Abstract
AIM: To examine the expression of leptin and its receptor, OB-R, in normal gastric mucosa and neoplasia.
METHODS: By immunohistochemical staining using specific antibodies, we evaluated the expression of leptin and OB-R in 207 gastric carcinomas (100 early and 107 advanced carcinomas) and analyzed their relationship with clinicopathological features.
RESULTS: Both normal gastric epithelium and carcinoma cells expressed a significant level of leptin. In cases with OB-R staining, carcinoma cells showed OB-R-positive expression, but the intensity was weaker than that in normal mucosa. The expression of OB-R showed a significant correlation with the level of leptin expression. The expression levels of both leptin and OB-R tended to increase as the depth of tumor invasion or TMN stage increased (P < 0.01). Lymph node metastasis was detected in 49.5% (47/95) of leptin-strong cases and in 50.5% (48/95) of OB-R-positive cases, and the rate was 33% (37/112) in leptin-weak cases and 17% (19/112) in OB-R-negative cases. Both venous and lymphatic invasion also tended to be observed frequently in positive tumors as compared with negative tumors. Interestingly, in the 96 leptin- or OB-R-positive tumors, hematogenous metastasis was detected preoperatively in 3 (3.1%) patients. In contrast, none of the carcinomas that lacked expression of leptin and OB-R showed hematogenous metastasis.
CONCLUSION: Overexpression of leptin and expression of OB-R may play a positive role in the process of progression in gastric cancer. Functional upregulation of leptin/OB-R may have a positive role in the development and initial phase of progression in gastric cancer.
Keywords: Leptin, OB-R, Gastric cancer
INTRODUCTION
Previous studies have shown a positive association between adiposity and increased risk of cancers of the endometrium, kidney, gallbladder (in women), breast (in postmenopausal women), and colon (particularly in men)[1-9]. Consistently, a recent large scale cohort study demonstrated that increased body weight is associated with increased mortality for all cancers combined, as well as for cancers at various specific sites[10].
The adipocyte-derived cytokine, leptin, is thought to play a key role in the control of satiety, energy expenditure, and various reproductive processes[11-13]. Leptin is a peptide hormone composed of 167 amino acids with a molecular mass of 16 kDa[14]. Generally, plasma leptin level is representative of body fat mass[15-18] and increases in a logarithmic fashion with an increase in body mass in mice[19]. Leptin controls body mass and metabolism by affecting the metabolic, neuroendocrine, reproductive and hematopoietic systems[20]. In cancer, there is regulatory dysfunction in metabolic, neuroendocrine and other systems. Although initially thought to be exclusively expressed in and secreted by adipocytes, recent studies have shown that leptin expression can be detected in a number of additional tissues, including the placenta[21], gastric mucosa[22], pituitary cells[23] and hepatic stellate cells[24]. More importantly, leptin has been shown to regulate cell proliferation in diverse normal and malignant tissues and to stimulate the proliferation of certain normal hematopoietic and epithelial cells. Leptin has also been shown to promote the invasiveness of premalignant colon and kidney epithelial cells in vitro[25]. These findings suggest the possibility that leptin may be critically involved in the promotion of cancer.
Leptin exerts its action through the leptin receptor (OB-R), a member of the cytokine family of receptors, which was also detected in both rat and human gastric mucosa. Further studies, however, have shown that leptin receptors are expressed in many other tissues, including the brain, placenta, pancreas, adrenal gland, hematopoietic cells, liver, lung and heart[21,24,26-28]. In addition, OB-R has been identified in malignant cells, including lung and gastric carcinoma and leukemic cells[22,29-32].
In this study, therefore, we used antibodies to leptin and OB-R, and immunohistochemically characterized the expression pattern of these two proteins in gastric carcinoma and evaluated the possible role of leptin in the tumorigenesis and spread of gastric cancer.
MATERIALS AND METHODS
Two hundred and seven carcinomas, which were surgically resected in the Department of Surgery, The University of Tokyo, from 1991 to 2002, were included in this study. In all cases, serial-step sections 3-mm wide were cut, fixed in 10% formalin solution, and then embedded in paraffin. All the resected primary tumors and regional lymph nodes were histologically examined by hematoxylin-eosin staining according to the Japanese Classification of Gastric Carcinoma[33]. Tumors were histologically classified into two types based on the predominant features: differentiated type (well and moderately differentiated adenocarcinoma) and undifferentiated type (poorly differentiated adenocarcinoma and signet ring cell carcinoma). In addition, we examined several discrete histological parameters, including lymphatic invasion, venous invasion and lymph node metastasis.
Immunohistochemical study of leptin and OB-R
We investigated the expression of OB-R and leptin with immunohistochemical staining using affinity purified rabbit polyclonal antibodies against leptin (Santa Cruz, Biotech, CA, USA) and goat polyclonal antibodies against OB-R (M-18, Santa Cruz Biotech)[30,32], respectively. Sections (3-μm thick) were deparaffinized in xylene, hydrated through a graded series of ethanol, and heated in a microwave oven for two 7-min cycles (500 W). After being rinsed in phosphate buffered saline (PBS), endogenous peroxidase activity was inhibited by incubation with 0.3% hydrogen peroxide in 100% methanol for 30 min. After 3 washes in PBS, nonspecific reaction was blocked by incubation with PBS containing 5% skimmed milk for 30 min at room temperature, and then the sections were incubated with normal rabbit or goat serum for 30 min. The sections were incubated overnight at 4°C in humid chambers with the primary antibody to leptin at 1/70 dilution or the primary antibody to OB-R at 1/100. After three washes with PBS, the sections were incubated with biotinylated rabbit anti-goat or anti-rabbit immunoglobulin for 30 min. After washing again with PBS, the slides were treated with peroxidase-conjugated streptavidin for 30 min, and developed by immersion in 0.01% H2O2 and 0.05% diaminobenzidine tetrahydrochloride for 3 min. Light counterstaining with Mayer’s hematoxylin was performed.
Statistical analysis
All statistical calculations were carried out using Stat View-J 5.0 statistical software (SAS Institute, USA). Student’s t-test and Wilcoxon’s test were used to analyze data. Differences with a P value of less than 0.05 were considered to be statistically significant.
RESULTS
Immunohistochemical detection of leptin and OB-R in normal mucosa and carcinoma
In all cases, the lower part of the fundic glands in the normal part of the mucosa expressed a significant level of leptin, suggesting that leptin is mainly produced in chief and parietal cells (Figure 1A). Leptin could be detected in the cytoplasm as well as the cell membrane, but not in the nucleus. However, the surface epithelium of normal gastric mucosa totally lacked expression of leptin. This staining pattern was similar to that described in the gastric epithelium in a previous report[22].
Figure 1.
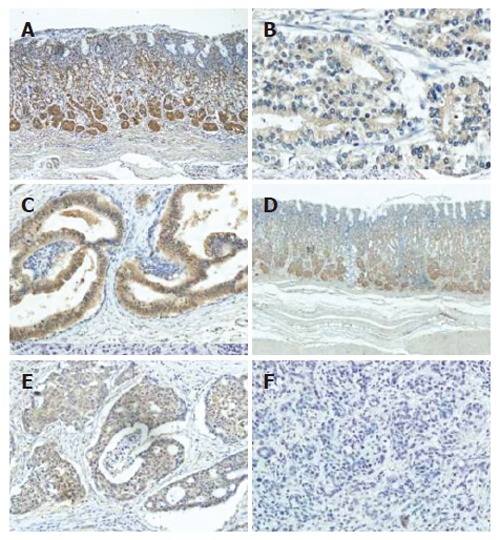
Immunohistochemical staining of leptin and OB-R in normal gastric mucosa and cancer. A: Leptin in normal mucosa; B: Leptin weak type in gastric cancer; C: leptin strong type in gastric cancer; D: OB-R in normal mucosa; E: OB-R positive type in gastric cancer; F: OB-R negative type in gastric cancer.
Gastric carcinoma cells mostly showed positive immunoreactivity, although the staining intensity varied among the samples. According to the staining pattern, tumors were subdivided into two groups. When investigators agreed that the staining intensity of carcinoma cells was significantly weaker than that of chief and parietal cells in corresponding normal mucosa, those tumors were categorized as having weak expression (Figure 1B). In contrast, when carcinoma cells stained to a similar degree or more strongly than normal gastric mucosa, those tumors were categorized as having strong expression (Figure 1C).
OB-R was also detected in normal mucosa, and the immunostaining pattern was mostly consistent with that of leptin staining (Figure 1D). In cancer tissue, however, some carcinoma cells showed significant expression while others were mostly negative for OB-R. In tumors positive for OB-R, most of the carcinoma cells were equally stained and heterogeneity was rarely observed in each sample (Figure 1E), while in negative tumors a few carcinoma cells were stained only faintly (Figure 1F).
The relationship between the expression patterns of leptin and OB-R is presented in Table 1. Among 74 carcinomas with strong expression of leptin, 45 (60.8%) also expressed OB-R strongly, while only 29 carcinomas (39.2%) lacked expression of OB-R. In the 133 carcinomas with weak leptin expression, 111 (79.3%) also lacked expression of leptin OB-R. Hence, the expression of leptin and OB-R was significantly correlated in gastric cancer (P < 0.001).
Table 1.
Relationship between expression of Ob-R and leptin
|
Ob-R expression |
|||
| Positive (67) | Negative (140) | P | |
| Leptin expression | |||
| Strong (74) | 45 | 29 | |
| Weak (133) | 22 | 111 | < 0.001 |
Clinicopathological features and relation to expression of leptin and OB-R
Table 2 shows the correlation between leptin and OB-R expression and clinicopathological data in the 207 carcinomas cases. No significant difference was observed in age, preoperative tumor markers or tumor size between the positive and negative groups. Interestingly, positive expression of leptin was found in 64 of 155 male patients (41.3%) versus 10 of 52 female patients (19.2%), and the difference was statistically significant (P < 0.05). The relationship of BMI with leptin expression in the two groups did not show a significant association. The stomach is anatomically divided into three portions; upper (U), middle (M), lower (L) parts[33]. Interestingly, the percentage of tumors with strong expression of leptin was higher in those located in the upper part (23/51; 45.1%) than in those in the middle (36/94; 38.3%) and lower (15/62; 24.2%) parts, and this was statistically significant (P < 0.05).
Table 2.
Expression of leptin and clinicopathologic characteristics of patients
|
Leptin expression |
OB-R expression |
|||||
| High (74) | Low (133) | P | Positive (67) | Negative (140) | P | |
| Age (yr) | 62.7 ± 8.9 | 61.3 ± 11.2 | 0.98 | 64.2 ± 9.9 | 61.6 ± 10.8 | 0.167 |
| Sex | ||||||
| Male | 64 | 91 | 53 | 102 | ||
| Female | 10 | 42 | 0.003 | 14 | 38 | 0.33 |
| BMI | 22.4 ± 3.1 | 22.7 ± 2.8 | 0.48 | 22.4 ± 3.2 | 22.7 ± 2.8 | 0.49 |
| Tumor markers | ||||||
| CEA | 8.6 ± 17.7 | 14.7 ± 111.3 | 0.65 | 9.9 ± 19.6 | 13.8 ± 108.3 | 0.79 |
| CA19-9 | 89.8 ± 453.5 | 75.4 ± 347.6 | 0.81 | 138.3 ± 561.4 | 53.8 ± 270.8 | 0.16 |
| Size (cm) | 6.0 ± 3.2 | 5.1 ± 3.6 | 0.13 | 5.8 ± 3.2 | 5.2 ± 3.6 | 0.25 |
| Location | ||||||
| Upper part (51) | 23 | 28 | 19 | 32 | ||
| Middle part (94) | 36 | 58 | 29 | 65 | ||
| Lower part (62) | 15 | 47 | 0.03 | 19 | 43 | 0.51 |
| Depth of tumor invasion | ||||||
| T1 | 24 | 76 | 20 | 80 | ||
| T2 | 28 | 29 | 27 | 37 | ||
| T3 | 18 | 28 | 16 | 30 | ||
| T4 | 4 | 0 | 0.001 | 4 | 0 | < 0.001 |
| Macroscopic type | ||||||
| Elevated | 57 | 62 | 48 | 71 | ||
| Depressed/flat | 17 | 71 | < 0.001 | 19 | 69 | 0.005 |
| Histological type | ||||||
| Differentiated | 45 | 43 | 43 | 45 | ||
| Undifferentiated | 29 | 90 | < 0.001 | 24 | 95 | < 0.001 |
| TNM stage | ||||||
| IA | 20 | 67 | 15 | 72 | ||
| IB | 17 | 23 | 15 | 25 | ||
| II | 8 | 12 | 9 | 11 | ||
| IIIA | 10 | 17 | 11 | 16 | ||
| IIIB | 5 | 7 | 7 | 5 | ||
| IV | 14 | 7 | 0.001 | 10 | 11 | < 0.001 |
| Lymphatic invasion | ||||||
| Positive | 39 | 45 | 39 | 46 | ||
| Negative | 34 | 87 | 0.002 | 28 | 94 | < 0.001 |
| Venous invasion | ||||||
| Positive | 46 | 49 | 45 | 50 | ||
| Negative | 28 | 84 | < 0.001 | 22 | 90 | < 0.001 |
| Lymph node metastasis | ||||||
| Positive | 47 | 48 | 48 | 47 | ||
| Negative | 37 | 85 | 0.052 | 19 | 93 | 0.001 |
| Hematogenous metastasis | ||||||
| Positive | 3 | 0 | 2 | 1 | ||
| Negative | 71 | 134 | 0.02 | 65 | 139 | 0.19 |
| Peritoneal dissemination | ||||||
| Positive | 5 | 1 | 2 | 4 | ||
| Negative | 69 | 133 | 0.01 | 65 | 136 | 0.95 |
The expression levels of both leptin and OB-R increased as the depth of tumor invasion or TMN stage increased (P < 0.01). When leptin expression was compared with histological type, 45 of 88 (51.1%) well differentiated carcinomas expressed leptin strongly, while 90 of 119 (75.6%) undifferentiated carcinomas showed weak staining (P < 0.001). Moreover, the percentage of tumors with OB-R-positive expression was significantly higher in differentiated carcinomas (48.9%) than in undifferentiated carcinomas (20.1%) (P < 0.001). Thus, both leptin and OB-R were expressed at a higher level in differentiated carcinomas as compared with undifferentiated carcinomas (P < 0.001).
Venous as well as lymphatic invasion was more frequently observed in tumors with high leptin and positive OB-R expression. Accordingly, lymph node metastasis was detected in 71.6% (48/67) of OB-R-positive cases, which was significantly higher than 31.3% (47/150) of OB-R-negative cases. Interestingly, in the 74 tumors with high leptin expression, hematogenous metastasis was detected preoperatively in 3 (4.1%) patients, and peritoneal dissemination was detected intraoperatively in 5 (6.8%) patients. However, in 133 tumors with low leptin expression, only one case showed peritoneal dissemination and none was associated with hematogenous metastasis (P < 0.05).
DISCUSSION
Leptin is well known to play a major role in the regulation of weight and adiposity. Recently, many studies have shown that increased body weight is associated with increased risk of cancer and cancer-related mortality, suggesting a possible role of leptin in the pathogenesis of cancer. Leptin is reported to be abundantly produced in the stomach[34,35]. In gastric carcinoma, some reports have shown that obesity is one of the main risk factors[36-42]. These findings suggest that leptin may be critically involved in the development and progression of gastric cancer. This idea encouraged us to evaluate the expression of leptin and its receptor in gastric cancer tissues.
In our series, leptin and OB-R were predominantly expressed in chief and parietal cells but not in the surface epithelium in normal parts of the gastric mucosa that were adjacent to cancer tissue, which is mostly consistent with data of previous studies[22,43,44]. However, carcinoma cells showed a variety of staining patterns for leptin or OB-R. Leptin was detected in all carcinoma cells, although the level of expression could be divided into two categories according to the staining intensity, whereas OB-R was detected in some tumors but not in others, and the level of expression of leptin showed a positive correlation with OB-R expression. This suggests the existence of a common regulatory mechanism in the expression of leptin and its receptor in the gastric epithelium.
The main finding in our study was that the expression levels of both leptin and OB-R tended to increase as the depth of tumor invasion or TMN stage increased (P < 0.01). Moreover, nodal and distant metastasis, as well as pathological lymphatic or vascular invasion, was frequently detected in leptin-strong and OB-R-positive tumors as compared with leptin-weak and OB-R-negative tumors. Shuneider et al[43] reported that leptin led to significantly increased proliferation in AGS cells, and the MAP-kinase-1 specific inhibitor U0126 blocked leptin-induced cell proliferation in a dose-dependent manner. Tessitore reported that in colorectal cancer patients, plasma leptin level in stage IV patients was significantly higher than that in stage I patients. In addition to stimulating proliferation, leptin has been shown to promote invasiveness of renal and colonic epithelial cells via PI3-kinase-, rho- and rac-dependent cascades[25]. All these findings support that leptin may have a promoting effect on cancer invasion and metastasis. Our findings were consistent with these results and suggest that leptin and OB-R might function as an autocrine growth factor during the development and progression of gastric cancer.
Another interesting finding was that the expression of leptin/OB-R was correlated with the differentiation of gastric cancer. In our series, cancers of undifferentiated type tended to have weak expression of leptin as well as negative OB-R expression as compared with differentiated cancers. In each type, expression of leptin/OB-R showed a positive association with stage and metastasis (data not shown). This suggests that the different expression of leptin/OB-R was determined at the early stage of carcinogenesis. The carcinogenic pathway of differentiated type carcinoma is considered to begin with H pylori infection, followed by atrophic gastritis and intestinal metaplasia, and inappropriate activation of gut specific transcription factor CDX2 has an important role in the early stage of carcinogenesis. In contrast, dysfunction of E-cadherin is considered to have critical roles in the development of undifferentiated carcinoma. The molecular interaction between leptin/OB-R and CDX2 or E-cadherin is an interesting subject for future research[45].
In conclusion, we confirmed that the expression level of leptin/OB-R showed a positive correlation with the depth of tumor invasion, stage, and metastasis as well as tumor differentiation. Our findings suggest that coexpression of leptin and OB-R may have a positive role in the progression in gastric cancer in an autocrine or paracrine manner. Functional inhibition of leptin/OB-R might effectively suppress the growth and metastasis of gastric cancer.
Footnotes
S- Editor Wang GP L- Editor Schreyer AG E- Editor Ma WH
References
- 1.Fund WCR. Food, nutrition and the prevention of cancer: a global perspective. American Institute for Cancer Research. 1997:371–373. doi: 10.1016/s0899-9007(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 2.Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids. 1998;33:1055–1059. doi: 10.1007/s11745-998-0305-8. [DOI] [PubMed] [Google Scholar]
- 3.Bergström A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421–430. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11 Suppl 2:S94–S100. [PubMed] [Google Scholar]
- 5.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 6.Correa P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981;41:3685–3690. [PubMed] [Google Scholar]
- 7.Carroll KK. Neutral fats and cancer. Cancer Res. 1981;41:3695–3699. [PubMed] [Google Scholar]
- 8.Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med. 1997;216:28–43. doi: 10.3181/00379727-216-44153b. [DOI] [PubMed] [Google Scholar]
- 9.Willett WC. Fat, energy and breast cancer. J Nutr. 1997;127:921S–923S. doi: 10.1093/jn/127.5.921S. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 12.Lindheim SR, Sauer MV, Carmina E, Chang PL, Zimmerman R, Lobo RA. Circulating leptin levels during ovulation induction: relation to adiposity and ovarian morphology. Fertil Steril. 2000;73:493–498. doi: 10.1016/s0015-0282(99)00578-6. [DOI] [PubMed] [Google Scholar]
- 13.Henson MC, Castracane VD. Leptin in pregnancy. Biol Reprod. 2000;63:1219–1228. doi: 10.1095/biolreprod63.5.1219. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 15.Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45:1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 16.Caro JF Leptin: from 1958 to the present. Can J Diabetes Care, 1998: 18-23 [Google Scholar]
- 17.Flier JS, Maratos-Flier E. Obesity and the hypothalamus: novel peptides for new pathways. Cell. 1998;92:437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 18.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- 19.Smith FJ, Rivera I, Tanenbaum R, et al. Weight loss reverses decreased sensitivity to exogenous ob protein (leptin) in diet induced obese mice. Int J Obesity. 1998:41. [Google Scholar]
- 20.Ganong WF. Review of Medical Physiology. 18th ed. New York: Appleton and Lange; 1997. p. 223. [Google Scholar]
- 21.Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci USA. 1997;94:11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Göke M, Beil W, Kuske M, Brabant G, Manns MP, et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47:481–486. doi: 10.1136/gut.47.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isono M, Inoue R, Kamida T, Kobayashi H, Matsuyama J. Significance of leptin expression in invasive potential of pituitary adenomas. Clin Neurol Neurosurg. 2003;105:111–116. doi: 10.1016/s0303-8467(02)00129-4. [DOI] [PubMed] [Google Scholar]
- 24.Briscoe CP, Hanif S, Arch JR, Tadayyon M. Leptin receptor long-form signalling in a human liver cell line. Cytokine. 2001;14:225–229. doi: 10.1006/cyto.2001.0871. [DOI] [PubMed] [Google Scholar]
- 25.Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 26.Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, Snodgrass HR. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2:585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 27.Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 1997;46:313–316. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 28.Glasow A, Haidan A, Hilbers U, Breidert M, Gillespie J, Scherbaum WA, Chrousos GP, Bornstein SR. Expression of Ob receptor in normal human adrenals: differential regulation of adrenocortical and adrenomedullary function by leptin. J Clin Endocrinol Metab. 1998;83:4459–4466. doi: 10.1210/jcem.83.12.5337. [DOI] [PubMed] [Google Scholar]
- 29.Morash B, Johnstone J, Leopold C, Li A, Murphy P, Ur E, Wilkinson M. The regulation of leptin gene expression in the C6 glioblastoma cell line. Mol Cell Endocrinol. 2000;165:97–105. doi: 10.1016/s0303-7207(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol. 1999;365:273–279. doi: 10.1016/s0014-2999(98)00884-x. [DOI] [PubMed] [Google Scholar]
- 31.Hino M, Nakao T, Yamane T, Ohta K, Takubo T, Tatsumi N. Leptin receptor and leukemia. Leuk Lymphoma. 2000;36:457–461. doi: 10.3109/10428190009148392. [DOI] [PubMed] [Google Scholar]
- 32.Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 33.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 34.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 35.Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Duda A, Pierzchalski P, Bielański W, Hahn EG. Leptin in gastroprotection induced by cholecystokinin or by a meal. Role of vagal and sensory nerves and nitric oxide. Eur J Pharmacol. 1999;374:263–276. doi: 10.1016/s0014-2999(99)00314-3. [DOI] [PubMed] [Google Scholar]
- 36.Augustin LS, Gallus S, Negri E, La Vecchia C. Glycemic index, glycemic load and risk of gastric cancer. Ann Oncol. 2004;15:581–584. doi: 10.1093/annonc/mdh130. [DOI] [PubMed] [Google Scholar]
- 37.Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, Dubrow R, Schoenberg JB, Mayne ST, Farrow DC, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–155. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 38.Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 39.Bremner CG, Lynch VP, Ellis FH Jr. Barrett's esophagus: congenital or acquired An experimental study of esophageal mucosal regeneration in the dog. Surgery. 1970;68:209–216. [PubMed] [Google Scholar]
- 40.Vaughan TL, Farrow DC, Hansten PD, Chow WH, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Rotterdam H, et al. Risk of esophageal and gastric adenocarcinomas in relation to use of calcium channel blockers, asthma drugs, and other medications that promote gastroesophageal reflux. Cancer Epidemiol Biomarkers Prev. 1998;7:749–756. [PubMed] [Google Scholar]
- 41.You WC, Blot WJ, Chang YS, Ershow AG, Yang ZT, An Q, Henderson B, Xu GW, Fraumeni JF Jr, Wang TG. Diet and high risk of stomach cancer in Shandong, China. Cancer Res. 1988;48:3518–3523. [PubMed] [Google Scholar]
- 42.Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol. 1998;27:173–180. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 43.Schneider R, Bornstein SR, Chrousos GP, Boxberger S, Ehninger G, Breidert M. Leptin mediates a proliferative response in human gastric mucosa cells with functional receptor. Horm Metab Res. 2001;33:1–6. doi: 10.1055/s-2001-12617. [DOI] [PubMed] [Google Scholar]
- 44.Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324–329. doi: 10.1136/gut.49.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]


