Abstract
AIM: To assess the clinical, biochemical, and virological outcome during long-term follow-up of chronic hepatitis C patients with sustained virological response following effective antiviral therapy.
METHODS: This study was a retrospective cohort study including 171 sustained responders defined as HCV RNA PCR negative at 6 mo after the end of effective antiviral treatment (SVR-6). Clinical signs and symptoms, biochemical hepatic parameters, ultrasonography and HCV RNA PCR were followed.
RESULTS: Mean follow-up period was 35.38 ± 22.2 mo after the end of treatment. Twenty-seven (15.8%) responders had evidence of cirrhosis before treatment. Forty-eight (28.1%), 107 (62.6%) and 6 (3.5%) patients were genotype 1, 3, and 6 respectively, while 10 patients (5.8%) were unclassified. There were no virological and biochemical relapses during the period of follow-up. None of the patients showed evidence of hepatic decompensation. However, there were 3 patients (1.8%) developing hepatocellular carcinoma at 14, 18, 29 mo after treatment discontinuation, two of whom had evidence of cirrhosis prior to therapy.
CONCLUSION: The study shows that during a follow-up interval for about 3 years in 171 chronic hepatitis C patients with sustained viral response after effective antiviral treatment there were no evident signs of either biochemical or clinical relapse of liver disease in all but three patients who developed hepatocellular carcinoma.
Keywords: Chronic hepatitis C, Sustained virological response, Long-term outcome
INTRODUCTION
Chronic hepatitis C viral (HCV) infection is well recognized as a major cause of cirrhosis and hepatocellular carcinoma. Spontaneous remission of the disease seems to be rare. After alfa-interferon was used as therapy for HCV infection in 1986[1], Interferon associated with Ribavirin became the standard treatment for this disease. Sustained virological response, defined as HCV RNA PCR negative at 6 mo after the end of treatment (SVR-6), is considered to be a useful predictor for long term response[2], since the probability of a late relapse among sustained responders was only 4.7%-8.7%[2-5], and a sustained response is associated with decreased histological activity on liver biopsy[6,12]. Moreover, the disappearance of detectable plasma HCV RNA has fostered the notion that sustained responders may be cured of the disease[4].
From most of the studies, SVR-6 can be obtained in 40%-60% of individuals infected with genotype 1 and in a higher percentage (75%-85%) of subjects with genotypes 2 and 3[5]. However, there are only few studies reporting long-term outcomes in patients who had SVR-6[1-4,6-8], especially in Asian population. In this study, we assessed the long-term clinical, biochemical and virological outcome of Thai HCV patients with sustained virological response.
MATERIALS AND METHODS
Study design
Chronic hepatitis C patients with SVR-6 from Hepatology Unit, Faculty of Medicine, Mahidol University (Siriraj Hospital), Bangkok, Thailand from 1995 to 2005 were included in this retrospective cohort study.
Patients
The criteria required for the study included: evidence of chronic HCV infection as defined by HCV RNA PCR positive by Roche Amplicor HCV Assay® (with a detection limit of 100 copies/mL), age of 18 years or older, and sustained virological response after effective antiviral treatment. Patients co-infected by chronic hepatitis B virus and/or human immunodeficiency virus and patients where complete data could not be retrieved were excluded from the analysis.
Data were obtained from 171 chronic hepatitis C patients who responded to interferon therapy. Data were collected from case record forms. Patients treated with conventional interferon, PEG-interferonα-2a and PEG-interferonα-2b were 54.4%, 17% and 28.6% respectively. Twenty-one patients (12.3%) required therapy twice to achieve SVR-6. Duration of therapy was 24 wk for responders with genotype 3 infection, while non-responders and other HCV genotypes patients were treated for 48 wk.
Data recorded
The investigators recorded demographic data, details of treatments (type of interferon used and duration of therapy), virological data (genotypes and viral loads) and biochemical data (bilirubin, transaminases and albumin levels) obtained from certified laboratories. Pre-treatment liver biopsies were done in 105 of the 171 patients using the Histology Activity Index (HAI) score. Mean histological score and fibrosis score were 7.72 ± 4.08 (range 1 to18) and 1.51 ± 1.3 (range 0 to 4) respectively.
Sustained virological response was defined as no detectable HCV RNA PCR at the end of treatment and after six months of follow up.
Patients were considered to have decompensation if they showed any of the following findings: ascites, bleeding varices, jaundice, or hepatic encephalopathy. Patients were classified as having cirrhosis on the basis of ultrasonography or liver biopsy. Hepatocellular carcinoma was diagnosed from ultrasonography or other imaging studies.
Sustained virological responders were considered to have a late virological relapse if HCV RNA PCR became detectable on any occasion after six months of follow up, confirmed by HCV viral load study.
Follow-up data was recorded every six or twelve months for clinical, biochemical and virological outcomes.
Statistical analysis
Statistical analyses were performed using SPSS version 10 for Windows. The descriptive statistics and Paired t-Test were used to analyse. The Kaplan-Meier method was used to determine the rate of hepatocellular carcinoma occurrence.
RESULTS
Study population
Data were obtained from 171 patients treated for chronic HCV infection who had SVR-6. The ages of the patients were 48 ± 10.5 years. Male to female ratio was 1.12:1. Routes of infection are shown in Figure 1. Characteristics of sustained virological responders are shown in Table 1.
Figure 1.
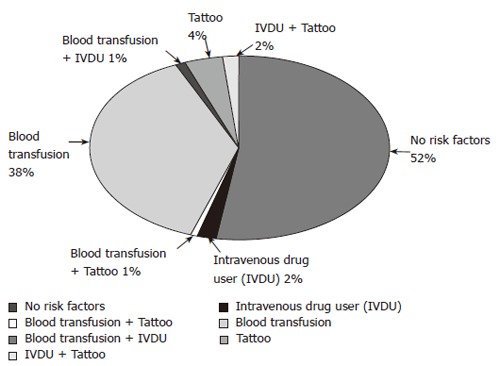
Routes of infection.
Table 1.
Characteristics of 171 HCV infection patients with SVR-6 (n = 171)
| Characteristics | |
| Age (yr) | |
| Mean ± SD | 48 ± 10.5 |
| Range | 21-75 |
| Sex | |
| Male | 91 (53%) |
| Female | 80 (47%) |
| Follow up (mo) | |
| Mean ± SD | 35.38 ± 22.2 |
| Range | 8-134 |
| Genotype | |
| 1 | 48 (28.1%) |
| 3 | 107 (62.6%) |
| 6 | 6 (3.5%) |
| Unclassified | 10 (5.8%) |
| Cirrhosis (before therapy) | 27 (25.8%) |
| HCV viral load (copies/mL) | 1 000-136 000 000 |
| Median HCV viral load | 903 000 |
Of 171 sustained responders, 27 patients (15.8%) had compensated cirrhosis (as determined by liver biopsy or imaging study) before the start of treatment.
Clinical, biochemical and virological studies were followed up to 35.38 ± 22.2 mo (range 8 to 134 mo) after the end of treatment.
Clinical outcomes
At the end of follow-up, all patients were fully active and alive. No new cases of cirrhosis appeared. Of the 27 patients with cirrhosis, none developed decompensated liver disease. Hepatocellular carcinoma, as assessed by abdominal ultrasonography every 6 mo were found in three patients at 14, 18 and 29 mo of follow-up. The details of these patients are shown in Table 2. Their HCV RNA values were still undetectable at the time of the diagnosis of hepatocellular carcinoma. Two of them had cirrhosis before treatment. From the statistical analysis of this data, it showed that, after a median follow-up period of 31 mo, the incidence of developing hepatocellular carcinoma in SVR-6 patients was no more than 1.8%.
Table 2.
Features of the three hepatocellular carcinoma patients
| Case1 | Case 2 | Case 3 | |
| Age (yr) | 50 | 75 | 63 |
| Sex | Male | Male | Male |
| Previous cirrhosis | Yes | Yes | No |
| Onset after the end of treatment (mo) | 14 | 18 | 29 |
| Alfa-fetoprotein (IU/mL) | 433.7 | 2.09 | 21.59 |
| Size at first detection | 3 cm | 1.5 cm | 1 cm, 3 lesions |
Biochemical and virological outcomes
There was a significant reduction of aminotransferase levels after SVR-6. The pre-treatment and post-treatment values of AST were 84.3 ± 48.9 and 27.7 ± 12.9, while ALT were 120.4 ± 81.2 and 25.2 ± 15.3 U/L (the normal values of AST: 0-37 and ALT 0-40 U/L).
No late virological relapse was found. Four (2.3%) of the 171 sustained responders had false positive HCV RNA PCR (confirmed by HCV RNA viral load less than 50 IU/mL).
DISCUSSION
We assessed the long term outcome of 171 patients with chronic hepatitis C who achieved sustained virological response by effective antiviral treatment. Our result demonstrated that SVR-6 was associated with a permanent absence of HCV viremia during the long-term follow-up in all cases. However, this finding showed better result than previous reports which had a late virological relapse between 4.7%-8.7%[2,3]. The reasons may due to: (1) the smaller number of patients included compared to previous studied ; (2) fewer HCV genotype 1 included compared; (3) more than half of our patients were infected by genotype 3 (this type is known to have a more favorable prognosis), and (4), the low sensitivity of HCV RNA PCR tests used in the studies before 2000 that may potentially account for few apparent early virological responses.
Disease progression such as development of cirrhosis seems to arrest after the stage of SVR-6, but a risk of developing hepatocellular carcinoma is still remaining. The incidence of hepatocellular carcinoma from our study at a mean follow-up period about 3 years is 1.8% which is slightly higher than the previous results reported from Japan (0.02%-0.5% per year)[3,9,10], while the incidence of hepatocellular carcinoma in western countries seems to be rare after SVR and limited only to patients with cirrhosis[4,11]. Therefore, regular ultrasonography should not be discarded for the management of cirrhotic patients, even in those showing persistently normal aminotransferase, alfa-fetoprotein and undetectable HCV RNA levels after interferon treatment.
In summary, this long-term study shows that in chronic HCV infection, sustained responders to interferon attain remarkable improvement of clinical outcomes. This supports the hypothesis that persons with sustained responses to interferon-therapy show a low risk for further relapse of HCV infection, development of cirrhosis and hepatocellular carcinoma[3,4,6,7,12]. However, the follow-up period of these patients was too short to allow a definite conclusion about the potential effect of the sustained response to interferon therapy for prevention of progressive liver disease .
Footnotes
S- Editor Wang J L- Editor Chiarioni G E- Editor Bi L
References
- 1.Reichard O, Glaumann H, Frydén A, Norkrans G, Wejstål R, Weiland O. Long-term follow-up of chronic hepatitis C patients with sustained virological response to alpha-interferon. J Hepatol. 1999;30:783–787. doi: 10.1016/s0168-8278(99)80129-9. [DOI] [PubMed] [Google Scholar]
- 2.Cammà C, Giunta M, Pinzello G, Morabito A, Verderio P, Pagliaro L. Chronic hepatitis C and interferon alpha: conventional and cumulative meta-analyses of randomized controlled trials. Am J Gastroenterol. 1999;94:581–595. doi: 10.1111/j.1572-0241.1999.00919.x. [DOI] [PubMed] [Google Scholar]
- 3.Veldt BJ, Saracco G, Boyer N, Cammà C, Bellobuono A, Hopf U, Castillo I, Weiland O, Nevens F, Hansen BE, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004;53:1504–1508. doi: 10.1136/gut.2003.038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno S, Battezzati PM, Bellati G, Manzin A, Maggioni M, Crosignani A, Borzio M, Solforosi L, Morabito A, Ideo G, et al. Long-term beneficial effects in sustained responders to interferon-alfa therapy for chronic hepatitis C. J Hepatol. 2001;34:748–755. doi: 10.1016/s0168-8278(01)00062-9. [DOI] [PubMed] [Google Scholar]
- 5.Teoh NC, Farrell GC. Management of chronic hepatitis C virus infection: a new era of disease control. Intern Med J. 2004;34:324–337. doi: 10.1111/j.1445-5994.2004.00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Larghi A, Tagger A, Crosignani A, Ribero ML, Bruno S, Portera G, Battezzati PM, Maggioni M, Fasola M, Zuin M, et al. Clinical significance of hepatic HCV RNA in patients with chronic hepatitis C demonstrating long-term sustained response to interferon-alpha therapy. J Med Virol. 1998;55:7–11. [PubMed] [Google Scholar]
- 8.Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, Kilani A, Areias J, Auperin A, Benhamou JP, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med. 1997;127:875–881. doi: 10.7326/0003-4819-127-10-199711150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394–1402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]
- 10.Imai Y, Kawata S, Tamura S, Yabuuchi I, Noda S, Inada M, Maeda Y, Shirai Y, Fukuzaki T, Kaji I, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998;129:94–99. doi: 10.7326/0003-4819-129-2-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 11.McHutchison JG, Esteban-Mur R. Durability of sustained virological response in patients with chronic hepatitis C after treatment with interferon a-2B alone or in combination with ribavirin. Hepatology. 2001;34:244A. [Google Scholar]
- 12.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]


