number of EGF-related growth factors have also been reported[15,16,49].
In our sufficiently large series of gastric cancer patients, a percentage of H pylori infection was demonstrated, either as IgG-circulating level or as cagA/vacA DNA, which is in agreement with the previous studies[50,51]. In addition, the neo-angiogenesis characteristics reported did not significantly differ from those of other series of gastric cancers[7,52,53]. It is also possible to verify a clear relationship between p53, TP, VEGF and the clinicopathological characteristics considered in our series, further stressing the impact that angiogenesis has on tumor aggressiveness[52,53]. In fact, TP expression and plasma VEGF levels were both associated with tumor size, while p53 expression was associated with metastatic status.
However, when addressing the main objective of our study, it is possible to demonstrate an association only between higher levels of plasma VEGF and high levels of IgG (Table 3). Conversely, H pylori-infected tumors did not show p53, MVD, TP, VEGF-R1 characteristics which obviously differed from those without presence of H pylori infection. These results only partially agree with previous data[17,54] which, however, were all obtained from experimental in vitro models, in some cases referring to mRNA analyses utilizing only quantitative molecular or immuno-enzymatic approaches[14], therefore not exploiting morphological antigen tissue distribution. Furthermore, the increase of gene expression induced by the co-culture of gastric tumor cells with H pylori has been reported to be generally modest with more evident positive modulation for angiogenic factors not investigated in the present study, such as interleukin-8[14]. Caputo et al[13] also recently suggested that vacA-induced up-regulation of VEGF expression could depend on the functionality of epidermal growth factor receptor (EGFR)-, mitogen-activated protein kinase (MAPK)- and COX-2-mediated pathways, the biological targets which are largely heterogeneous in human gastric cancer. In conclusion, our study seems to suggest that the relationship between the H pylori toxic effect and angiogenic factors demonstrated in vitro could be influenced in human gastric tumor tissues by other key biological factors not considered in the present study.
A last comment regarding IgG and VEGF association concerns the blood of gastric cancer patients. The level of IgG antibodies has been suggested to be useful, not for diagnosis of infection, but for monitoring the outcome of H pylori infection over time, and specifically the efficacy of therapies aiming to eradicate H pylori infection. Thus, an elevated serum level before primary surgery for gastric cancer could be a signal of long-lasting and probably H pylori infection resistant to antimicrobial therapy[55].
The results of our study indicate that, from the angiogenic point of view, H pylori-related gastric cancers do not differ from those in which exposure to the bacterium cannot be demonstrated. Different explanations for these findings can be proposed: an angiogenetic relationship, if any, could be only induced by a long-lasting H pylori infection demonstrated by high IgG levels in the plasma; an alternative hypothesis might regard the ability of H pylori to modulate angiogenesis only during early phase of disease genesis and progression to be then lost during the clinically evident disease phases. This hypothesis would concord with the presumed role that angiogenesis plays, especially during the extremely early phases of cancer[9,17,56].
ACKNOWLEDGMENTS
We thank Mr. Baldassarre Stea for statistical analysis, Mr. Cesare Salvatore for technical support and Mrs. Paulene Maselli-Campagna for her help in the elaboration of the manuscript.
Footnotes
Supported by grants from A I R C Project 2005, Italy
S- Editor Wang GP L- Editor Kumar M E- Editor Ma WH
References
- 1.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89:S116–S128. [PubMed] [Google Scholar]
- 2.Lambert JR, Lin SK, Aranda-Michel J. Helicobacter pylori. Scand J Gastroenterol Suppl. 1995;208:33–46. doi: 10.3109/00365529509107760. [DOI] [PubMed] [Google Scholar]
- 3.Linsalata M, Russo F, Notarnicola M, Berloco P, Di Leo A. Polyamine profile in human gastric mucosa infected by Helicobacter pylori. Ital J Gastroenterol Hepatol. 1998;30:484–489. [PubMed] [Google Scholar]
- 4.Ierardi E, Francavilla A, Balzano T, Traversa A, Principi M, Monno RA, Amoruso A, Ingrosso M, Pisani A, Panella C. Effect of Helicobacter pylori eradication on gastric epithelial proliferation. Relationship with ras oncogene p21 expression. Ital J Gastroenterol Hepatol. 1997;29:214–219. [PubMed] [Google Scholar]
- 5.Dunn BE, Sung CC, Taylor NS, Fox JG. Purification and characterization of Helicobacter mustelae urease. Infect Immun. 1991;59:3343–3345. doi: 10.1128/iai.59.9.3343-3345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giatromanolaki A, Koukourakis MI, Stathopoulos GP, Kapsoritakis A, Paspatis G, Kakolyris S, Sivridis E, Georgoulias V, Harris AL, Gatter KC. Angiogenic interactions of vascular endothelial growth factor, of thymidine phosphorylase, and of p53 protein expression in locally advanced gastric cancer. Oncol Res. 2000;12:33–41. doi: 10.3727/000000001108747426. [DOI] [PubMed] [Google Scholar]
- 7.Feng CW, Wang LD, Jiao LH, Liu B, Zheng S, Xie XJ. Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: correlation with clinical features. BMC Cancer. 2002;2:8. doi: 10.1186/1471-2407-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuccillo C, Cuomo A, Rocco A, Martinelli E, Staibano S, Mascolo M, Gravina AG, Nardone G, Ricci V, Ciardiello F, et al. Vascular endothelial growth factor and neo-angiogenesis in H. pylori gastritis in humans. J Pathol. 2005;207:277–284. doi: 10.1002/path.1844. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994–998. doi: 10.3748/wjg.v8.i6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenderenda M, Rutkowski P, Jesionek-Kupnicka D, Kubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res. 2001;7:129–134. doi: 10.1007/BF03032579. [DOI] [PubMed] [Google Scholar]
- 11.Shimaoka S, Matsushita S, Nitanda T, Matsuda A, Nioh T, Suenaga T, Nishimata Y, Akiba S, Akiyama S, Nishimata H. The role of thymidine phosphorylase expression in the invasiveness of gastric carcinoma. Cancer. 2000;88:2220–2227. [PubMed] [Google Scholar]
- 12.Tse V, Yung Y, Santarelli JG, Juan D, Hsiao M, Haas M, Harsh G 4th, Silverberg G. Effects of tumor suppressor gene (p53) on brain tumor angiogenesis and expression of angiogenic modulators. Anticancer Res. 2004;24:1–10. [PubMed] [Google Scholar]
- 13.Caputo R, Tuccillo C, Manzo BA, Zarrilli R, Tortora G, Blanco Cdel V, Ricci V, Ciardiello F, Romano M. Helicobacter pylori VacA toxin up-regulates vascular endothelial growth factor expression in MKN 28 gastric cells through an epidermal growth factor receptor-, cyclooxygenase-2-dependent mechanism. Clin Cancer Res. 2003;9:2015–2021. [PubMed] [Google Scholar]
- 14.Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue N, Yasui W, Aihara M, Imagawa K, Haruma K, Chayama K. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun. 2003;311:809–814. doi: 10.1016/j.bbrc.2003.10.077. [DOI] [PubMed] [Google Scholar]
- 15.Romano M, Ricci V, Di Popolo A, Sommi P, Del Vecchio Blanco C, Bruni CB, Ventura U, Cover TL, Blaser MJ, Coffey RJ, et al. Helicobacter pylori upregulates expression of epidermal growth factor-related peptides, but inhibits their proliferative effect in MKN 28 gastric mucosal cells. J Clin Invest. 1998;101:1604–1613. doi: 10.1172/JCI1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuccillo C, Manzo BA, Nardone G, D'Argenio G, Rocco A, Di Popolo A, Della VN, Staibano S, De Rosa G, Ricci V, et al. Up-regulation of heparin binding epidermal growth factor-like growth factor and amphiregulin expression in Helicobacter pylori-infected human gastric mucosa. Dig Liver Dis. 2002;34:498–505. doi: 10.1016/s1590-8658(02)80108-6. [DOI] [PubMed] [Google Scholar]
- 17.Berloco P, Russo F, Cariola F, Gentile M, Giorgio P, Caruso ML, Valentini AM, Di Matteo G, Di Leo A. Low presence of p53 abnormalities in H pylori-infected gastric mucosa and in gastric adenocarcinoma. J Gastroenterol. 2003;38:28–36. doi: 10.1007/s005350300003. [DOI] [PubMed] [Google Scholar]
- 18.Wu MS, Chen SY, Shun CT, Lee WJ, Wang HP, Wang TH, Chen CJ, Lin JT. Increased prevalence of Helicobacter pylori infection among patients affected with intestinal-type gastric cancer at non-cardiac locations. J Gastroenterol Hepatol. 1997;12:425–428. doi: 10.1111/j.1440-1746.1997.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 19.Deguchi R, Takagi A, Kawata H, Inoko H, Miwa T. Association between CagA+ Helicobacter pylori infection and p53, bax and transforming growth factor-beta-RII gene mutations in gastric cancer patients. Int J Cancer. 2001;91:481–485. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1088>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Teh M, Tan KB, Seet BL, Yeoh KG. Study of p53 immunostaining in the gastric epithelium of cagA-positive and cagA-negative Helicobacter pylori gastritis. Cancer. 2002;95:499–505. doi: 10.1002/cncr.10697. [DOI] [PubMed] [Google Scholar]
- 21.Lin TT, Yeh CT, Yang E, Chen PC. Detection of Helicobacter pylori by polymerase chain reaction assay using gastric biopsy specimens taken for CLOtest. J Gastroenterol. 1996;31:329–332. doi: 10.1007/BF02355020. [DOI] [PubMed] [Google Scholar]
- 22.Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992;30:192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 25.Goossens H, Glupczynski Y, Burette A, Van den Borre C, DePrez C, Bodenmann J, Keller A, Butzler JP. Evaluation of a commercially available complement fixation test for diagnosis of Helicobacter pylori infection and for follow-up after antimicrobial therapy. J Clin Microbiol. 1992;30:3230–3233. doi: 10.1128/jcm.30.12.3230-3233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, et al. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 27.Ranieri G, Labriola A, Achille G, Florio G, Zito AF, Grammatica L, Paradiso A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int J Oncol. 2002;21:1317–1323. [PubMed] [Google Scholar]
- 28.Paradiso A, Ranieri G, Simone G, Silvestris N, Costa A, De Lena M, Leone A, Vallejo C, Lacava J. mdm2-p53 Interaction: lack of correlation with the response to 5-fluorouracil in advanced colorectal cancer. Oncology. 2002;62:278–285. doi: 10.1159/000059576. [DOI] [PubMed] [Google Scholar]
- 29.Paradiso A, Volpe S, Iacobacci A, Marubini E, Verderio P, Costa A, Daidone MG, Marchetti A, Mottolese M, Amadori D, et al. Quality control for biomarker determination in oncology: the experience of the Italian Network for Quality Assessment of Tumor Biomarkers (INQAT) Int J Biol Markers. 2002;17:201–214. doi: 10.1177/172460080201700310. [DOI] [PubMed] [Google Scholar]
- 30.Ranieri G, Coviello M, Chiriatti A, Stea B, Montemurro S, Quaranta M, Dittadi R, Paradiso A. Vascular endothelial growth factor assessment in different blood fractions of gastrointestinal cancer patients and healthy controls. Oncol Rep. 2004;11:435–439. [PubMed] [Google Scholar]
- 31.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 33.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 34.Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457–466. doi: 10.1038/35073084. [DOI] [PubMed] [Google Scholar]
- 35.Cover TL, Tummuru MK, Cao P, Thompson SA, Blaser MJ. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 36.Miehlke S, Kirsch C, Agha-Amiri K, Günther T, Lehn N, Malfertheiner P, Stolte M, Ehninger G, Bayerdörffer E. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322–327. [PubMed] [Google Scholar]
- 37.Atherton JC, Cover TL, Papini E, Telford JL. Vacuolating Cytotoxin. In: Mobley HLT, Mendz GL, Hazell SL eds. Helicobacter pylori. Physiology and Genetics; 2001. pp. 97–109. [Google Scholar]
- 38.Magalhães PP, Queiroz DM, Camargo AA, Simpson AJ. CagA status of Helicobacter pylori infection and p53 gene mutations in gastric adenocarcinoma. Carcinogenesis. 2003;24:145; author reply 147. doi: 10.1093/carcin/24.1.145. [DOI] [PubMed] [Google Scholar]
- 39.Shibata A, Parsonnet J, Longacre TA, Garcia MI, Puligandla B, Davis RE, Vogelman JH, Orentreich N, Habel LA. CagA status of Helicobacter pylori infection and p53 gene mutations in gastric adenocarcinoma. Carcinogenesis. 2002;23:419–424. doi: 10.1093/carcin/23.3.419. [DOI] [PubMed] [Google Scholar]
- 40.van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 41.Yamaoka Y, Kodama T, Graham DY, Kashima K. Comparison of four serological tests to determine the CagA or VacA status of Helicobacter pylori strains. J Clin Microbiol. 1998;36:3433–3434. doi: 10.1128/jcm.36.11.3433-3434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 43.Rudi J, Kolb C, Maiwald M, Zuna I, von Herbay A, Galle PR, Stremmel W. Serum antibodies against Helicobacter pylori proteins VacA and CagA are associated with increased risk for gastric adenocarcinoma. Dig Dis Sci. 1997;42:1652–1659. doi: 10.1023/a:1018849112533. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y, Yoshida T, Hayashi K, Sekiya T, Yokota J, Hirohashi S, Nakatani K, Nakano H, Sugimura T, Terada M. p53 gene mutations in gastric cancer metastases and in gastric cancer cell lines derived from metastases. Cancer Res. 1991;51:5800–5805. [PubMed] [Google Scholar]
- 45.Tamura G, Kihana T, Nomura K, Terada M, Sugimura T, Hirohashi S. Detection of frequent p53 gene mutations in primary gastric cancer by cell sorting and polymerase chain reaction single-strand conformation polymorphism analysis. Cancer Res. 1991;51:3056–3058. [PubMed] [Google Scholar]
- 46.Lauwers GY, Wahl SJ, Melamed J, Rojas-Corona RR. p53 expression in precancerous gastric lesions: an immunohistochemical study of PAb 1801 monoclonal antibody on adenomatous and hyperplastic gastric polyps. Am J Gastroenterol. 1993;88:1916–1919. [PubMed] [Google Scholar]
- 47.Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 48.Franceschi F, Genta RM, Gasbarrini A, Gentiloni Silveri N, Gasbarrini G, Sepulveda AR. Helicobacter pylori infection and expression of the angiogenic factor platelet-derived endothelial cell growth factor by pre-neoplastic gastric mucosal lesions and gastric carcinoma. Dig Liver Dis. 2002;34:621–625. doi: 10.1016/s1590-8658(02)80203-1. [DOI] [PubMed] [Google Scholar]
- 49.Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, Acquaviva AM, Del Vecchio Blanco C, Bruni CB, Zarrilli R. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560–28563. doi: 10.1074/jbc.273.44.28560. [DOI] [PubMed] [Google Scholar]
- 50.Iaquinto G, Todisco A, Giardullo N, D'Onofrio V, Pasquale L, De Luca A, Andriulli A, Perri F, Rega C, De Chiara G, et al. Antibody response to Helicobacter pylori CagA and heat-shock proteins in determining the risk of gastric cancer development. Dig Liver Dis. 2000;32:378–383. doi: 10.1016/s1590-8658(00)80256-x. [DOI] [PubMed] [Google Scholar]
- 51.Enroth H, Kraaz W, Engstrand L, Nyrén O, Rohan T. Helicobacter pylori strain types and risk of gastric cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:981–985. [PubMed] [Google Scholar]
- 52.Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996;2:1679–1684. [PubMed] [Google Scholar]
- 53.Huang SP, Wu MS, Shun CT, Wang HP, Lin JT. Tumor angiogenesis increases with nuclear p53 accumulation in gastric carcinoma. Hepatogastroenterology. 2002;49:1453–1456. [PubMed] [Google Scholar]
- 54.Ławniczak M, Starzyńska T. [Helicobacter pylori CagA(+) infection in gastric cancer patients] Pol Merkur Lekarski. 2002;13:216–220. [PubMed] [Google Scholar]
- 55.Glupczynski Y, Burette A, Goossens H, DePrez C, Butzler JP. Effect of antimicrobial therapy on the specific serological response to Helicobacter pylori infection. Eur J Clin Microbiol Infect Dis. 1992;11:583–588. doi: 10.1007/BF01961663. [DOI] [PubMed] [Google Scholar]
- 56.Shiao YH, Rugge M, Correa P, Lehmann HP, Scheer WD. p53 alteration in gastric precancerous lesions. Am J Pathol. 1994;144:511–517. [PMC free article] [PubMed] [Google Scholar]


