Abstract
AIM: To examine the existence of Nitric oxide/cGMP sensitive store-operated Ca2+ entry in mouse fibroblast NIH/3T3 cells and its influence on matrix metalloproteinase (MMP) production and adhesion ability of fibroblasts.
METHODS: NIH/3T3 cells were cultured. Confocal laser scanning microscopy was used to examine the existence of thapsigargin-induced store-operated Ca2+ entry in fibroblasts. Gelatin zymography and semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) were employed to detect the involvement of [Ca2+]i and NO/cGMP in MMP secretion. The involvement of NO/cGMP-sensitive Ca2+ entry in adhesion was determined using matrigel-coated culture plates.
RESULTS: 8-bromo-cGMP inhibited the thapsigargin-induced Ca2+ entry in 3T3 cells. The cGMP-induced inhibition was abolished by an inhibitor of protein kinase G, KT5823 (1μmol/L). A similar effect on the Ca2+ entry was observed in 3T3 cells in response to a NO donor, (±)-S-nitroso-N-acetylpenicillamine (SNAP). The inhibitory effect of SNAP on the thapsigargin-induced Ca2+ entry was also observed, indicating NO/cGMP-regulated Ca2+ entry in 3T3 cells. Results of gelatin zymography assay showed that addition of extracellular Ca2+ concentration induced MMP release and activation in a dose-dependent manner. RT-PCR also showed that cGMP and SNAP reduced the production of MMP mRNA in 3T3 cells. Experiments investigating adhesion potentials demonstrated that cGMP and SNAP could upgrade 3T3 cell attachment rate to the matrigel-coated culture plates.
CONCLUSION: NO/cGMP sensitive store-operated Ca2+ entry occurs in fibroblasts, and attenuates their adhesion potentials through its influence on MMP secretion.
Keywords: cGMP, Nitric oxide, Protein kinase G, Store-operated Ca2+ entry, Matrix metalloproteinase, Fibroblast
INTRODUCTION
Members of matrix metalloproteinase (MMP) family have been broadly implicated in both physiological and pathophysiological tissue remodeling[1], and play a key role in tumor invasion and metastasis. Since degradation of extracellular matrix (ECM) components by MMP is critical for tumor cell invasion and metastasis[2,3], MMP-2 degrades type IV collagen, a major component of the basement membrane because of its activity. Recent studies have revealed that tumor cells utilize MMP-2 produced by neighboring stromal cells including fibroblasts rather than by tumor cells themselves[4,5] for tumor progression, invasion, and metastasis, and that tumor cells can stimulate MMP production by stromal cells via soluble factors such as cytokines[6-9] or through cell-cell interaction mediated by cell adhesion molecules such as CD147[10,11].
Studies have proved that stromal cells mainly release MMP around tumor cells, and that calcium is a second intracellular messenger which mediates a wide range of cellular responses. These studies aroused our interest in whether calcium ions are involved in MMP secretion. In non-excitable cells, the influx of Ca2+ is a biphasic process, which consists of an initial transient phase followed by a large and sustained phase. Under Ca2+-free conditions, the response is only a small and transient rise in [Ca2+]i, which should reflect the release of intracellular Ca2+ stores. When extracellular Ca2+ is reintroduced in the absence of the agonist, there is a large rise in [Ca2+]i. Since the depletion of intracellular Ca2+ stores is apparently the single mechanism at work under such a circumstance, it has been hypothesized that the Ca2+ entry is activated by the depletion of Ca2+ stores and this Ca2+ entry is thus called store-operated Ca2+ entry (SOC)[12].
Nitric oxide (NO) is also known to inhibit Ca2+ entry through L-type Ca2+ channels (LTCC) in SMCs via cGMP-dependent mechanisms or via membrane hyperpolarization due to cGMP-dependent activation of Ca2+-dependent K+ channels. Besides the direct effects of NO on Ca2+ entry, it can also activate heme-containing soluble guanylyl cyclase (sGC) and catalyze the production of cGMP from GTP, thus playing a role in NO/cGMP/protein kinase G (PKG) pathway. Although many reports have described the regulating mechanisms on the calcium channels, they all focused on the end result-- increase in [Ca2+]i. Thus further investigation is certainly required to clarify the role of NO/cGMP in SOC and MMP secretion.
The aim of the present study was to demonstrate the existence of NO/cGMP-regulated SOC in NIH/3T3 cells and to further explore the influence of store-operated Ca2+ entry on MMP secretion and adhesion potential in NIH/3T3 cells.
MATERIALS AND METHODS
Cell culture and reagents
NIH/3T3 cells (CRL-1658, obtained from American Tissue Culture Collection, ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 100 mL/L fetal calf serum. Cultures were maintained at 37°C in a humidified incubator under an atmosphere of 950 mL/L air and 50 mL/L CO2. 8-bromo-cGMP, (±)-S-nitroso-N-acetylpenicillamine (SNAP), N-methyl-(8R,9S,11S)-(-)-9-methoxy-9-methoxycarbonyl-8-methyl-3,10-dihydro-8-11-epoxy-1H, 8H, 11H-2.7b, 11a-triazadibenzo(a,g)cycloocta(cde) trinden-1-one (KT5823), NG-nitro-L-arginine methyl ester (L-NAME) and thapsigargin were obtained from Calbiochem (La Jolla, CA). DMEM and fetal bovine serum were purchased from Life Technologies, Inc. Fluo3/AM was obtained from Molecular Probes, Inc. (Eugene, OR). One Step RNA PCR kit (AMV) was purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. MMP-2 primers (Accession no. NM008610; 5’ACCATCGAGACCATGCGG3’, 5’CTCCCCCAACACCAGTGC3’, 334 bp), MMP-9 primers (Accession no. NM013599; 5’TTCTGCCCTACCCGAGTGGA3’, 5’CATAGTGGGAGGTGCTGTCGG3’, 426 bp), β-actin primers (5’CTCACTGTCCACCTTCCAG3’, 5’CGACCATCCTCCTCTTAGG3’, 494 bp) were obtained from SaiBaiSheng Co. Trizol for total RNA isolation was from Life Technologies, Inc. Matrigel was obtained from Becton Dickinson Laboratory (Bedford, MA). Other reagents were from Sigma-Aldrich (St. Louis, MO).
Measurement of [Ca2+]i in single cells by confocal laser scanning microscopy
After an overnight attachment, the cells were rinsed in 0.01 mol/L PBS and loaded with 5 μmol/L Fluo3/AM for 45 min in dark at 37°C in normal PBS (NPBS) containing 2 mmol/L CaCl2, pH 7.4. The cells were then washed and resuspended in NPBS. To start the experiment, the cells were pretreated with 4 μmol/L thapsigargin for 2 min. Then the cells were washed with and maintained briefly in PBS containing no Ca2+ and 2 mmol/L EGTA. Unless stated otherwise, the cells were pretreated with or without chemicals (i.e. 8-bromo-cGMP, KT5823, SNAP, L-NAME or NiCl2) for 1-2 min. The fluorescent signal of cytoplasmic calcium ion concentration in 3T3 cells was determined by fluorescence using Bio-Rad MRC 1024 visible light CLSM (Bio-Rad, Hercules, CA) and argon (488/526 nm) laser light.
Gelatin zymography
The conditioned media were collected by centrifugation, concentrated and dialyzed. The dialyzed samples containing an equal amount (20 μg) of total protein were mixed with the sample buffer, incubated in water bath (about 55°C) for 3-5 min and loaded onto the zymographic 100 g/L SDS-polyacrylamide gel containing gelatin (1g/L) and run under standard conditions (2 h at constant voltage of 100V). Afterwards, the gels were washed once with 50 mmol/L Tris (pH 7.4), containing 25 mL/L Triton X-100 for 30 min, and twice with 50 mmol/L Tris (pH 7.4). Gels were then incubated for 16-18 h in 50 mmol/L Tris (pH 7.5), 0.15 NaCl, 10 mmol/L CaCl2, 1 mL/L Triton X-100 and 0.2 mg/L sodium azide. Finally, the gels were stained with Coomassie blue and washed with 75 mL/L acetic acid containing 200 mL/L methanol. The gels were subjected to densitometric analysis to quantitate the gelatinase activity by obtaining volumograms on a photo documentation system from UVItec (Cambridge, UK) using the UVIchrom acquisition software.
Semiquantitative reverse transcriptase-polymerase chain reaction
Total RNA was extracted from cGMP-pretreated 3T3 cells using Trizol agents. First-strand cDNA synthesis was performed using 1 μg of total RNA, 40 MU/L of RNase inhibitor, 20 MU/L sense and anti-sense primers of MMP-2, MMP-9 and β-actin, 10 mmol/L dNTP mixture, 25 mmol/L MgCl2, 5 MU/L AMV Rtase XL, 5 MU/L AMV-optimized Taq. MMP-2, MMP-9 and β-actin primers were reported in Material section. According to One Step RNA PCR kit (AMV) instructions, the following conditions were used for MMP-2: 28 cycles of PCR amplification, denaturation at 94°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 1 min; MMP-9: 30 cycles of PCR amplification, denaturation at 94°C for 50 s, annealing at 60°C for 1 min, and extension at 72°C for 2 min; β-actin: 23 cycles of PCR amplification, denaturation at 94°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 1 min. Amplification was carried out in a GeneAmp 2400 PCR system (Perkins-Elmer, Foster City, CA). PCR products were resolved on 25 g/L agarose gel in the presence of 0.6 mg/L ethidium bromide. The intensities of the cDNA bands for each protein were normalized to β-actin band intensities. Images of the ethidium bromide (EB)-stained agarose gels were acquired with a digital Kodak camera (Eastman Kodak Company, Rochester, NY, USA) and quantification of the bands was performed by imaging analysis software from Kodak.
Cell adhesion assay
The wells of 96-well culture plates were coated with matrigel at a concentration of 5 mg/L and incubated at 4°C overnight. The coated wells were blocked with PBS containing 20 g/L bovine serum albumin for 30 min and then washed with PBS. Cell suspension in serum-free medium containing 1 g/L bovine serum albumin was added to the wells (2 × 104/well) and incubated at 37°C in 50 mL/L CO2 for 30-60 min with or without test agents (8-bromo-cGMP or SNAP). After the medium and nonattached cells were removed, 2 g/L crystal violet was added for 10 min. The plate was gently washed with tap water and dried in air for 24 h. Then 0.1mL of 50 g/L SDS with 500 mL/L ethanol was added for 20 min, the plate was read at 540 nm on an ELISA reader (Microplate Co., EL311SX).
Statistical analysis
Data were expressed as mean ± SD. Intracellular Ca2+ fluorescence ratio and percentages of attached cells were estimated with Student′s t-test or analysis of variance followed by Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
RESULTS
Thapsigargin-induced store-operated Ca2+ entry in fibroblast NIH/3T3 cells
Thapsigargin was used to deplete intracellular Ca2+ stores and induce Ca2+ entry from extracellular space. After treatment with 4 μmol/L thapsigargin in Ca2+-free and 2 mmol/L EGTA-containing medium for 2 min, the addition of 2 mmol/L CaCl2 induced a rise in [Ca2+]i about 120 later (Figure 1). The thapsigargin-induced elevation of [Ca2+]i was completely blocked by Ni2+ (3 mmol/L), a potent blocker of Ca2+ entry that competes for Ca2+-binding sites in 3T3 cells (Figure 2), confirming the presence of capacitive Ca2+ entry in this cell type.
Figure 1.
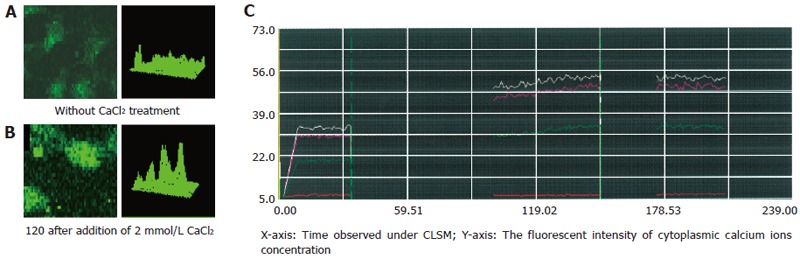
Tg-induced intracellular calcium ion concentration changes in 3T3 cells. A: The fluorescent intensity in 3T3 cells was 25-30 in Ca2+-free and 2 mmol/L EGTA-containing medium after treatment with 4 μmol/L Tg; B: The fluorescent intensity rose to 50-55 in 3T3 cells after adding 2 mmol/L CaCl2; C: Similar intracellular free Ca2+ concentration dynamic changes in 3T3 cells and Tg-induced elevation of [Ca2+]i in 3T3 cells 120 min after treatment.
Figure 2.
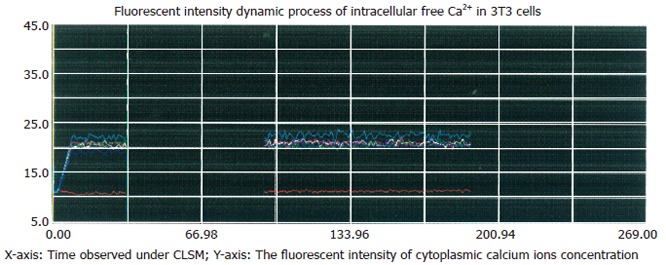
Inhibitory effect of Ni2+ on thapsigargin-induced rise in [Ca2+]i. The cells were incubated in NPBS with 4 μmol/L thapsigargin and 3 mmol/L NiCl2 for 90 s, then the media were replaced with respective media containing 2 mmol/L CaCl2 without EGTA, no significant change of [Ca2+]i occurred after 120 s.
Effect of NO/cGMP/PKG signal pathway on store-operated Ca2+ entry
It has been reported that capacitive Ca2+ entry in hepatoma cells could be inhibited by cGMP, an activator of protein kinase G (PKG), and enhanced by KT5823, an inhibitor of PKG[13-15]. The present study examined the effect of cGMP and KT5823 on the capacitative Ca2+ entry in 3T3 cells. The [Ca2+]i fluorescence ratios were obtained in cells without chemical treatment (control) or treated with 2 mmol/L cGMP or 1 μmol/L KT5823, respectively. Results showed that 2 mmol/L cGMP inhibited the Ca2+ entry with 25.3% ± 1.3% inhibition compared with 3T3 cells not pretreated (53.0% ± 4.4% ) (P < 0.05, Figure 3C). KT5823 (1 μmmol/L) stimulated the Ca2+ entry by 72.3% ± 3.9% (P < 0.05, Figure 3C).
Figure 3.
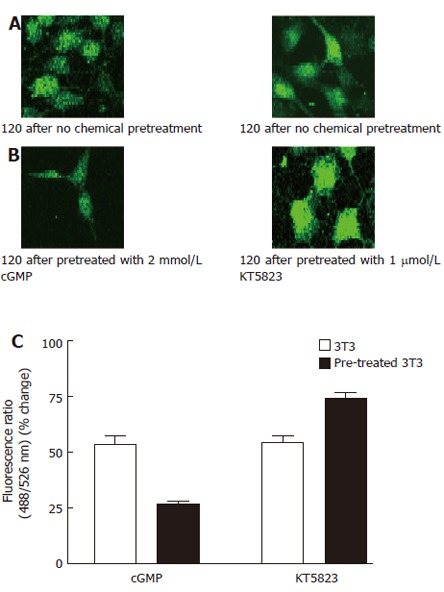
Effect of 8-bromo-cGMP and KT5823 on thapsigargin-induced Ca2+ influx. A: 3T3 cells were incubated in NPBS with 4 μmol/L thapsigargin for 2 min, then 2 mmol/L CaCl2 was added into the medium; B: 3T3 cells were incubated in NPBS with 4 μmol/L thapsigargin for 2 min, 8-bromo-cGMP(2 mmol/L) or KT5823 (1 μmol/L) was introduced 1 min prior to the measurement, then 2 mmol/L CaCl2 was added into the medium; C: Effects of cGMP and KT5823 on thapsigargin-induced Ca2+ influx.. The Ca2+ entry fluorescence ratios were obtained in cells without chemical treatment (control) or treated with 2 mmol/L 8-Br-cGMP (cGMP) or 1 μmol/L KT5823. The cells were incubated in NPBS with 4 μmol/L thapsigargin for 2 min prior to the chemical treatment. One mmol/L CaCl2 was introduced to the medium to obtain the fluorescent signal of Ca2+ entry. The values are the mean ± SE (n = 4-6).
NO is an important intracellular signal molecule that activates soluble guanylye cyclase to synthesize cGMP[16]. The present study was carried out to investigate the involvement of NO in regulation of the capacitive Ca2+ entry using a NO donor (SNAP) and L-NAME (a specific NOS inhibitor) to trigger production of endogenous cGMP. Results showed that SNAP also inhibited the thapsigargin-indued Ca2+ influx, 26.7% ± 6.4% inhibition was observed at 100 μmol/L SNAP compared with control (48.1% ± 5.2% ) (P < 0.05, Figure 4C). L-NAME (200 μmol/L) could excite the thapsigargin-indued Ca2+ influx in 3T3 cells too, 71.6% ± 3.9% stimulation was observed in 3T3 cells compared with control (49.6% ± 5.8%) (P < 0.05, Figure 4C).
Figure 4.
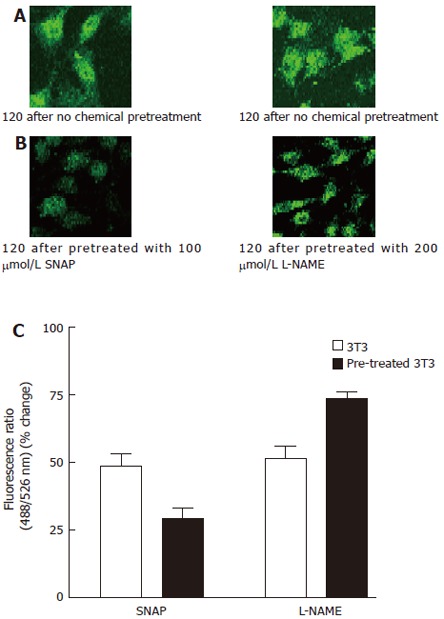
Effect of SNAP and L-NAME on thapsigargin-induced Ca2+ influx. A: 3T3 cells were incubated in NPBS with 4 μmol/L thapsigargin for 2 min, then 2 mmol/L CaCl2 was added into the medium; B: 3T3 cells were incubated in NPBS with 4 μmol/L thapsigargin for 2 min, 100 μmol/L SNAP or 200 μmol/L L-NAME was introduced 1 min prior to the measurement, then 2 mmol/L CaCl2 was added into the medium; C:.Ca2+ entry fluorescence ratios were obtained in cells without chemical treatment (control) or treated with 100 μmol/L SNAP or 200 μmol/L L-NAME. The values are the mean ± SE (n = 4-6).
Effect of SOC on MMP mRNA expression
The expression and release of MMPs (such as MMP-2 and MMP-9) work through kinase signaling pathways[17-19], like NO-cGMP-Ca2+ signaling pathway[20-22]. The present study tested the involvement of SOC in regulating MMP release in vitro. RT-PCR showed that synthesis of MMP-2 and MMP-9 mRNA in cGMP-pretreated 3T3 cells was less than that in 3T3 cells not pretreated. Gelatin zymography showed that production of MMP-2 and MMP-9 in 3T3 medium culture was more than that in cGMP- pretreated 3T3 medium culture (Figure 5).
Figure 5.
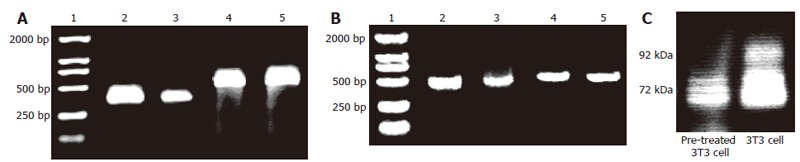
Expression of MMP in pretreated and not pretreated 3T3 cells. A: RT-PCR results of MMP-2 mRNA in both cells (1: DL2000 marker; 2: MMP-2 cDNA of 3T3 cells; 3: MMP-2 cDNA of 8-Br-cGMP (2 mmol/L) pre-treated 3T3 cells; 4: β-actin cDNA of 3T3 cells; 5: β-actin cDNA of pre-treated 3T3 cells); B: RT-PCR results of MMP-9 mRNA in pretreated and not pretreated 3T3 cells (1: DL2000 marker; 2: MMP-9 cDNA of 3T3 cells; 3: MMP-9 cDNA of 8-Br-cGMP (2 mM) pre-treated 3T3cells; 4: β-actin cDNA of 3T3 cells; 5: β-actin cDNA of pre-treated 3T3 cells); C: Gelatin zymography showing constitutive secretion of MMP-2 (72 kDa) and MMP-9 (92 kDa) into serum-free media by pretreated and not pretreated 3T3 cells.
Involvement of [Ca2+]i and NO/cGMP in MMP secretion
Zymography results showed that the increasing extracellular calcium concentrations (0, 0.09, 0.4, 0.8, 1.2 mmo/L) enhanced MMP-2 and MMP-9 secretion in a dose-dependent manner (Figure 6). In the present study, 4 μmol/L thapsigargin was used to deplete the intracellular Ca2+ store in order to induce Ca2+ entry. Zymography results showed pretreatment with 8-bromo-cGMP (2 mmol/L) or SNAP (200 μmol/L) significantly reduced the production of MMP-2 and MMP-9 in 3T3 cells, and 1 μmol/L KT5823 or 100 μmol/L L-NAME enhanced the production of MMP-2 and MMP-9 in 3T3 cells. These results indicated that NO/cGMP sensitive SOC was involved in MMP secretion of fibroblasts (Figure 7).
Figure 6.
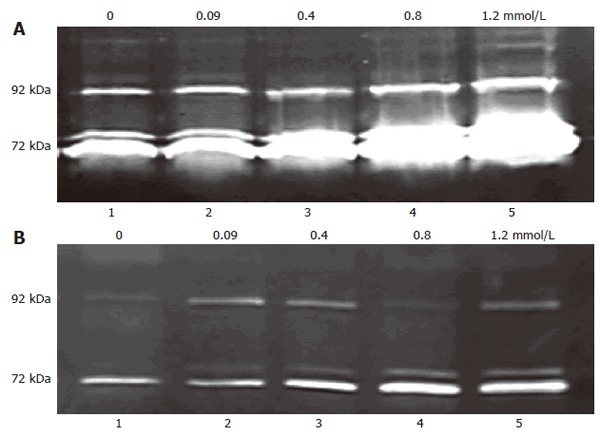
Dose-dependent effect of extracellular Ca2+ on MMP release. 8-bromo-cGMP (2 mmol/L) pre-treated and not pretreated 3T3 cells were plated in media with serial doses of Ca2+ (0, 0.09 ,0.4, 0.8, 1.2 mmol/L) and incubated for 16-18 h, 200 μL conditioned media was used as sample. The 72 kDa gelatinolytic bands corresponded to the MMP-2, the 92 kDa band to MMP-9. A: 3T3 cell mean ratio of 72 kDa in groups 1-5 was 0.198 ± 0.094, 0.287 ± 0.114, 0.486 ± 0.079, 1.247 ± 0.089 and 2.487 ± 0.179, respectively (n = 4-6); B: 8-bromo-cGMP (2 mmol/L) pre-treated 3T3 cell mean ratio of 72 kDa in groups 1-5 was 0.104 ± 0.084, 0.126 ± 0.086, 0.257 ± 0.102, 0.490 ± 0.173 and 0.784 ± 0.187, respectively (n = 4-6).
Figure 7.
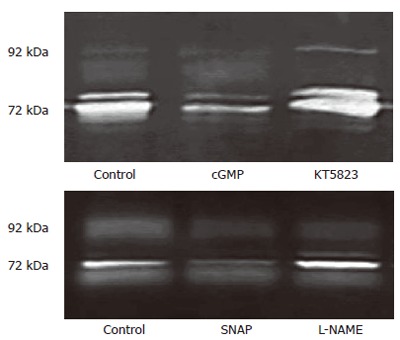
cGMP/KT5823 and SNAP/L-NAME affecting MMP release 3T3 cells via Tg-induced SOC. 3T3 cells were incubated in NPBS with 4 μmol/L thapsigargin for 2 min. Each kind of chemical reagents (2 mmol/L cGMP, 1 μmol/L KT5823, 100 μmol/L SNAP or 200 µmol/L L-NAME) was introduced 1 min prior to the measurement, then 2 mmol/L CaCl2 was added into each medium and incubated for 16-18 h. The 72 kDa gelatinolytic band (MMP-2) was obtained in cells without chemical treatment (control) or treated with cGMP, KT5823, SNAP, L-NAME.
Involvement of NO/cGMP-sensitive Ca2+ entry in adhesion
We used matrigel-coated plates as models for investigation of possible signaling pathways involved in extracellular matrix-induced metastasis. Matrigel is a soluble basement membrane preparation containing almost all extracellular matrix components[23]. The present study was carried out with matrigel as an adhesion substratum. A significant difference in the amount of cells attached to the matrigel-coated plates was observed in 3T3 cells pre-treated and not pre-treated with cGMP (2 mmol/L) for 30 min (71.4% ± 0.5% vs 44.7% ± 2.5%) (P < 0.05). Pre-treatment with SNAP (200 μmol/L) showed that the percent of attached and non-attached 3T3 cells was 64.7% ± 1.2% and 46.3% ± 2.0%, respectively (P < 0.05, Figure 8).
Figure 8.
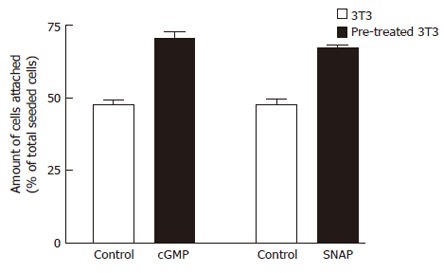
Effects of cGMP or SNAP on adhesion potentials of 3T3 cells to matrigel by adhesion assay. 3T3 cells pretreated with 4 μmol/L Tg were suspended in serum-free medium supplemented with no chemical reagent (control) or cGMP (2 mmol/L) or SNAP (200 μmol/L), 2 mmol/L CaCl2 was added to induce SOC, then seeded into the matrigel (5 mg/L)-coated wells. After incubation for 30 min at 37°C, the percentage of adhered cells was determined using colorimetric crystal violet assay. The values are the mean ± SE (n = 6-8).
DISCUSSION
Collagenolytic degradation of endothelial and parenchymal basement membranes is a necessary step in the process of tumor invasion and angiogenesis. Neutral MMP-2 and MMP-9 mainly secreted locally by stromal cells, degrade the basement membrane and extracellular matrix collagens. However, little is known about the signaling pathways that regulate the production of these enzymes. Previous studies have demonstrated that transforming growth factor-β1 (TGF-β1) plays a role in transmembrane signal transduction of MMP-2 regulation[24]. The present study described a role of calcium mobilization in the regulation of MMP expression.
The present study demonstrated that the existence of NO/cGMP-sensitive store-operated Ca2+ entry in mouse fibroblast NIH/3T3 cells, which is allergic to NO/cGMP. The thapsigargin-induced SOC appears to be evidenced by the magnitude of the Ca2+ entry and its blockage by Ni2+, cGMP or SNAP inhibits the thapsigargin-induced Ca2+ entry in 3T3 cells and can be reversed by KT5823 (highly specific PKG inhibitor), indicating a negative regulatory mechanism involving NO/cGMP via a PKG-dependent pathway.
Characterization of the regulation of interstitial collagenase (MMP-1) and stromelysidtransin (MMP-3) indicates that there are at least two known regulatory arms for the secretion of these MMPs. Treatment with TGF-β1 inhibits the production of these MMP genes through TIE, a novel CIS-acting transcriptional element[25,26], whereas phorbol ester-mediated stimulation of transcription is through phorbol ester-responsive elements (TREs) and AP-1 sites[27,28]. Regulation of matrix metalloproteinases has also been linked to the second cAMP messenger signal transduction pathway[29,30]. Several investigators have demonstrated the induction of MMP-1 production in response to cAMP either directly or after cell stimulation with cytokines that signal their effects through cAMP[29,31]. However, in contrast to MMP-1 and MMP-3, neither MMP-2 nor MMP-9 has TIE or cAMP-regulated elements defined in their 5’ sequences[27,32]. The TRE mechanism has been implicated in the regulation of MMP-9 but not MMP-2[27]. AP-1 sites have not been documented for MMP-2, although AP-1 and AP-2 sites are in the upstream of MMP-9[27,32,33]. It was reported that there is a NF-κB binding site in the upstream sequence of MMP-9[34]. Besides the factors influencing MMP secretion, calcium-activated signal transduction steps are also a common thread underlying important signal transduction pathways, including protein kinase C, phosphoinositide metabolism, and generation of arachidonic acid. However, few calcium-mediated transcriptional pathways have been identified for MMP secretion.
The present study was to identify NO/cGMP-sensitive production and activation of MMP through SOC. In our study, adding extracellular Ca2+ concentration induced release and activation of MMP (MMP-2, MMP-9) in a dose-dependent manner, cGMP and SNAP reduced MMP secretion in 3T3 cells. RT-PCR displayed that MMP-2 and MMP-9 mRNA synthesis in cGMP- pretreated 3T3 cells was significantly less than that in non cGMP-pretreated 3T3 cells. Tumor invasion is greatly dependent on the permissive action of the microenvironment. One critical factor is the production of proteolytic enzymes involved in the degradation and remodeling of ECM. Among these enzymes, MMPs represent a large family playing a key role in cell proliferation, angiogenesis, tumor invasion and metastasis[6]. These enzymes principally degrade the ECM components and attenuate cell-cell adhesion ability so as to promote tumor invasion and metastasis.
Our experiments investigating adhesion potentials demonstrated that the attachment rate of 3T3 cells living in high extracellular Ca2+ concentration of the matrigel-coated culture plates was lower than that in low extracellular Ca2+ concentration of the matrigel-coated culture plates, suggesting that increased cellular Ca2+ concentration attenuates the adhesion potentials in fibroblasts by enhancing MMP secretion. The attachment rate could be upgraded by cGMP and SNAP in 3T3 cells, indicating that interference with NO/cGMP/PKG pathway sensitivity of fibroblasts reduces SOC and inhibits MMP secretion at a considerable level, thus strengthening the adhesion potential of fibroblasts and decreasing the chance of HCC metastasis.
In conclusion, store-operated Ca2+ channels exist in mouse fibroblast cells and are involved in MMP secretion. NO/cGMP/PKG pathway negatively regulates this Ca2+ entry. Increased extracellular Ca2+ concentration may promote degradation of ECM components by increasing MMP secretion and attenuating fibroblast adhesion potential.
Footnotes
Supported by the Major State Basic Research Development Program (973 Program) of China, No.2003CB515507
S- Editor Pan BR L- Editor Wang XL E- Editor Liu WF
References
- 1.Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 2.Gauthier MC, Racine C, Ferland C, Flamand N, Chakir J, Tremblay GM, Laviolette M. Expression of membrane type-4 matrix metalloproteinase (metalloproteinase-17) by human eosinophils. Int J Biochem Cell Biol. 2003;35:1667–1673. doi: 10.1016/s1357-2725(03)00136-5. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo Y, Hashimoto S, Koga T, Yonemitsu Y, Yoshino I, Sugimachi K, Honda H, Masuda K, Sueishi K. Growth pattern correlates with the distribution of basement membrane and prognosis in lung adenocarcinoma. Pathol Res Pract. 2004;200:517–529. doi: 10.1016/j.prp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Kim JR, Kim CH. Association of a high activity of matrix metalloproteinase-9 to low levels of tissue inhibitors of metalloproteinase-1 and -3 in human hepatitis B-viral hepatoma cells. Int J Biochem Cell Biol. 2004;36:2293–2306. doi: 10.1016/j.biocel.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995;55:3263–3266. [PubMed] [Google Scholar]
- 6.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Lohi J, Lehti K, Westermarck J, Kähäri VM, Keski-Oja J. Regulation of membrane-type matrix metalloproteinase-1 expression by growth factors and phorbol 12-myristate 13-acetate. Eur J Biochem. 1996;239:239–247. doi: 10.1111/j.1432-1033.1996.0239u.x. [DOI] [PubMed] [Google Scholar]
- 8.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA. 1995;92:2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simi L, Andreani M, Davini F, Janni A, Pazzagli M, Serio M, Orlando C. Simultaneous measurement of MMP9 and TIMP1 mRNA in human non small cell lung cancers by multiplex real time RT-PCR. Lung Cancer. 2004;45:171–179. doi: 10.1016/j.lungcan.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Sato M, Senoo H, Ishikawa K. Direct cell-cell interaction enhances pro-MMP-2 production and activation in co-culture of laryngeal cancer cells and fibroblasts: involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 2004;293:259–266. doi: 10.1016/j.yexcr.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Koono M, Wakisaka S. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2000;157:177–184. doi: 10.1016/s0304-3835(00)00485-7. [DOI] [PubMed] [Google Scholar]
- 12.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 13.Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN, Chan HC. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem. 2001;276:46870–46877. doi: 10.1074/jbc.M108291200. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Star RA, Tortorici G, Muallem S. Depletion of intracellular Ca2+ stores activates nitric-oxide synthase to generate cGMP and regulate Ca2+ influx. J Biol Chem. 1994;269:12645–12653. [PubMed] [Google Scholar]
- 15.Yao X, Kwan HY, Chan FL, Chan NW, Huang Y. A protein kinase G-sensitive channel mediates flow-induced Ca(2+) entry into vascular endothelial cells. FASEB J. 2000;14:932–938. doi: 10.1096/fasebj.14.7.932. [DOI] [PubMed] [Google Scholar]
- 16.Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee SD, Wu CC, Chang YC, Chang SH, Wu CH, Wu JP, Hwang JM, Kuo WW, Liu JY, Huang CY. Porphyromonas gingivalis-induced cellular hypertrophy and MMP-9 activity via different signaling pathways in H9c2 cardiomyoblast cells. J Periodontol. 2006;77:684–691. doi: 10.1902/jop.2006.050070. [DOI] [PubMed] [Google Scholar]
- 18.Lim M, Martinez T, Jablons D, Cameron R, Guo H, Toole B, Li JD, Basbaum C. Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett. 1998;441:88–92. doi: 10.1016/s0014-5793(98)01474-4. [DOI] [PubMed] [Google Scholar]
- 19.Tang W, Hemler ME. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J Biol Chem. 2004;279:11112–11118. doi: 10.1074/jbc.M312947200. [DOI] [PubMed] [Google Scholar]
- 20.Eto M, Bock R, Brautigan DL, Linden DJ. Cerebellar long-term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron. 2002;36:1145–1158. doi: 10.1016/s0896-6273(02)01107-8. [DOI] [PubMed] [Google Scholar]
- 21.Goette A, Lendeckel U, Klein HU. Signal transduction systems and atrial fibrillation. Cardiovasc Res. 2002;54:247–258. doi: 10.1016/s0008-6363(01)00521-1. [DOI] [PubMed] [Google Scholar]
- 22.Jurasz P, Sawicki G, Duszyk M, Sawicka J, Miranda C, Mayers I, Radomski MW. Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Res. 2001;61:376–382. [PubMed] [Google Scholar]
- 23.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 24.Saed GM, Zhang W, Diamond MP. Effect of hypoxia on stimulatory effect of TGF-beta 1 on MMP-2 and MMP-9 activities in mouse fibroblasts. J Soc Gynecol Investig. 2000;7:348–354. [PubMed] [Google Scholar]
- 25.Edwards DR, Leco KJ, Beaudry PP, Atadja PW, Veillette C, Riabowol KT. Differential effects of transforming growth factor-beta 1 on the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in young and old human fibroblasts. Exp Gerontol. 1996;31:207–223. doi: 10.1016/0531-5565(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 26.White LA, Mitchell TI, Brinckerhoff CE. Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochim Biophys Acta. 2000;1490:259–268. doi: 10.1016/s0167-4781(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 27.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about. Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 28.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 29.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 30.Duca L, Debelle L, Debret R, Antonicelli F, Hornebeck W, Haye B. The elastin peptides-mediated induction of pro-collagenase-1 production by human fibroblasts involves activation of MEK/ERK pathway via PKA- and PI(3)K-dependent signaling. FEBS Lett. 2002;524:193–198. doi: 10.1016/s0014-5793(02)03057-0. [DOI] [PubMed] [Google Scholar]
- 31.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 32.McCawley LJ, Li S, Benavidez M, Halbleib J, Wattenberg EV, Hudson LG. Elevation of intracellular cAMP inhibits growth factor-mediated matrix metalloproteinase-9 induction and keratinocyte migration. Mol Pharmacol. 2000;58:145–151. doi: 10.1124/mol.58.1.145. [DOI] [PubMed] [Google Scholar]
- 33.Pan MR, Chuang LY, Hung WC. Non-steroidal anti-inflammatory drugs inhibit matrix metalloproteinase-2 expression via repression of transcription in lung cancer cells. FEBS Lett. 2001;508:365–368. doi: 10.1016/s0014-5793(01)03118-0. [DOI] [PubMed] [Google Scholar]
- 34.Takahra T, Smart DE, Oakley F, Mann DA. Induction of myofibroblast MMP-9 transcription in three-dimensional collagen I gel cultures: regulation by NF-kappaB, AP-1 and Sp1. Int J Biochem Cell Biol. 2004;36:353–363. doi: 10.1016/s1357-2725(03)00260-7. [DOI] [PubMed] [Google Scholar]


