Abstract
Research on the therapeutic modulation of cardiac autonomic tone by electrical stimulation has yielded encouraging early clinical results. Vagus nerve stimulation has reduced the rates of morbidity and sudden death from heart failure, but therapeutic vagus nerve stimulation is limited by side effects of hypotension and bradycardia. Sympathetic nerve stimulation that has been implemented in the experiment may exacerbate the sympathetic-dominated autonomic imbalance. In contrast, concurrent stimulation of both sympathetic and parasympathetic cardiac nerves increases myocardial contractility without increasing heart rate. This review assesses the current state of electrical stimulation of the cardiac autonomic nervous system to treat heart failure.
Heart disease is the leading cause of death worldwide [1]. Progress in developing pharmacologic therapies, interventional and surgical cardiovascular procedures, heart transplants, and mechanical circulatory support devices to treat heart failure (HF) has slowed the increase in HF-associated mortality. In spite of this improvement, aging of the population has led to a steadily increasing incidence of HF in the United States [2]. Currently available HF therapies have limited efficacy. Thus, innovative therapies are needed to reduce the incidence of the costly morbidity and devastating mortality associated with HF.
HF pathophysiology is associated with neurohormonal activation of the sympathetic nervous and renin-angiotensin systems, resulting in increased plasma levels of norepinephrine (NE), angiotensin II, and endothelin-1. In addition, plasma levels of inflammatory biomarkers and cytokines increase (eg., tumor necrosis factor-α and C-reactive protein), as do markers of systemic and cardiac oxidant stress [3]. The sympathetic and parasympathetic autonomic nervous systems exert opposing influences on cardiac function; thus, addressing the imbalance is crucial in finding effective new modes of intervention. To address these factors, a number of efforts in translational research are being made to evaluate the therapeutic potential of modulating cardiac function by stimulating cardiac autonomic nerves [4–7].
Use of vagus nerve stimulation (VNS) as a medical therapy to counter sympathetic nervous system activation in HF has yielded encouraging results [8–12]. In both animal and early clinical studies, it is clear that optimal cardiac neurostimulation therapy varies, based on the type and severity of HF and on the individual balance of sympathetic and parasympathetic autonomic activity.
This article reviews cardiac innervation and sympathovagal cardiac autonomic imbalance in chronic HF and highlights the state of the art with regard to the use of electrical stimulation of the cardiac autonomic nervous system as a treatment for both chronic and acute HF. Efforts to use vagal and sympathetic cardiac nerve stimulation, as well as new results from concomitant stimulation of both sympathetic and parasympathetic cardiac nerves at the cardiac plexus, are summarized. We focus on the impact of these therapies on cardiac function and cardiovascular physiology and compare and contrast these with conventional HF treatment algorithms.
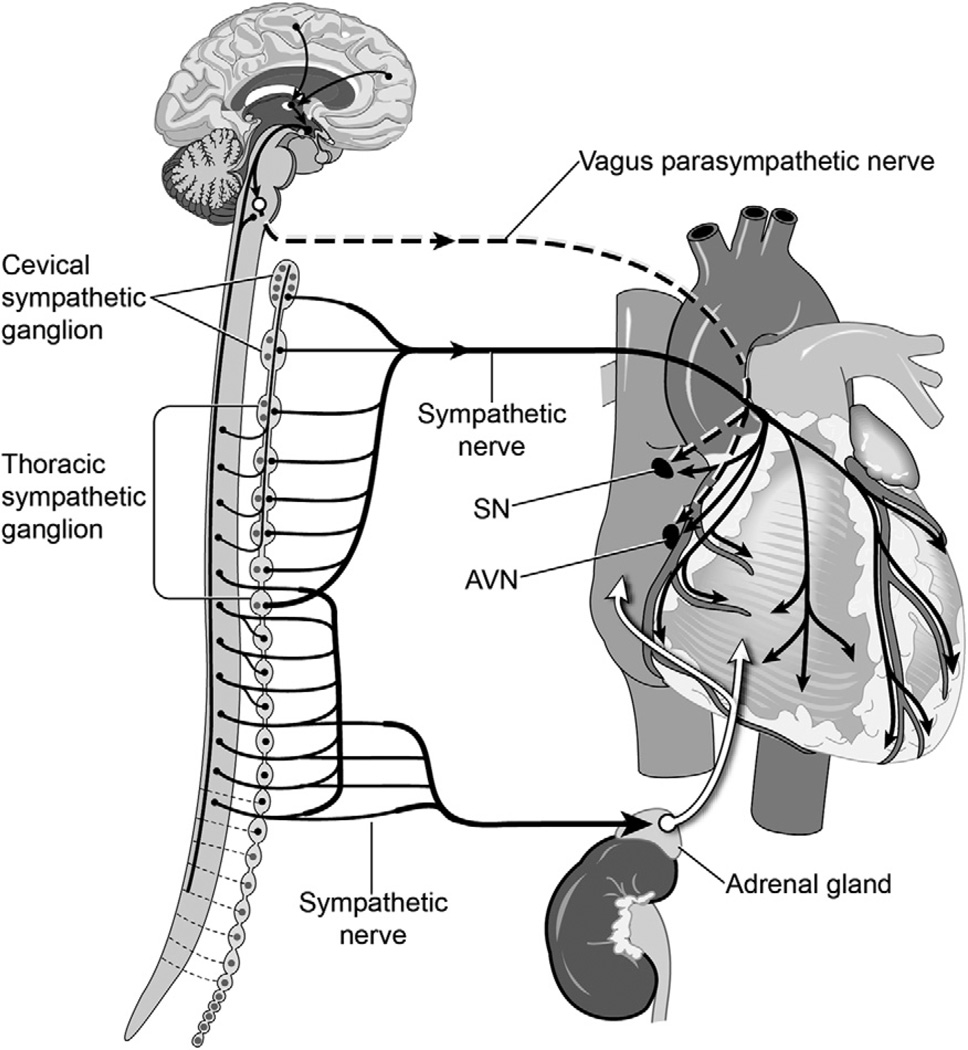
Cardiac Innervation
Cardiac autonomic innervation originates from the central nervous system as parasympathetic preganglionic nerve fibers from the vagus nerve (10th cranial nerve) and from sympathetic preganglionic nerves originating from the spinal cord. Vagal preganglionic fibers travel directly to an array of ganglia located on the epicardium. Sympathetic preganglionic nerves communicate with sympathetic paravertebral and prevertebral ganglia located close to the spinal cord. Sympathetic postganglionic peripheral nerves then travel to the heart (Fig 1).
Fig 1.
Innervation of the heart. (AVN = atrioventricular node; SN = sinus node.)
Cardiac autonomic nerves are concentrated in the cardiac plexus, a network of autonomic nerves formed by postganglionic sympathetic nerve fibers from the cervical and thoracic ganglion and preganglionic parasympathetic branches from the vagus nerves, parasympathetic ganglion, and parasympathetic postganglionic fibers. The cardiac plexus is embedded in the adventitia of the great vessels, in the concavity of the aortic arch, in front of the right pulmonary artery and of the bifurcation of the trachea, above the point of division of the pulmonary artery, and behind the aortic arch. Sympathetic postganglionic nerves that are extensions of the cardiac plexus run along the coronary arteries (coronary plexus). Intrinsic cardiac neurons are present throughout the atria (especially in the sinoatrial node) and ventricles, including the conduction system of the heart.
A second important pathway modulating cardiac activity by sympathetic innervation is the sympatho-adrenal response, by which the secretion of catecholamines (adrenaline and NE) into the bloodstream is the result of activation of preganglionic sympathetic fibers innervating the adrenal medulla. This response results in a strong positive inotropic effect on the heart (β1 adrenergic agonist) accompanied by peripheral vasodilation in the skeletal muscle vasculature at low blood levels (β2-adrenergic dominant), with generalized peripheral vasoconstriction at higher levels (α-adrenergic dominant).
Cardiac neurons interact through a number of synaptic mechanisms to influence heart rate (HR), ventricular function, and secretion of natriuretic peptides [13]. The primary sympathetic neurotransmitter (NE) produces an increase in HR, myocardial conduction velocity, and contractility. Conversely, the parasympathetic neurotransmitter acetylcholine (ACh) reduces HR.
From the perspective of cardiac function, sympathetic stimulation increases cardiac output, myocardial work, and myocardial oxygen consumption (MVo2), whereas parasympathetic stimulation increases cardiac efficiency by slowing HR to take advantage of preload effects, decreases MVo2, and increases coronary diastolic perfusion times with a much less significant effect on cardiac output. In canine studies using concurrent sympathetic and parasympathetic cardiac nerve stimulation, Levy [8] and Levy and Zieske [14] concluded that vagal effects on HR predominate over sympathetic effects, showing that the positive chronotropic effects produced by strong sympathetic stimulation can be inhibited by relatively weak vagal stimulation. This observation was later supported in studies by Brack and colleagues [15].
Cardiac autonomic control requires a dynamic balance of sympathetic and parasympathetic tone. Baseline cardiac sympathetic tone is adjusted in response to inputs from cardiopulmonary receptors, arterial baroreceptors, and afferent central nervous system chemoreceptors. Adjustments are immediate in response to changes in blood pressure. However, they occur over minutes in response to changes in blood volume as cardiac sympathetic nerve activity increases with increased blood volume and occur chronically in response to stress, alterations in hormonal levels, or chronic stimuli (eg, obesity, hypertension, and persistent HF) [16]. These chronic factors are additive with respect to their effects on plasma NE and sympathetic nerve activity [17]. Parasympathetic (vagal) tone may protect cardiac function by decreasing HR and mitigating sympathetic arousal [18].
As stated above, the sympathetic and parasympathetic autonomic systems affect cardiac function differently, but they also have direct inhibitory effects on each other at the neurotransmitter level. The NE release from sympathetic nerve terminals inhibits ACh release from neighboring vagal fibers, whereas ACh can prevent the release of NE [8, 19]. With concurrent sympathetic and parasympathetic cardiac stimulation, the initial response is expected to be primarily parasympathetic because of the slower rate of release of NE and slower rate of adrenergic signal transduction [20]. Also, ACh acts almost immediately on release and can markedly suppress the release of NE from the sympathetic nerves very soon after the onset of stimulation.
The baroreflex is one of the body’s homeostatic mechanisms for maintaining blood pressure and its primary physiologic role is in afterload control. Baroreceptors located in the aortic arch, carotid sinus, systemic vein, pulmonary vessel, atria, and ventricles sense blood pressure and increase or decrease vascular resistance and cardiac output through the autonomic nervous system. Baroreflex electrical stimulation inducing parasympathetic activation and sympathetic inhibition has been reported to decrease hypertension and has been recently applied to HF as summarized by Lopshire and Zipes [11].
Cardiac Sympathovagal Imbalance in Heart Failure
Imbalance of the cardiac autonomic nervous system is characterized by marked sympathetic activation and abnormally low levels of parasympathetic activity under conditions of obesity, stress, hypertension, and cardiovascular diseases, including coronary artery disease [13, 16, 17, 21, 22]. In the early stages of HF, activation of cardiac sympathetic nerves provides a compensatory physiologic response that improves cardiac function by its positive inotropic and chronotropic effects during transient periods of increased physiologic demand. However, in later stages of HF, excessive upregulation of sympathetic neural tone has pathologic effects [13, 21–23]. Persistent sympathetic activation and parasympathetic withdrawal in HF patients is believed to contribute to the progression and pathogenesis of HF [24]. The level of sympathetic activity is closely linked to the severity of symptoms and hemodynamic derangement in HF. Elevated plasma NE levels, due in part to release from the adrenal medulla, are highly predictive of short-term mortality [23]. Sympathetic excitation can lead to peripheral vasoconstriction and acutely shorten the refractory period of ventricles, reducing the threshold of ventricular fibrillation [13]. Sympathetic-dominant autonomic imbalance increases the incidence of life-threatening arrhythmia and sudden cardiac death in chronic HF [21, 22].
Effect of Autonomic Imbalance on the Renin-Angiotensin System and Systemic Inflammatory Mediators
In chronic HF exacerbated by increased sympathetic tone, rebalancing cardiac autonomic control attenuates systemic inflammation and slows HF progression [5, 13]. The pathophysiology of HF is also associated with activation of the renin-angiotensin system and elevation of plasma biomarkers of inflammatory activation, cytokines, and systemic and cardiac oxidant stress [3]. The pathophysiologic effects of a decrease in parasympathetic tone may reflect the vagus nerve’s role as the efferent arm of the “cholinergic anti-inflammatory pathway” [25]. Studies have shown that the parasympathetic nervous system acts as a physiologic regulator of the inflammatory response to immune system activation. The parasympathetic nervous system senses and attenuates inflammation by efferent neural outflow to the reticuloendothelial system. It has been postulated that parasympathetic stimulation reduces production of systemically active cytokines by spleen cells and activates ACh receptors on the surface of macrophages within the spleen, resulting in reduced expression of inflammatory mediators and adhesion molecules [26].
Parasympathetic nervous system inhibition of the renin-angiotensin system has been demonstrated in animal studies: one showed that vagal efferent nerves inhibit release of renin by the kidneys [27] and another that vagal blockade significantly increased plasma renin activity [28]. Our studies showed that VNS treatment significantly reduced elevated levels of plasma NE, angiotensin II, and C-reactive protein in HF dogs [5]. Current HF pharmacologic treatment utilizes angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and aldosterone antagonists [29, 30], all of which decrease the systemic inflammatory response [31, 32].
Cardiac Autonomic Nerve Stimulation Therapy
Vagus Nerve Stimulation: Animal Model and Clinical Heart Failure Studies
Although direct VNS primarily has a negative chronotropic effect, some animal and human studies have shown a decrease in left ventricular (LV) work and efficiency with VNS [33–35]. There is general agreement, however, on its potential benefit in reestablishing cardiac autonomic balance in response to the increased sympathetic tone in HF animal models and in HF patients [5, 13, 36–38]. Along with its hemodynamic effects, VNS attenuates the systemic inflammatory response and activation of the renin-angiotensin system in animal HF models, which has been shown to be beneficial in HF treatment [8, 28]. The most therapeutic effect of VNS in treating chronic HF is its reduction of elevated HR created by increased sympathetic tone, which improves diastolic filling and coronary perfusion and reduces MVo2. These VNS effects combine to slow or reverse the progression of chronic HF. In patients with severe coronary artery disease, VNS reduced sympathetic tone by inhibiting the release of NE, resulting in improved microcirculation and LV contractility. Vagal activation has also been shown to have a powerful antifibrillatory effect [39].
In recent pilot VNS clinical studies, patients diagnosed with New York Heart Association (NYHA) class II/III HF were treated with an implantable VNS system (CardioFit, BioControl Medical USA, New Hope, MN) that delivered pulses to the right cervical vagus nerve through a multiple contact bipolar nerve cuff electrode [4, 38]. At 1-year follow-up, VNS was associated with a reduction in HR (p = 0.003), increase in LV ejection fraction (p < 0.0001), and improvements in NYHA class (p < 0.001) and 6-minute walk (p = 0.012).
Pharmacologic Therapy Versus Vagus Nerve Stimulation
The negative chronotropic effects of VNS mimic the actions of anti-adrenergic beta-blockers, which have been proved effective in reducing mortality and improving cardiac function in chronic HF patients [40]. However, systemic side effects, such as arterial hypotension and bradycardia, limit the use of beta-blockers in the acute HF setting. The recent systolic HF treatment with the If inhibitor ivabradine trial (SHIFT) evaluated the effects of ivabradine, a selective inhibitor of the If current in the sinoatrial node, without inotropic effects [41]. This randomized, double-blind study in NYHA class II–III HF patients demonstrated that HR in patients on ivabradine fell by 15.4 ± 10.7 beats per minute at 28 days and that hospital admission rates for worsening HF were significantly lower in patients taking ivabradine than placebo (21% placebo versus 16% ivabradine; p < 0.0001) with significantly fewer deaths attributable to HF (5% vs 3%; p = 0.014).
Although the effects of VNS on HR are similar to those of ivabradine or beta-blockers, VNS also attenuates the systemic inflammatory activation and the effects of renin-angiotensin system activation that are associated with the progression of heart disease. It is notable that, relative to adjusting medication levels, the effects of VNS can be much more quickly titrated by changing stimulation parameters. As noted above, VNS significantly improved NYHA functional class and LV ejection fraction in initial clinical trials in NYHA class II–III HF patients [37]. As a result, VNS is being proposed as an adjuvant to defibrillator and resynchronization therapies for this population [42]. Both VNS and pharmacologic HF therapies target stable chronic HF patients to achieve long-term therapeutic effects and are not recommended for endstage or acute-phase severe HF patients in need of increased cardiac output.
Sympathetic Stimulation: Experimental Studies
Cardiac sympathetic nerve stimulation (SNS) alone produces a cardioselective positive inotropic effect without significant effects on systemic vascular tone. In ex vivo studies using isolated rabbit and canine hearts, cardiac SNS increased LV pressure by increased contractility [43, 44]. The SNS at the subclavian artery also increased the adrenergic neural tone of the heart [7]. Unfortunately, SNS also significantly increases HR, leading to a significant increase in MVo2, the same adverse effect associated with the use of inotropic drugs such as dobutamine to override beta-receptor downregulation and acutely augment LV contractility in patients with acute and endstage HF [45, 46]. The mortality rate for inotrope-dependent patients at 6 months is more than 50%, with few survivors at 1 year [44, 47]. Positive inotropic agents are no longer recommended for long-term treatment of HF, even in its advanced stages, and are in some cases reserved for the palliative care of endstage HF patients, in whom the increased mortality risks are traded for an improved quality of life [48, 49]. As a result, there has been no significant clinical application of direct cardiac SNS as HF therapy.
It has long been known that the positive inotropic effects of SNS are stronger with stimulation of the left-sided cervical sympathetic nerves, whereas the chronotropic effects are stronger with right-sided SNS [44]. Future research into the identification of separate cardiac sympathetic neural elements that selectively affect LV contractility over HR may allow sympathetic neuro-modulation in HF therapy. More recent animal studies have investigated the impact of selective SNS by stimulation directly at the heart in isolated heart experiments (ex vivo) and by in vivo deployment of stimulating catheters into the coronary sinus [6, 7].
Concurrent Parasympathetic and Sympathetic Nerve Stimulation
Efforts to therapeutically manipulate the balance of parasympathetic and sympathetic nerve activity are complicated by the range of organ systems and noncardiac functions that these nerves regulate (systemic arterial pressure, body temperature, gastrointestinal motility, glandular secretion, respiration, renal and urinary bladder function, etc). Pharmacologic manipulation has been performed using NE, Ach, and their analogs to mimic the effects of autonomic nerve stimulation, yet it remains difficult to determine the extent to which such manipulation models the effects of activating neural pathways. Previous end-organ studies using concurrent sympathetic and parasympathetic stimulation have focused on direct stimulation of the vagus nerve and its branches while also stimulating either cervical sympathetic nerves or sympathetic paravertebral and prevertebral ganglia [4–7, 13, 36–38, 43, 44]. An ex vivo study focusing on the chronotropic effects of SNS and VNS was performed on isolated rabbit hearts [15]. Background VNS reduced the overall positive chronotropic effect of SNS with the level of inhibition increasing with increasing background VNS frequency. Background SNS, however, acted to enhance the negative chronotropic effects of VNS. These observations reinforce previously discussed studies showing that VNS activity has a dominant role in regulating chronotropic effects on the heart and that the cardiac parasympathetic system has an important protective role in limiting sympathetically driven physiologic effects.
In reviewing the literature, we could find no in vivo animal studies that focused on concurrent stimulation of parasympathetic and sympathetic cardiac nerves to affect cardiac and hemodynamic function. In recent studies, we have examined the cardiac effects of stimulation at the cardiac plexus, which contains both sympathetic and parasympathetic nerves traversing the adventitia of the great vessels at the base of the heart. Stimulation at this site produces localized cardiac effects that minimize or eliminate the other end-organ side effects that are associated with stimulation of autonomic nerves above the level of the heart. We stimulated the cardiac plexus of healthy dogs using a single bipolar electrode at the epivascular surface of the right branch of the pulmonary artery. We reported increased LV contractility secondary to sympathetic stimulation with no increase in HR in all (12 of 12) dogs. This study demonstrated that, during use of concurrent sympathetic and parasympathetic cardiac nerve stimulation, parasympathetic stimulation has a dominant-negative chronotropic effect over that of sympathetic cardiac stimulation [50]. Although this reproducible response to cardiac plexus stimulation (CPS) was demonstrated in healthy dogs, it also has potentially therapeutic applications in the treatment of advanced HF. Clinical evaluation of simultaneous stimulation of parasympathetic and sympathetic cardiac nerves has not been reported, and its potential therapeutic effects are still unclear for HF patients.
Stratification of Cardiac Autonomic Nerve Stimulation in the Treatment of Heart Failure
Jessup and Brozena [42] stratified patient treatment options relative to their American College of Cardiology/American Heart Association stage of systolic HF. Stage A is a high-risk group with no symptoms. Stage B covers structural heart disease with no symptoms; angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers are recommended for all patients, and beta-blockers in selected patients. Treatment for stage C patients with structural disease with previous or current symptoms includes angiotensin-converting enzyme inhibitors, beta-blockers, cardiac resynchronization therapy for bundle branch block, revascularization and mitral valve surgery, aldosterone antagonists and nesiritide. Stage D patients have symptoms refractory to the listed treatments that require inotropes or special interventions such as the implantation of a ventricular assist device or even heart transplantation. Although VNS is being proposed as an adjuvant therapy to defibrillator and resynchronization therapies for stage C patients (or chronic NYHA class II/III patients), we propose that concurrent parasympathetic stimulation and SNS may also be useful as an alternative treatment even for stage D/NYHA class IV patients.
Conclusions and Future Perspective
Results from recent cardiac neurostimulation studies in both HF animal models and early clinical studies are encouraging. Efforts to optimize stimulation sites and stimulation parameters may lead to increased use of cardiac neurostimulation that can be tailored to the type and severity of HF, based on the balance of sympathetic and parasympathetic autonomic control, the individual patient’s hemodynamics and myocardial reserve capacity, and the degree of myocardial ischemia and dysfunction. Sympathovagal imbalance is associated with both hemodynamic and systemic effects, resulting in the activation of inflammatory and hypertensive mediators. To date, VNS is the only form of cardiac neuromodulation that has reached clinical application and thus far, only for the treatment of chronic early stage HF. VNS seems likely to decrease rates of long-term morbidity and mortality by alleviating the hemodynamic and systemic chronic pathology related to sympathetic-dominant cardiac autonomic imbalance.
Pharmacologic manipulations that seek to mimic the effect of nerve stimulation have significant limitations and adverse systemic effects that may be overcome by use of cardiac nerve stimulation or by direct CPS. The ultimate answer may lie in unraveling the molecular mechanisms of autonomic imbalance in HF, but for now cardiac neuromodulation is a near-term option with great promise for the treatment of HF. To enhance the efficacy of CPS, additional studies are needed to develop dedicated electrode arrays that can cover a greater proportion of the epicardial and endocardial surfaces of the cardiac plexus and to better define the stimulation parameters and stimulation sites that can be used in both ischemic and nonischemic chronic HF models.
The increased incidence of morbidity and mortality associated with the use of inotropes in treatment of both acute and chronic severe HF are well documented. Intra-operative epivascular CPS placement (post surgeries) can be used to eliminate or reduce inotrope use in immediate postoperative acute HF as well as chronic HF, or at the onset of acute HF in previously stable chronic HF patients. The CPS effect of increased cardiac output while limiting the high metabolic demand of increased heart rate can be advantageous for both advanced and post-surgical HF patients in whom increased sympathetic tone can exacerbate cardiac ischemia.
Proof of its therapeutic effects of CPS could lead to its elective use in stable advanced HF patients by minimally invasive delivery techniques. The programmable graded cardiac response to varying levels of CPS demonstrated in this study would enable clinicians to select stimulation parameters based on the current stage of HF, increasing maintenance stimulation parameters in a chronic HF patient to either prevent onset of or treat acute cardiac decompensated HF.
We hope to extend this rapidly growing knowledge base by developing neuromodulation algorithms targeted to the type and severity of HF that utilize VNS, SNS, and concurrent stimulation of both parasympathetic and sympathetic cardiac nerves.
The effectiveness of CPS therapy may be limited in those patients with little functional cardiac reserve, and any adverse effect on existing cardiac rhythm disturbances may present a complicating factor with the progression of ischemic heart disease. The tolerance of the cardiac plexus and vascular tissues to chronic stimulation is yet to be studied and may present a tradeoff of effectiveness versus safety and long-term stability. Potential problems include loss of tissue sensitivity to stimulation, depletion of neurotransmitters, reflex cardiac autonomic stimulation, and loss of natural cardiac autonomic tone.
Abbreviations and Acronyms
- ACh
acetylcholine
- CO
Cardiac output
- CPS
cardiac plexus stimulation
- HF
heart failure
- HR
heart rate
- LV
left ventricular
- MVo2
myocardial oxygen consumption
- NE
norepinephrine
- NYHA
New York Heart Association
- PNS
parasympathetic nervous system
- SNS
sympathetic nerve stimulation
- VNS
vagus nerve stimulation
References
- 1.Gaziano TA, Gaziano JM. Global burden of cardiovascular disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Chap. 1. Philadelphia, Pennsylvania: Saunders/Elsevier Inc; 2012. [Accessed September 19, 2012]. Available at http://www.mdconsult.com/books/page.do?eid=4-u1.0-B978-1-4377-0398-6..00001-9&isbn=978-1-4377-0398-6&uniqId=364060231-4#4-u1.0-B978-1-4377-0398-6..00001-9. [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Van Wagoner DR. Chronic vagal nerve stimulation for the treatment of human heart failure: progress in translating a vision into reality. Eur Heart J. 2011;32:788–790. doi: 10.1093/eurheartj/ehq424. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, De Ferrari GM, Sanzo A, et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884–891. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 6.Meyer C, Rana OR, Saygili E, et al. Augmentation of left ventricular contractility by cardiac sympathetic neural stimulation. Circulation. 2010;121:1286–1294. doi: 10.1161/CIRCULATIONAHA.109.874263. [DOI] [PubMed] [Google Scholar]
- 7.Zarse M, Plisiene J, Mischke K, et al. Selective increase of cardiac neuronal sympathetic tone: a catheter-based access to modulate left ventricular contractility. J Am Coll Cardiol. 2005;46:1354–1359. doi: 10.1016/j.jacc.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29:437–445. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- 9.Hauptman PJ, Mann DL. The vagus nerve and autonomic imbalance in heart failure: past, present, and future. Heart Fail Rev. 2011;16:97–99. doi: 10.1007/s10741-010-9222-2. [DOI] [PubMed] [Google Scholar]
- 10.Upton AR, White AM. Autonomic stimulation. Pacing Clin Electrophysiol. 1991;14:50–69. doi: 10.1111/j.1540-8159.1991.tb04049.x. [DOI] [PubMed] [Google Scholar]
- 11.Lopshire JC, Zipes DP. Device therapy to modulate the autonomic nervous system to treat heart failure. Curr Cardiol Rep. 2012;14:593–600. doi: 10.1007/s11886-012-0292-8. [DOI] [PubMed] [Google Scholar]
- 12.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–122. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Zamotrinsky AV, Kondratiev B, de Jong JW. Vagal neuro-stimulation in patients with coronary artery disease. Auton Neurosci. 2001;88:109–116. doi: 10.1016/S1566-0702(01)00227-2. [DOI] [PubMed] [Google Scholar]
- 14.Levy MN, Zieske H. Autonomic control of cardiac pacemaker activity and atrioventricular transmission. J Appl Physiol. 1969;27:465–470. doi: 10.1152/jappl.1969.27.4.465. [DOI] [PubMed] [Google Scholar]
- 15.Brack KE, Coote JH, Ng GA. Interaction between direct sympathetic and vagus nerve stimulation on heart rate in the isolated rabbit heart. Exp Physiol. 2004;89:128–139. doi: 10.1113/expphysiol.2003.002654. [DOI] [PubMed] [Google Scholar]
- 16.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 17.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension. 2003;42:873–877. doi: 10.1161/01.HYP.0000098660.26184.63. [DOI] [PubMed] [Google Scholar]
- 18.Porges SW. Vagal tone: a physiologic marker of stress vulnerability. Pediatrics. 1992;90(3 Pt 2):498–504. [PubMed] [Google Scholar]
- 19.Grodner AS, Lahrtz HS, Pool PE, Braunwald E. Neurotransmitter control of sinoatrial pacemaker frequency in isolated rat atria and in intact rabbits. Circ Res. 1970;27:867–873. doi: 10.1161/01.res.27.6.867. [DOI] [PubMed] [Google Scholar]
- 20.Mokrane A, Nadeau R. Dynamics of heart rate response to sympathetic nerve stimulation. Am J Physiol. 1998;275(3 Pt 2):H995–H1001. doi: 10.1152/ajpheart.1998.275.3.H995. [DOI] [PubMed] [Google Scholar]
- 21.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm. 2006;3:108–113. doi: 10.1016/j.hrthm.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 23.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 24.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 25.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zitnik RJ. Treatment of chronic inflammatory diseases with implantable medical devices. Ann Rheum Dis. 2011;70(Suppl 1):i67–i70. doi: 10.1136/ard.2010.138677. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Romero JC, Shepherd JT. Continuous inhibition of renin release in dogs by vagally innervated receptors in the cardiopulmonary region. Circ Res. 1975;36:529–535. doi: 10.1161/01.res.36.4.529. [DOI] [PubMed] [Google Scholar]
- 28.Elsner D, Kromer EP, Riegger GA. Effects of vagal blockade on neurohumoral systems in conscious dogs with heart failure. J Cardiovasc Pharmacol. 1990;15:586–591. doi: 10.1097/00005344-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 30.Elder DH, Wei L, Szwejkowski BR, et al. The impact of renin-angiotensin-aldosterone system blockade on heart failure outcomes and mortality in patients identified to have aortic regurgitation: a large population cohort study. J Am Coll Cardiol. 2011;58:2084–2091. doi: 10.1016/j.jacc.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24:33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 32.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 33.Lewis ME, Al-Khalidi AH, Bonser RS, et al. Vagus nerve stimulation decreases left ventricular contractility in vivo in the human and pig heart. J Physiol. 2001;534(Pt 2):547–552. doi: 10.1111/j.1469-7793.2001.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickerson LW, Rodak DJ, Fleming TJ, et al. Parasympathetic neurons in the cranial medial ventricular fat pad on the dog heart selectively decrease ventricular contractility. J Auton Nerv Syst. 1998;70:129–141. doi: 10.1016/s0165-1838(98)00048-4. [DOI] [PubMed] [Google Scholar]
- 35.Matsuura W, Sugimachi M, Kawada T, et al. Vagal stimulation decreases left ventricular contractility mainly through negative chronotropic effect. Am J Physiol. 1997;273(2 Pt 2):H534–H539. doi: 10.1152/ajpheart.1997.273.2.H534. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 37.Klein HU, Ferrari GM. Vagus nerve stimulation: a new approach to reduce heart failure. Cardiol J. 2010;17:638–644. [PubMed] [Google Scholar]
- 38.De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz PJ, De Ferrari GM. Vagal stimulation for heart failure: background and first in-man study. Heart Rhythm. 2009;6(Suppl 11):S76–S81. doi: 10.1016/j.hrthm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Swedberg K, Kjekshus J, Snapinn S. Long-term survival in severe heart failure in patients treated with enalapril. Ten year follow-up of CONSENSUS I. Eur Heart J. 1999;20:136–139. doi: 10.1053/euhj.1998.1098. [DOI] [PubMed] [Google Scholar]
- 41.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 42.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 43.Brack KE, Coote JH, Ng GA. The effect of direct autonomic nerve stimulation on left ventricular force in the isolated innervated Langendorff perfused rabbit heart. Auton Neurosci. 2006;124:69–80. doi: 10.1016/j.autneu.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Miyano H, Nakayama Y, Shishido T, et al. Dynamic sympathetic regulation of left ventricular contractility studied in the isolated canine heart. Am J Physiol. 1998;275(2 Pt 2):H400–H408. doi: 10.1152/ajpheart.1998.275.2.H400. [DOI] [PubMed] [Google Scholar]
- 45.Steen PA, Tinker JH, Pluth JR, Barnhorst DA, Tarhan S. Efficacy of dopamine, dobutamine, and epinephrine during emergence from cardiopulmonary bypass in man. Circulation. 1978;57:378–384. doi: 10.1161/01.cir.57.2.378. [DOI] [PubMed] [Google Scholar]
- 46.Stephens J, Ead H, Spurrell R. Haemodynamic effects of dobutamine with special reference to myocardial blood flow. A comparison with dopamine and isoprenaline. Br Heart J. 1979;42:43–50. doi: 10.1136/hrt.42.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson LW. Clinical use of inotropic therapy for heart failure: looking backward or forward? Part II: chronic inotropic therapy. Circulation. 2003;108:492–497. doi: 10.1161/01.CIR.0000078349.43742.8A. [DOI] [PubMed] [Google Scholar]
- 48.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 49.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi M, Sakurai S, Takaseya T, et al. Effect of epivascular cardiac autonomic nerve stimulation on cardiac function. Ann Thorac Surg. 2012;94:1150–1156. doi: 10.1016/j.athoracsur.2012.04.092. [DOI] [PubMed] [Google Scholar]