Abstract
Traumatic brain injury (TBI) patients would benefit from the identification of reliable biomarkers to predict outcomes and treatment strategies. In our study, cerebrospinal fluid (CSF) from patients with severe TBI was evaluated for oxidant stress-mediated damage progression after hospital admission and subsequent ventriculostomy placement. Interestingly, substantial levels of peroxiredoxin VI (Prdx6), a major antioxidant enzyme normally found in astrocytes, were detected in CSF from control and TBI patients, and were not associated with blood contamination. Functionally, Prdx6 and its associated binding partner glutathione S-transferase pi (GSTP1-1, also detected in CSF) act in tandem to detoxify lipid peroxidation damage to membranes. We found Prdx6 was fully active in CSF of control patients but becomes significantly inactivated (oxidized) under TBI. Furthermore, significant and progressive oxidation of “buried” protein thiol in CSF of TBI patients (as compared to that of non-trauma control) were detected over a 24h period following hospital admission, with increased oxidation correlating with severity of trauma. Conversely, recovery of Prdx6 activity after 24h indicated more favorable patient outcome. Not only is this the first report of an extracellular form of Prdx6 but also the first report of its detection at a substantial level in CSF. Taken together, our data suggest a meaningful correlation between TBI-initiated oxidation of Prdx6, its specific phospholipid hydroperoxide peroxidase activity, and severity of trauma outcome. Consequently, we propose that Prdx6 redox status detection has the potential to be a biomarker for TBI outcome and a future indicator of therapeutic efficacy.
Keywords: Peroxiredoxin VI (Prdx6), TBI, CSF, oxidant stress, protein thiol, GSH, lipid peroxidation, choroid plexus (CP) epithelial cells, ependymal cells, antioxidant protection
Introduction
TBI is a spectrum of injuries ranging from mild concussion to severe traumatic brain damage, the symptoms and severity of which can be influenced by multiple factors [1]. The pathophysiology of TBI is complex and starts with a primary injury which can be defined by two phases: immediate damage due to the physical impact (direct neuronal or axonal disruption) and secondary injury, corresponding with impaired cerebral energy metabolism, oxidative/nitrosative stresses, inflammation and other adverse events affecting brain homeostasis [2]. Severe TBI (Glasgow Coma Scale (GCS) score ≤ 8) is also linked with a reduction of total antioxidant reserve [3], primarily glutathione (GSH) and protein thiols, each of which contributes to redox buffering [4, 5]. In a rat model of TBI, the redox state of the brain is altered due to trauma but [6] limitations in collecting brain tissue samples in humans necessitates the use of CSF and/or plasma for analyses. Direct contact of CSF with the brain makes it a primary candidate for assessment of TBI.
The major functions of CSF include: (i) regulation of intracranial pressure (ICP), (ii) removal of metabolic by products, and (iii) delivery of physiologically important compounds to various parts of the brain [2]. Secretion of CSF occurs mainly (≥90%) in the choroid plexus (CP), where the epithelium is highly adapted to its secretory role, with numerous cytoplasmic mitochondria and an apical membrane containing microvilli and cilia that faces CSF. Additionally, the apical membrane of CP epithelium participates in limited absorption of materials from CSF [7] and the ciliary beating of CP epithelial/ependymal cells supports CSF circulation [2]. Thus, damage to the apical membrane of the CP epithelial/ependymal cells may be a critical factor that disturbs brain homeostasis following TBI.
One critical type of homeostatic response found in the brain is lipid peroxidation, which is a complex process involving the interaction of oxygen-derived free radicals with polyunsaturated acyl chains of lipids, resulting in formation of –OOH group. Lipid peroxidation is one of the major sources of free radical-mediated injury that directly damages neuronal membranes and yields a number of secondary products responsible for extensive cellular damage [8]. The brain is particularly vulnerable to lipid peroxidation because brain lipids are rich in polyunsaturated fatty acids and the concentration of oxygen in the lipid bilayer is at millimolar levels [9]. Furthermore, there is a known link between lipid peroxidation, neurodegeneration, and neurodegenerative disease [10]. Consequently, understanding the mechansims by which the brain mediates oxidative response has important pathophysiological implications.
Peroxiredoxin VI (Prdx6) is a dual-functioning antioxidant enzyme [11, 12] that is found primarily in astrocytes and has been described as a critical enzyme in neurodegenerative diseases. For example, in Alzheimer’s patients Prdx6 is markedly elevated in astrocytes of both white and gray matter [13]. The presence of this enzyme in astrocytes appears to be involved in the detoxification of diffuse plaques and to a lesser extent, neuritic plaques. Similarly, dementia patients with Lewy bodies also have Prdx6 as a major antioxidant enzyme in their neural tissue [14]. Collectively, these findings indicate a protective role for Prdx6 in maintaining brain homeostasis.
Enzymatically, Prdx6 combines the activities of glutathione peroxidase [15] and acidic calcium-independent PLA2 [16, 17], providing protection against lipid peroxidation in membranes. The glutathione peroxidase activity of Prdx6 is mediated and activated by its hetero-dimerization with glutathione S-transferase pi (GSTP1-1, [12, 15]). The resulting phospholipid peroxidation is associated with reorientation of acyl chain containing –OOH groups towards membrane surfaces, which affects lipid-lipid and lipid-protein interactions in bilayers. Prdx6 specifically binds to peroxidized phospholipids in bilayers, either reducing them to alcohols, or hydrolyzing peroxidized fatty acids [16], thus eliminating perturbation of bilayers caused by flotation of the -OOH group to the surface [18], and restoring lipid homeostasis.
Levels of Prdx6 expression are highest in lung > brain > liver > testis [12]. Intracellularly, Prdx6 is mainly cytosolic, lysosomal or present in lamellar bodies [12]. However, there are no existing reports of Prdx6 being present in an extracellular physiologically relevant locale. We now report for the first time the presence of extracellular Prdx6 in CSF and demonstrate that it potentially serves to maintain homeostasis during TBI. We hypothesize that the presence of Prdx6 in CSF could provide antioxidant protection of apical membranes of the CP epithelial/ependymal cells. Consequently, our present study is focused on identifying a role for oxidative damage in CSF during TBI, and we demonstrate that Prdx6 could be a reliable biomarker that accurately predicts the outcome of TBI-associated injury.
Materials and Methods
TCEP (Tris[2-carboxyethyl] phosphine), GSH, Guanidine hydrochloride (GndHCl), phosphate buffer and hydrogen peroxide were purchased from Sigma (St. Louis, MO, USA). PLPC (1-palmitoyl-2-linolenoyl-sn-glycero-3-phosphoryl choline) was from Avanti Polar lipids (USA). ThioGlo-1 (3H-Naphthol[2,1-b]pyran-s-carboxylic Acid, (10-(2,5-Dihydro-2,5-dioxo-1H-pyrrol-1-yl)-9-methoxy-3-oxo-, Methyl Ester; TG1) was purchased from Calbiochem (CA, USA). BioSpin-6 micro columns were purchased from BioRad (CA).
Sample collection, processing and storage
Samples were collected following an institutional IRB approved protocol. Legally authorized representatives of patients, with severe TBI (GCS≤8) were consented for sample collection. Such patients were undergoing external ventricular drain (EVD) for clinical care within 6 hours of severe TBI. Exclusion criteria included the following: operation prior to EVD placement, penetrating TBI, pregnancy, being held in police custody, DNR or inability to obtain consent.
CSF samples were obtained at the time of EVD placement (time 0) and 24 hours later. CSF was removed from the drain, aliquoted and immediately frozen at −80°C. Plasma samples at the same time points were obtained from blood in Lithium Heparin or EDTA tubes, centrifuged at 1200 rpm for 10 min at 4°C, aliquoted, and frozen at −80°C for future analysis. Control CSF was obtained from ED patients undergoing lumbar puncture for other clinical (non-trauma) evaluations. None of the patients had meningitis, subarachnoid hemorrhage or any significant findings from a cellular or a microbial analysis (cell count, gram stain and culture). Clinical outcome was graded on a scale indicating neurologic deficits at discharge or death (Table 1).
Table 1.
Demographics, initial injury assessment, and clinical outcomes of patients with severe TBI.
| TBI | |||||
|---|---|---|---|---|---|
| patients | Age/Sex | ISS | GCS | Neurological Outcome | SNDD |
| 1 | 17 yom | 25 | 5 | DC to LTAC, localizing in RUE, LUE, LLE | 2 |
| 2 | 21 yom | 41 | 3T | Death, deterioration quickly to brain death | 1 |
| 3 | 33 yom | 50 | 3T | DC home with HHN, PT/OT, cane | 3 |
| 4 | 15 yom | 25 | 3T | DC home w/o neurologic deficits | 4 |
| 5 | 17 yom | 41 | 7T | DC to rehab, with CNIII palsy, trach, PEG | 2 |
| 6 | 22 yom | 34 | 5 | Death after prolonged respiratory failure, high ICPs, cerebral edema | 1 |
| 7 | 56 yof | 38 | 3T | Death after IR embolization of multiple pelvic bleeding sites, deteriorated overnight | 1 |
| 8 | 49 yom | 25 | 3T | DC to LTAC, quadriplegic, movement of head, neck, shoulder shrug | 2 |
| 9 | 47 yom | 38 | 5 | DC to rehab, GCS 9T, trach, PEG, intermittently following commands | 2 |
| 11 | 68 yom | 35 | 8 | DC to LTAC, FC in RLE, resists LLE, non-purposeful movements in bilateral UES, aphasic ---readmitted for hydrocephalus, requiring VP shunt, UTI | 2 |
| 12 | 26 yom | 45 | 3T | Death, elevated ICPs throughout stay, pentobarb coma, several EVD changes | 1 |
| 14 | 24 yom | 26 | 6 | DC home with PT/OT, dysphagia (+PEG), walker | 3 |
| 16 | 16 yof | 9 | 10 | DC to rehab, cognitive deficits as well as balance problems | 2 |
| 17 | 50 yom | 21 | 6 | DC to home with OT, PT | 3 |
| 18 | 63 yom | 24 | 3 | Death. | 1 |
| 19 | 47 yom | 17 | 3 | DC home w OT, PT, speech therapy | 3 |
| 20 | 5 yof | 43 | 3T | Death from massive head injury | 1 |
| 21 | 21 yom | 29 | 3 | Death | 1 |
Abbreviations: yom(f) – years old male(female); ISS- Injury Severity Score; GCS - Glasgow Coma Scale; T – intubation; DC –discharged; SNDD - Scale of Neurological Deficits at Discharge: 1-death; 2 - severe deficits, 3 - mild deficits, 4 - normal.
Cell culture
Collecting duct cells derived from the orpk mouse model expressing a hypomorphic allele of the Tg737 gene [pCDNA cells, cilia (−)] and genetically rescued cells with the wild-type orpkTg737 gene [BAP2 cells, cilia (+)] were provided by P. Darwin Bell., Ph.D., Department of Nephrology of the Medical University of South Carolina [19]. Cells were grown to confluence in DMEM/F12 media (Mediatech, Manassas, VA) with 0.2 μg/ml dexamethasone, 10 nM triiodothreonine, 1x insulin-transferrin-sodium selenite, 12 U/ml IFN-γ, 268 μg/ml G418, 1% penicillin-streptomycin, and 5% FBS at 33°C, 5% CO2. When confluent, cells were placed at 37°C, 5% CO2 in complete media without IFN-γ or G418 for 5 days until differentiated. All media additives were from Sigma (St. Louis, MO) except for FBS (Thermo Scientific, Waltham, MA). For the generation of cilia movement, differentiated cells were subjected to shear stress on an orbital rotator at 1 Hz [20].
Fluorescent detection of protein and low molecular weight thiol redox status
We used the fluorescent detection of thiol sulfhydryls in the CSF and the plasma of TBI and control (non-trauma) patients by their specific reaction with fluorescent maleimide probe TG-1 (Calbiochem, CA) as reported earlier [15, 21, 22]. Samples of CSF or plasma were diluted in a quartz cuvette 1:100 (by volume) with 20 mM PB (pH=7.4) at 37°C under constant stirring in a sample holder of QM-4 fluorometer (PTI, NJ). After an addition of TG-1 (DMSO solution, final concentration 5 μM), the fluorescent detection (Em = 513 nm, Ex = 379 nm) of sulfhydryl labeling was performed using a standard kinetic mode of QM-4 with a resolution of 0.1 sec to reach saturation. The fluorescence of CSF or plasma, the effect of DMSO, and the background fluorescence of TG-1 in 20 mM PB at 37°C were subtracted from the final results. The final emission values were averaged for 50 sec (500 time points) using SigmaPlot 10.0 software (Systat Software, Inc., MA) and presented as mean±SD.
A separation of CSF and plasma protein thiols was performed using size exclusion chromatography with the BioSpin-6 micro column (6 kDa “cut-off”, BioRad, CA). The subtraction of the surface protein thiols (SPT) from the total intact CSF (plasma) thiols, is a measure of the low molecular weight thiols (LMWT, predominantly GSH). In separate experiments TG-1 (10 μM) in 20 mM PB (pH=7.4, 37°C) was incubated with increased (0, 1.0, 2.0, 4.0, 5.0 and 7.5 μM) of GSH and its emission was detected. The TG-1 emission show linear correlation with concentration of GSH (R2=0.992). Actual LMWT content in CSF and plasma samples was recalculated using calibration curve and presented in μM. The samples of CSF and plasma protein thiol were treated with 5.4M guanidine hydrochloride (pH~7.4, Sigma) before sulfhydryl detection to show any apparent effects of TBI-related oxidant stress on the cysteines buried inside the proteins (BPT). The TG-1emission values corresponding to SPT and BPT content in CSF and plasma were normalized for protein concentrations. Protein concentration in CSF and plasma was determined using the standard Bradford (BioRad, CA) assay.
Western Blot detection of Prdx6 in CSF and plasma
SDS PAGE of CSF and plasma samples (~15 μg of total protein per lane) was performed using 12% Tris-Glycine 1.5mm/15 well precast gels and XCell SureLock™ Electrophoresis Cell and Novex Protein analysis solutions (all from Invitrogen, CA). Electrophoretically resolved proteins were transferred onto PVDF membranes (Bio-Rad; Hercules, CA). Non-specific binding was blocked by incubating membranes in TBS-T (Tris-buffered saline with 0.1% Tween-20) containing 5% non-fat dried milk or 5% bovine serum albumin Fraction V. Polyclonal rabbit Prdx6 antibody (1:4,000 dilutions, Strategic Biosolutions, Ramona, CA), anti-GSTP-1-1 (1:500 dilution, MBL, MA) and anti-Prdx6-SO2(3)H (1:1,000 dilution, Lab Frontier, Japan) were incubated for two hours at room temperature or overnight at 4°C. After extensive washing of membranes with TBS-T, the secondary HRP- or green (IRDye 800) and (or) red (IRDye 700) chromophore - conjugated (Li-Cor Biosciences, Lincoln, NE) antibodies were incubated for one to two hours at room temperature. The HRP blots were developed with ECL (GE Healthcare Bio-Sciences Corp.) and visualized with x-ray film. The blots were scanned and quantified with the use of ChemiDoc system (BioRad). The blots with green and (or) red fluorescent secondary antibodies were imaged and quantified with the dual-color IR-excited fluorescence imager Odyssey CLx, using Image Studio 3.1 software (all from Li-Cor Biosciences).
In some experiments the particular blots were stripped with NewBlot™ PVDF Stripping Buffer (Li-Cor Biosciences) according to manufacturer recommendations. The quality of stripping was controlled by imaging using Odyssey Clx apparatus. The stripped blot was reprobed with appropriate primary (secondary) antibodies and visualized with Odyssey Clx imager (Li-Cor Biosciences).
Recombinant purified human Prdx6 (PRDX6, ProSpec- Tany TechnoGene Ltd., Israel) and E. Coli expressed glutathione S-transferase π (GSTP-1-1) - both His-tagged, homogeneous (>95%) proteins were used in our in vitro experiments.
Immunodetection of increasing amount of purified Prdx6 under reducing (1.0 mM TCEP) condition was used for generation of calibration curve (linear regression) to quantify this protein content in CSF samples.
Hemoglobin (Hb) analysis in CSF samples of TBI patients
Hb analysis of CSF from TBI patients was performed using standard Hemoglobin Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer recommendations. Actual measurements were performed in 96-wells microplates using Modulus® Microplate Multimode Reader (Turner BioSystems, Sannyvale, CA). All analyses were in triplicate and the results are presented as mean±SD.
Fluorescent analysis of Prdx6 peroxidase activity
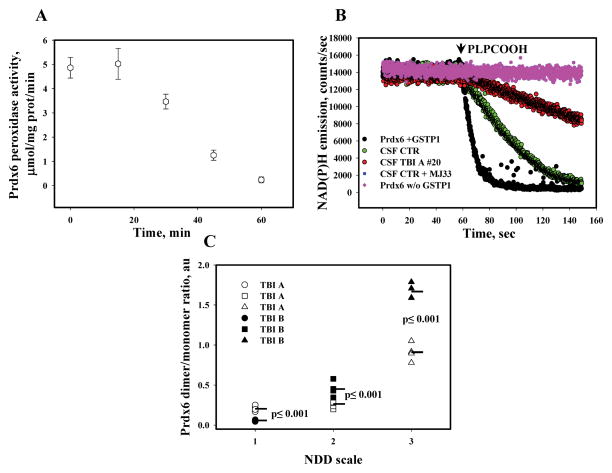
We used a standard GR/GSH/NADPH-coupled assay [11] with either1-palmitoyl-2-linolenoyl-sn-glycerol-3-phosphoryl choline hydroperoxide (PLPCOOH) or H2O2 as a substrate to study Prdx6 peroxidase activity in vitro and in CSF of the control or the TBI patients. Fluorescent (Ex/Em =340/460 nm) detection of the kinetics of NADPH oxidation to NADP was measured using a QM-4 fluorometer (PTI, NJ). For in vitro controls E. coli-expressed and purified GSTP1-1 (1.0 μg) was incubated with a reaction buffer, containing 1 mM GSH, 25 μM NADPH, and 23 U/ml GR [11] for 5 min at room temperature in a quartz cuvette under constant stirring in the dark. After the addition of purified recombinant Prdx6 (1.0 μg, ProSpec-TanyTechnoGene), the samples were incubated for an additional 5 min under the same conditions in the dark, and a recording of the NADPH emission was started 2 min before and 10 min after an indicated peroxide substrate addition. Aliquots (~60.0 μg of total protein) of CSF from control or TBI patients were used instead of purified proteins for analysis of the peroxidase activity under similar conditions. PLPCOOH was freshly prepared using a lipoxygenase-mediated oxidation of PLPC(Sigma) with purification using C18 Sep-Pack micro columns (Waters, Milford, MA) [11]. To show specificity of Prdx6 peroxidase activity, we used a specific competitive Prdx6 inhibitor, MJ33 (~50 μM) [16]. The original traces representative for at least 3 independent experiments are presented in Fig. 5, panel A. The peroxidase activities of Prdx6/GSTP1-1 in vitro and in CSF are presented in Table 2 as mean±SD for three independent experiments.
Fig. 5. Effects of H2O2 (in vitro) and TBI (ex vivo) on Prdx6 peroxidase function and neurological deficits at discharge.
Panel A: Peroxidase activity of continuously oxidized with H2O2 (2.5 μM) purified Prdx6 (0.5 μg, see Fig. 3E, with addition of GSH-loaded GSTP1-1) using H2O2 (20 μM) as a substrate in vitro. Panel B: peroxidase activity of purified Prdx6 in vitro and in CSF of control (#10) and TBI A (#20) patients using PLPCOOH or H2O2 (both 20 μM), as a substrate. Prdx6 specific inhibitor MJ33 (~50 μM) was used for peroxidase specificity control. The traces are representative of three independent experiments. Panel C: Effect of TBI-mediated Prdx6 oxidation on neurological deficit of patients at discharge. Data represent mean for: n=8(NDD=1), n=12(NDD=2) and n=4(NDD=3) independent experiments and statistical relevance of differences (ANOVA).
Table 2.
Prdx6 peroxidase activity in CSF of control (non-trauma) and TBI patients and in vitro using purified E. Coli- expressed Prdx6 and GSH-loaded GSTP1-1 proteins (see Materials and Methods). Data represent mean±SD for 3 independent experiments. Patient number is shown in parenthesis. ND-not detected.
| Sample | Activity (PLPCOOH), μmol/min μg prot | Activity (H2O2), μmol/min μg prot |
|---|---|---|
| Prdx6+GSTP1-1/GSH (in vitro) | 5.4±0.5 | 5.5±0.5 |
| Prdx6+GSTP1-1 (in vitro) | ND | ND |
| CSF CONTROL (#10) | 0.37±0.05 | 1.44±0.15 |
| CSF TBI A (#20) | 0.132±0.02 | 0.44±0.15 |
| CSF CONTROL (#10) +MJ33 | ND | 1.27±0.15 |
Statistical analysis
All experimental results were performed in triplicate and a statistical analysis was performed using SigmaStat 10.0 (Systat Software, Inc., MA) software. The relevance of statistical differences was evaluated using one-way ANOVA.
Results
Oxidation of thiols is a measure of oxidant stress due to TBI but there is a differential response in that between CSF and plasma
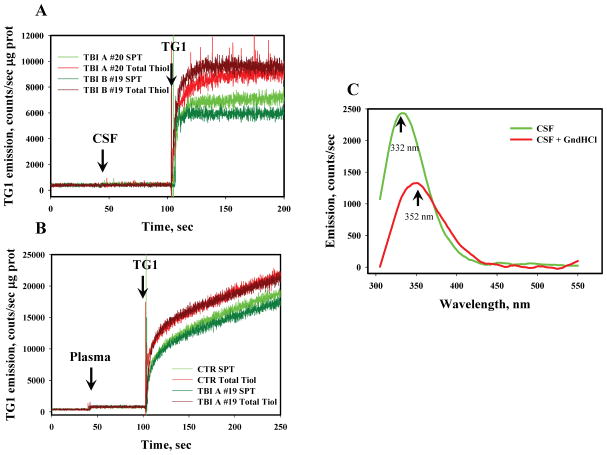
CSF and plasma are the two main physiological fluids that have potential utility for monitoring TBI in human patients. Therefore, as a measure of oxidant stress, the redox status of thiols in CSF and plasma samples from control (non-trauma) and TBI patients was compared. We used size-exclusion chromatography (SEC) to separate the low molecular weight thiols (LMWT) corresponding to GSH from surface protein thiols (SPT) and used the thiol-specific maleimide dye ThioGlo-1 (TG-1), which fluoresces after a specific reaction with sulfhydryls, to measure redox status of thiols in the samples (Fig. 1, panels A and B). Our data show a faster reaction of TG-1 with both LMWT and SPT in CSF (Fig. 1, panel A), as compared to that in plasma (Fig. 1, panel B). Chemical (~ 5.4 M GndHCl) denaturation of proteins was used to evaluate the redox status of buried (inside of protein globule) thiol (BPT) in CSF and plasma. Protein denaturing was confirmed by the tryptophanyl fluorescence analysis (Fig. 1, Panel C). Exposure of protein tryptophanyls to polar media upon denaturing resulted in batochromic shift of emission maximum and, consequently, in a decrease of emission intensity.
Fig. 1. Analysis of total- and protein-thiols in CSF and plasma of control and TBI patients.
Panel A: kinetics of TG-1 (final concentration 5 μM) emission with TBI CSF samples (#19 and 20) diluted (1:100) in 20 mM phosphate buffer (pH=7.4, 37°C) before and after SEC with BS6. Panel B: kinetics of TG-1 emission after plasma sample (TBI #19 and control) dilution (1:100) in 20 mM phosphate buffer (pH=7.4, 37°C) before and after SEC with BS6. Panel C: Effect of GnHCl (~5.4 M) on CSF protein denaturation through analysis of protein tryptophanyl fluorescence (Ex. 295 nm). Data are representative of 3 independent experiments.
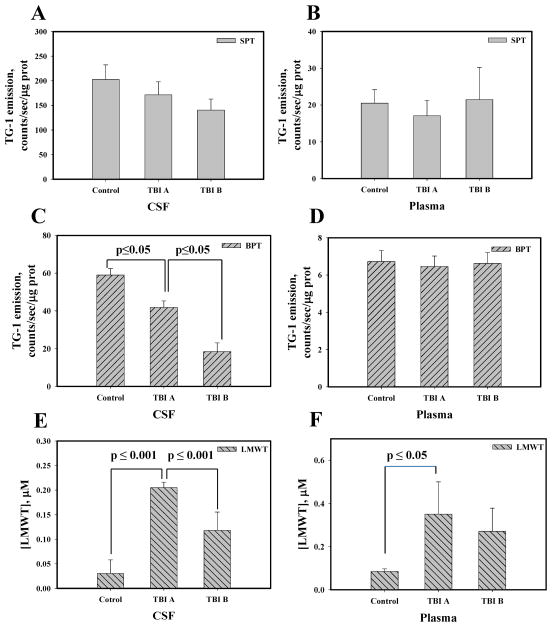
From these analyses, substantial differences between SPT and BPT in CSF from TBI patients were found (Fig. 2, panels A, C and E) when compared to those in plasma (Fig. 2, panels B, D and F). We found a progressive, but not statistically significant, SPT oxidation in CSF of TBI patients in the first 24 hours after their admission that did not occur in plasma (Fig. 2, panel A - CSF, panel B - plasma) suggesting that (1) CSF and plasma may provide different read-outs of TBI-related oxidative stress response, and that (2) perhaps SPTs are not an adequate measure of oxidant stress levels following TBI. However, what we found especially striking was a significant progression in oxidation of BPT in CSF under TBI (Fig. 2, panel C). Such differences were not detected in plasma (at least in the first 24 h) after trauma (Fig. 2, panel D). The lack of the BPT oxidation in the plasma samples corresponds with a variety of major antioxidant enzymes are present in plasma (superoxide dismutase, catalase, glutathione peroxidases etc.), lowering the level of diffusible and relatively stable oxidant (presumably H2O2) able to penetrate inside of the protein globule. These data are compatible with a model that suggests that TBI-mediated specific oxidant stress in CSF progresses after an injury and results in BPT oxidation.
Fig. 2. Redox status of thiols in CSF and plasma of control and TBI patients.
Panels A and B: surface protein thiol (SPT) in CSF and plasma, respectively. Panels C and D: buried protein thiols (BPT) in CSF and plasma, respectively. Panels E and F: low molecular weight thiols (LMWT, mainly GSH) in CSF and plasma, respectively. CSF and plasma matching samples were collected at the time of EVD placement (TBI A) and 24 hours later (TBI B). TG-1 emission was normalized for protein content. For LMWT TG-1 emission was recalculated into μM concentration using linear calibration curve with GSH. Data presented as mean±SD for 3 independent experiments.
Interestingly, the concentration of reduced LMWT (mainly GSH) in CSF is ~2-fold lower than that in plasma (Fig. 2, panels E and F), becomes elevated in CSF after TBI, and then progressively decreases in the 24h after trauma (Fig. 2, panel E). Although at a higher starting concentration, similar changes in reduced LMWT were detected in plasma from acute TBI patients (TBI-A) but any decline in LMWT in 24h samples (TBI-B) was not significant (Fig. 2, panel F). These findings indicate that there is a correlative increase in LMWT in the acute stage of injury that can be measured in both CSF and plasma, and could be a consequence of general injury that releases GSH from damaged tissues. However, a rapid reduction in LMWT occurs 24h post-injury only in CSF (Fig. 2, panel E and F). Together our findings regarding BPT and LMWT suggest that there is a specific differential oxidative response in CSF that is less pronounced in plasma and related to the acute stage of TBI. Therefore, CSF is a potentially more accurate source of diagnostic information related to oxidant stress-related neurological outcomes of TBI that can be monitored temporally.
Prdx6 detection in CSF is not a result of blood contamination and its oxidation correlates with protein monomerization in vitro
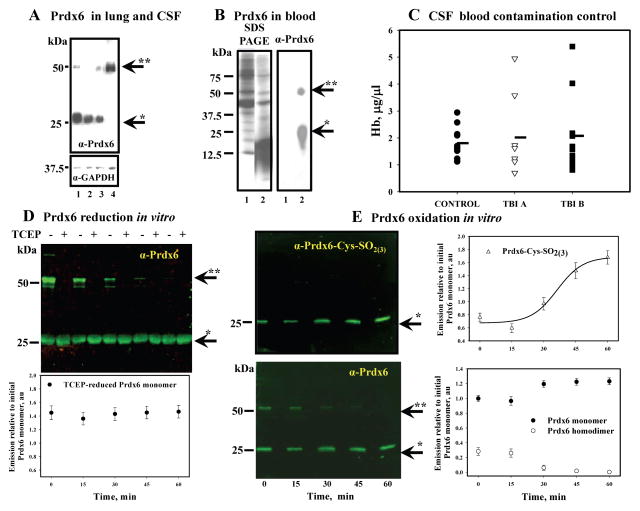
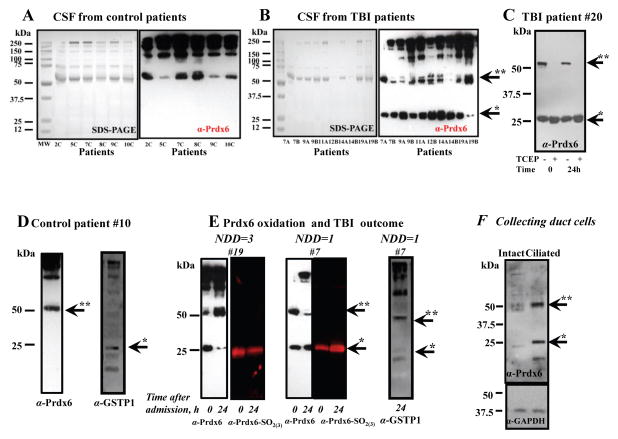
We next hypothesized that specific TBI-induced oxidant stress in CSF could be associated with the (patho)physiological functions of the plasma membrane of HP epithelial (ependymal) cells, of which the basolateral portion comprises a blood-CSF barrier and of which its apical portion is in contact with CSF. Prdx6 is the antioxidant enzyme that directly reduces PLOOH in biomembranes and thus potentially protects the functionality of HP epithelial (ependymal) cell plasma membranes. It is also a major antioxidant enzyme in astrocytes and its catalytic Cys47 is buried inside of the protein globule. Therefore, the presence of Prdx6 in CSF would be ideal, due to its protective effect on cellular membranes. To test this idea, we first sought to examine whether Prdx6 is found in CSF and/or plasma and whether it is active in our CSF samples from TBI patients. Our analysis shows substantial Prdx6 levels in CSF of TBI and control patients, similar to that in lung (the highest Prdx6 expressing organ, Fig. 3A). As severe trauma may result in massive infiltration of blood in CSF, we also looked for the presence of Prdx6 in blood and found it is expressed primarily in red blood cells but not in plasma [23] (Fig. 3B).
Fig. 3. Prdx6 detection in CSF, blood, its oxidation by H2O2 in vitro and CSF blood contamination control.
Panel A: comparison of Prdx6 level in: 1- lung homogenate; 2- TBI CSF; 3 - TBI CSF; 4- control CSF (all lanes contained 20.0 μg of total protein). Panel B: Prdx6 detection in: 1 – plasma (see Materials and Methods) and 2 - RBC lysates from the blood of control patients (SDS PAGE is presented as loading control, 20.0 μg protein per lane). Panel C: dot plot of Hb content in the CSF of control (n=9) and TBI (nTBI A=8, nTBI B=10) patients. Panel D: purified Prdx6 (1.0 μg) monomerization as a result of continuous incubation with 4.0 μM of H2O2 at 37°C in vitro. TCEP reduction of disulfide-based Prdx6 homodimer and quantification (Odyssey Clx, Image Studio 3.1 software) of total reduced monomer normalized for initial (starting material) monomer content before reduction. Panel E: purified Prdx6 (0.5 μg) overoxidation (upper panel, Ab against Prdx6-SO2(3)H) and monomerization (lower panel, same blot with Ab against Prdx6) as a result of its continuous incubation with 2.5 μM of H2O2 at 37°C in vitro. Quantification of Prdx6 overoxidation was fit with sigmoid curve using SigmaPlot 10.0 (SyStat, MA). Quantification of Prdx6 monomeric and dimeric forms was done similar to that in panel D. Data presented as mean±SD for 3 independent experiments. Arrows indicate monomeric (*) and dimeric (**) forms of Prdx6.
Since high levels of Prdx6 in CSF could be a result of its release from RBC, we next determined the haemoglobin (Hb) content in CSF from control and TBI patients in order to assess the level of blood contamination in CSF and how this corresponds with Prdx6 detection. Our analysis shows similar average levels of Hb (~2.0 μg/μl) in CSF from control and TBI patients (Fig. 3C), although individual TBI samples had higher levels of Hb. The latter most likely indicates some level of RBC infiltration/hemolysis caused by trauma. It is known that RBC lysate contains ~ 6.5 – 7.0 mM of Hb and its MW is ~65.5–65.7 kDa [24]. Therefore, using these data we can estimate that the Hb concentration is ~436.05 μg/μl in RBC lysate. Taking this into consideration, our data for Hb detection indicates that ~ 0.5% of RBC is present in our control (non-trauma) CSF. This result corresponds with the known fact that CSF contains some small amount of RBC [2] and validates the accuracy of our analysis. The western blot of RBC lysate (Fig. 3B, lane 2), which was diluted 200 times (to match our actual Hb detection in control CSF) with 10 mM PB (pH=7.4), had non-detectable levels of Prdx6 (data not shown). Alternatively, we found very clear detection of Prdx6 in CSF from control patients (Fig. 3A and 4A). This analysis suggests that RBC contamination/hemolysis is not likely to be a source of Prdx6 in CSF. Moreover, a substantial level of Prdx6 exists in control CSF (Fig. 3A and 4A) and is similar to the level detected in lung (Fig. 3A), suggesting that this enzyme resides in CSF under normal physiological conditions. Furthermore, this finding is the first report of an extracellular Prdx6 in a physiologically relevant setting.
Fig. 4. Detection of Prdx6 oxidation/monomerization and GSTP1-1 oxidation/dimerization dynamics in CSF from control and TBI patients.
Panel A and B: Prdx6 detection in CSF of control and TBI patients, respectively (matching SDS PAGE images are presented as a loading control). Panel C: effects of TCEP (~1.0 mM) reduction on Prdx6 monomerization in CSF of TBI patient (#20). Panel D: Prdx6 dimer and GSTP1-1 monomer detection in CSF of control patient #10. Panel E: Prdx6 monomerization/oxidation(red color) and GSTP1-1 dimerization dynamics detection in CSF of TBI patients with mild (#19) and severe (#7) outcome at the time of ER admission and 24 hours later. Prdx6 blots for patients #7 and #19 were stripped and re-probed anti Prdx6-SO2(3)H Ab and detected with red fluorescent secondary Ab. Panel F: Prdx6 detection in collecting duct cells (40 μM of total protein/lane). Arrows indicate monomeric (*) and dimeric (**) forms of Prdx6 and GSTP1-1. The blots (~15 μg of total protein/lane) are representative of three independent experiments. NDD: Neurological Deficits at Discharge.
Working on the principle that the homeostatic composition of CSF is determined by secretion/absorption of its constituents through apical membranes of CP epithelial/ependymal cells, we also decided to measure the expression of the only other known phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in CSF of both control and TBI patients. Although GPx4 is a seleno-enzyme that protects membranes against lipid peroxidation [25], it was undetectable in all samples (data not shown), adding to our assertion that Prdx6 may be the major mediator of extracellular antioxidant protection of HP epithelial (ependymal) cells apical membranes in CSF.
Our western blot analysis of Prdx6 detected two major bands (~25.0 and ~50.0 kDa) in lung and CSF under non-reducing conditions (Fig. 3A). Therefore, we sought to understand what these form of Prdx6 represent. Continuous treatment of expressed purified Prdx6 (≥ 97.5% homogeneity; 0.4 μM) with 10x molar excess of H2O2 resulted in a progressive decrease of Prdx6 homodimer and a concomitant increase of its monomer (Fig. 3D). In addition, we detected a progressive oxidation of Prdx6 (0.15 μM) to sulfinic (sulfonic) acids by 10x molar excess of H2O2 using specific anti-Prdx6-SO2(3) Ab (LabFrontier, Korea) (Fig. 3E). This blot was stripped and consequently re-probed with anti-Prdx6 Ab. Quantification of Prdx6 homodimer and monomer from these blots show that the increase in Prdx6 oxidation correlates with a decrease of its dimeric form (Fig. 3E). Reduction of Prdx6 with thiol-free reductant TCEP, caused a disappearance of the Prdx6 homodimer, confirming its disulfide origin (Fig. 3D). Additionally our analysis shows that the monomer which results from TCEP reduction represents the sum of homodimer and monomer before reduction (Fig. 3D). These results serve to illustrate that Prdx6 is not overoxidazed in its homodimeric form and its monomerization is consistent with overoxidation.
Prdx6 as a “sensor” of oxidant stress level in CSF
We next examined ex vivo Prdx6 oxidation in CSF from 12 control (non-trauma) samples and from 18 TBI patient samples. Consistent with our findings in Fig. 3A, we detected substantial levels of Prdx6 in CSF from both control and TBI patients (Fig. 4A and B). General pattern of Prdx6-positive high molecular weight bands were similar for CSF from control and TBI patients. Interestingly, only TBI samples had substantial levels of Prdx6 in its monomeric form suggesting overoxidation of this enzyme occurs shortly following trauma. The disulfide nature of the Prdx6 oligomers was confirmed by treating CSF with the reducing agent TCEP showing that oligomeric Prdx6 in CSF can be reduced (Supplemental Fig. S1A, B, and Fig. 4C), similar to our biochemical in vitro analysis (Fig. 3D and E). We used almost double amount of CSF (~25 μg of total protein) from the control patients for Prdx6 detection under non-reducing condition to be sure that an absence of Prdx6 monomer is not a result of its low level (Supplemental Fig. S1A). Our data show similar level of total Prdx6 in CSF from control and TBI patients (Supplemental Fig. S1A, B). We estimated the concentration of total (reduced, reversibly and irreversibly oxidized) Prdx6 using calibration curve from immunodetection of purified expressed protein. Our data show ~ 0.532±0.095 μg/ml for control patients, ~ 0.559±0.068 μg/ml for TBI patients upon their admission to the ER (TBI A), and ~ 0.485±0.052 μg/ml for the patients 24 hours after admission (TBI B) (Supplemental Fig. S2).
Our patient sample analysis revealed an absence of monomeric Prdx6 and the presence of monomeric reduced GSTP1-1 in the CSF from the control (non-trauma) patients (Fig. 4A, D and Supplemental Fig. S1A). Conversely, the oxidized (monomeric) Prdx6 and oxidized (dimeric) GSTP1-1 were found in the CSF of TBI patients (Fig. 4B), also representatively shown with patient #7 (Fig. 4E), who sustained severe TBI trauma. For this patient (who died as a result of trauma, GCS=3T, multiple pelvic fractures and a high ISS, NDD=1), Prdx6 in CSF was progressively oxidized during the 24 hours after admission (Fig. 4E). Under milder trauma, Prdx6 in CSF of TBI patients, represented here with patient #19 (discharged home with OT, PT, and speech therapy, GCS=3 and NDD=3), was initially oxidized (monomeric) but became reduced (dimeric) in the first 24 h after admission (Fig. 4E). These data show that Prdx6 oxidation (monomerization) in CSF is associated with the severity of brain trauma and the dynamics of its dimerization could potentially be predictive for TBI outcomes, where dimeric Prdx6 acts as an indicator of post-trauma recovery.
Furthermore, CSF is in direct contact with the brain and its circulation is directed by the apical membrane microvilli and motile cilia of CP epithelium/ependymal cells, which is consistent with our hypothesis that Prdx6 could be a protective enzyme for cell membrane in contact with CSF. Therefore, we next sought to determine if in general “ciliation” of epithelial cells was linked with Prdx6 expression. Indeed, although not related to CSF, collective duct kidney epithelial cells showed a substantial elevation of intracellular Prdx6 after their “ciliation”, implying that Prdx6 could potentially play an important role in the antioxidant protection of ciliated membrane (Fig. 3F). By association, Prdx6 is likely of importance in CP epithelial/ependymal cells and although we presently do not have evidence to support this assertion, these cells could be a source of Prdx6 for CSF.
Prdx6 glutathione peroxidase function and dynamics of its modulation correlates with TBI outcome
To confirm our idea that Prdx6 activity could act as biomarker to predict patient outcome, we determined if the peroxidase activity of Prdx6 in CSF was affected by TBI. To confirm that the peroxidase activity of Prdx6 decreases upon its oxidation (monomerization), we first assessed samples in vitro (Fig. 3E) to demonstrate that a decrease in activity corresponds with a decrease in its homodimer content (Fig. 5A). We next measured general and specific Prdx6 peroxidase activities in CSF of control and TBI patients using H2O2 and PLPCOOH as substrates, respectively. Our data showed a similar decrease (~60%) of both general and peroxidase activities in CSF as a consequence of TBI (Fig. 5B and Table 2), when compared to that in control (non-trauma) CSF. These results are compatible with TBI-mediated oxidation of Prdx6’s catalytic Cys47, which is buried inside of protein globule and denotes disruption of Prdx6 dimerization in the CSF of TBI patients.
The ratio of Prdx6 bands corresponding with its dimeric (under non-reducing condition) to monomeric (under reducing condition) forms in the same CSF samples correlates with the degree of enzyme oxidation (inactivation) (Fig. 4B and Supplemental Fig. S1B). Using this ratio we evaluated the correlation between Prdx6 oxidation (inactivation) upon admission and 24 hours later, relating this ratio to neurological deficits at discharge generating a scale which we propose could be used to determine severity of TBI outcome (Table 1). Based on our assessment, we ranked severity of neurological deficits, with “1” being the most severe and “4” being the least severe according to our scale. Our data show that extensive oxidation (i.e. loss of activity) of Prdx6 acutely after TBI (TBI A) and its continued oxidation in the following 24h after trauma (TBI B) correlates with increased severity of neurological deficits which could be a reason or a consequence of death (Fig. 5C). Alternatively, reduction (reactivation) of Prdx6 in CSF in the first 24h after TBI appeared to correlate with milder neurological deficits (Fig. 5C).
In aggregate our results demonstrate that analyzing oxidant stress in CSF can be utilized clinically to rapidly define outcomes for TBI patients within the first 24h after trauma. Moreover, we not only identify the critical enzyme, Prdx6, that performs the peroxidase functions associated with TBI injury and recovery but, we also showed that Prdx6 is present in a physiologically relevant extracellular locale, CSF, that can be used for patient assessment following injury. Intriguingly, these findings pave the way for the development of future diagnostic and therapeutic elements which currently do not exist for TBI.
Discussion
The diagnosis and prognosis of TBI is influenced by the extent of acute impact at the time of injury and the corresponding challenge to the brain’s antioxidant capacity [26]. Our present study focuses on the assessment of plasma and CSF from TBI patients to analyze the relationship between oxidative stress damage and recovery from TBI. By measuring the LMWT, the SPT, and the BPT in CSF and plasma of 12 non-trauma and 18 TBI patients, we concluded that the outcome of patients with severe TBI may be linked with the progressive and significant oxidation of BPT in CSF (Fig. 2C). This type of oxidant stress is compatible with the extensive generation of low molecular weight, diffusible, and relatively stable oxidant that is generated in CSF under TBI. There is evidence that a likely candidate is H2O2. First, H2O2 was used in multiple studies for oxidation of protein thiol and has a reasonable reaction rate with a low pK cysteines [27]. Second, H2O2 effectively oxidizes GSH into GSSG [28], which corresponds with our detection of dramatic LMWT (mainly GSH) oxidation under TBI (Fig. 2E). The immediate LMWT increase in CSF following TBI could conceivably be a result of some RBC infiltration/hemalysis presented in this study (Fig. 3C). The magnitude of this effect may reflect the substantial content of GSH in RBC (~10 mM) and relatively small volume of CSF(~150 ml). A similar situation was detected in plasma but with smaller effects, considering its substantial volume and a presence of multiple antioxidant enzymes (Fig. 2F). The minimal (if any) effect of TBI we observed on SPT and BPT in plasma (Fig. 2B and D) could be explained by similar reasons with the addition of substantially higher protein and GSH content as compared to that in CSF.
We further determined that Prdx6 is likely the major peroxidase in CSF, since this enzyme was quite abundant in CSF—as high as in lung (Fig. 3A) —and we were unable to detect the only other known enzyme of this type, GPX4, in CSF of both control and TBI patients. We additionally found in CSF that Prdx6 peroxidase activity is supported by GSH and monomeric GSTP1-1 (Fig. 2E and Fig. 4D), resulting in the detoxification of lipid peroxides (Fig. 5B). However, precisely how Prdx6 and GSTP1-1 are released into the CSF compartment is presently unclear. We postulate that there may be a host response to the TBI injury that mediates an excessive release of these proteins by CP epithelial/ependymal (or other) cells. This idea is supported by our observation that ciliated epithelial cells overexpress Prdx6 (Fig. 4F). The high molecular weight bands with positive Prdx6 detection are similar for control and TBI CSF samples and most likely represent some aggregates of Prdx6 with membrane-associated proteins from CP epithelial (ependymal) cells and/or RBC. It is plausible that these oligomers could also be involved in Prdx6 secretion/absorption (Supplemental Fig. S1A, B). However, further studies are required to determine the mechanism(s) of these enzymes secretion into and absorption from CSF.
Functionally, the presence of Prdx6 and GSTP1-1 in CSF could presumably provide antioxidant protection for the associated CP epithelial/ependymal cell apical (ciliated) membranes. One can imagine a model where active Prdx6 can prevent lipid peroxidation, serving to remove peroxidized acyl chains of cilia or villi that are exposed to CSF. Consequently, this would support a continuous beating of CP epithelial/ependymal cilia for the constant flow of CSF that is necessary for proper brain homeostasis.
Because Prdx6 and GSTP1-1 are found in the CSF of non-trauma patients, an enhanced detection of these proteins in CSF could serve as a predictive biomarker of the severity of TBI or a surrogate for treatment and resuscitation. This concept is supported by our findings that Prdx6 becomes monomeric (overoxidized/inactive) in CSF of TBI patients and that dimerization (reduction/reactivation) of Prdx6 correlates with recovery (Figs. 4E and 5C). We surmise that formation of the Prdx6 dimers is a chemical reaction between sulfhydryl and sulfenate forms of Prdx6 Cys47 which becomes exposed after protein denaturing upon boiling in SDS-containing loading buffer without reducing agent. Moreover, there are at least two reactions of Prdx6 monomers condensation into homodimer with reasonable rate [29]:
The reasonable probability of such reactions corresponds with fact that cysteine sulfenic acid is generally very reactive and in the presence of O2 rapidly undergoes oxidation into cysteine sulfinic(sulfonic) acids. Oxidation of Prdx6 catalytic Cys47 into sulfenic acid (reduceble by heterodimerization with GSH-loaded GSTP1-1) is relatively stable (detected upon crystalization of this protein) [30]. To prevent additional oxidation of Prdx6 we saturated the loading buffer with nitrogen before boiling. TCEP-mediated reduction of CSF results in the disappearance of all Prdx6-positive bands except for the monomer. These results correspond with the disulfide-based nature of the Prdx6-oligomer formation and apparently corroborate with our hypothesis that Prdx6 sulfenate (after protein denaturation) mediates this interaction (Supplementary Fig. S1 A, B). Alternatively, irreversible oxidation of Prdx6 cysteine into sulfinic(sulfonic) acids are very stable, need specific conditions for activation (25), and most likely remains monomeric under non-reducing and reducing SDS PAGE. Thus, the dimeric form of Prdx6 apparently represents its active portion and the monomeric form of Prdx6 corresponds with its inactive portion. Our analysis of Prdx6 peroxidase activity (Fig. 5A) in vitro using aliquots of protein (used for WB analysis, Fig. 3E) with addition of equimolar amount of GSH-loaded GSTP1-1 and H2O2 as a substrate shows its deactivation, which corresponds with both: protein overoxidation and monomerization (Fig. 3E). The exact mechanism of Prdx6 dimerization under non-reducing SDS PAGE is unknown and requires an additional studies. TCEP-mediated reduction of -SOS- bond to the best of our knowledge also was not reported. Although, -SOS- bond could isomerizes into –S(O)-S- and disulfide could be effectively reduced by TCEP. This apparently corresponds with our complete reduction of Prdx6 dimeric form (Figs. 3D, 4C and Supplementary Fig. S1A, B). Finally, we detected Prdx6 overoxidation in CSF from TBI patients and its dynamics correlates with severity of neurological deficiency outcome (Fig. 4E).
Removal of lipid peroxides in the apical membranes of CP epithelial/ependymal cells is time-dependent, thus a secretion/absorption of CSF metabolic components (including both reduced as well as over-oxidized Prdx6 and GSTP1-1) could be compromised during TBI. According to our model, oxidant stress occurs due to the acute injury caused by TBI and resulting in an inactivation of Prdx6. However, during the time post-injury (i.e. 24h after hospitalization), Prdx6 activity stabilizes resulting in a decrease of oxidant stress. Under this schematic, it is possible that when damage reaches a certain threshold, Prdx6 activity cannot be recovered and thus, damage could become irreversible causing CNS impairment. Alternatively, below this threshold (mild TBI) Prdx6 activity could be recovered, CNS functions became less compromised indicating that the patient will eventually recover, as we illustrate in our findings (Fig. 4E). However, additional studies are needed to further evaluate this hypothesis.
Our current model is further supported by findings that another peroxiredoxin, Prdx4, (the only known secretory member of the peroxiredoxin superfamily) was suggested as a “novel biomarker of oxidative stress in patients with “sepsis shock” [31] and this may be an indication of the general involvement of the peroxiredoxins in diseases that are linked with the “compartmentalized” antioxidant protection. Our discovery of Prdx6 in CSF fits well with this concept; however, the actual mechanism of how this enzyme is trafficked in CSF remains unclear. Moreover, the relevance of the dual-functionality of Prdx6 as a specific phospholipid hydroperoxide peroxidase/PLA2 has not previously been related to be of a particular physiological significance and our present data support the idea that its presence in CSF provides a unique combination of phospholipid hydroperoxide elimination activities. Severe TBI results in the inactivation of Prdx6 peroxidase function, but also in a CSF acidosis [2]. This can activate the Ca++-independent acidic Prdx6 PLA2 functions, while still providing protection to the apical membranes of CP epithelial/(ependymal) cells.
While not considered in the present study, we have recently reported that the allelic variation of GSTP1-1 results in specific differences in activation of Prdx6 [32]. Conceivably, GSTP1-1 polymorphisms could be of consequence in determining individual TBI susceptibility and its outcomes. In this regard the Prdx6/GSTP1-1 axis in CSF could potentially play an important role in the etiology of a number of neurological diseases and future consideration could be given to the individual GSTP1-1 genotyping. Our last remaining consideration is the type of the low molecular weight, diffusible oxidant under TBI, which we postulate could be H2O2, and its source. We theorize that H2O2 may be the most probable candidate because we showed that it effectively oxidizes Prdx6 in vitro (Fig. 3, panel B). Furthermore, additional studies show that a brief exposure of the ventricular surface of the brain to the high concentrations of H2O2 (3.0%; 1.1 M) results in a cessation of ciliary beating and extensive damage of the ependymal cells (disappearance of individual cilia) [33]. In TBI, lipid peroxidation amplifies as a chain reaction and can cause temporal accumulation of damage localized to cell membranes [34]. Moreover, elevated oxidant stress in CSF of TBI patients results in progressive oxidation of both Prdx6 and GSTP1-1 (Fig. 4B, C and E), as well as in its inactivation (Fig. 5B; Table 2), compromising antioxidant protection of apical membranes of CP epithelial/ependymal cells. Thus, hypothetically the extent of Prdx6 oxidation/inactivation could specifically correlate with H2O2, the levels of which in CSF, could be associated with severity of TBI-related neurological deficits (Fig. 5C). The source of H2O2 is more difficult to determine. It is plausible that under TBI, CSF contains elevated levels of H2O2 corresponding to CSF flow changes and (or) blood flow cessation, both of which correspond with the severity of TBI. Could brain vasculature be a source? According to the Munro-Kellie doctrine [35], the total intracranial volume equals the sum of the brain, intracranial blood and CSF volumes. The brain is the largest component of this isolated system and therefore, has the highest inertia. Since intracranial volume is held constant due to the fixed dimensions of the skull, any inertial displacement of the brain will cause simultaneous compression/relaxation of CSF and consequently, compression of the vasculature.
Idealizing an intracranial space as a “closed hydrodynamic system” and considering a constant flow of CSF, one can apply the Bernoulli law postulating that the sum of dynamic and static pressures for any flow of liquid is a constant. As a result, an increased cross-section of the CSF flow results in an increase of the intracranial (“static”) pressure and a consequent decrease of its flow. The precise response of CP epithelial and (or) ependymal cells (lining all spaces perfused by CSF) to the CSF flow and the intracranial pressure changes is not known. However, ischemia in the brain microvascular endothelial cells does activate the NADPH oxidase-mediated generation of intra- or extra-cellular superoxide anion radicals (O2*−, SO) [36, 37], which spontaneously (or under SOD catalysis) dismutates into the more stable H2O2. Human aquaporin AQP1 found in apical membranes of the ependymal cells [38] can mediate H2O2 transport across membranes [39]. Moreover, the blood-CSF barrier, which consists of microvascular endothelial cells, pia matter, and special epithelial CP/ependymal cells, is penetrable for both water and H2O2 [40]. Therefore it is mechanistically plausible for TBI to cause localized accumulation of H2O2 that is a substrate for Prdx6 found in CSF.
Currently there are limited treatment options for TBI. In severe TBI, the primary goal is to maintain neurological parameters, adequate perfusion and normal intracranial pressure. Neuroprotectants and hypothermia have been explored as possible treatments for severe TBI but results have not been promising with the exception of progesterone currently being under investigation in two major studies [41]. Therefore it is an actual necessity in reliable biomarkers of TBI severity outcome as well as of applied therapy efficacy control.
Conclusions
Our present data indicate that Prdx6 is a physiologically significant redox-sensitive antioxidant component of CSF. The fact that Prdx6 protects biological membranes against lipid peroxidation and is a major antioxidant enzyme in astrocytes, may provide a cause-effect relationship as a prognostic predictor of severity of TBI outcome. There is a meaningful correlation between TBI-initiated oxidation of Prdx6, its specific phospholipid hydroperoxide peroxidase activity, and severity of trauma outcome. Consequently, we propose that Prdx6 redox status detection has the potential to be a biomarker for TBI outcome and a future indicator of therapeutic efficacy. Prdx6 and its physiological activator GSTP1-1 in CSF could serve as potential targets for therapeutic interventions in TBI patients.
Supplementary Material
Highlights.
TBI induces fast rise and decay of GSH and oxidation of buried protein thiol in CSF
Prdx6 was detected in CSF at substantial levels
TBI results in Prdx6 oxidation and deactivation of its peroxidase function in CSF
Prdx6 redox state correlates with TBI neurological outcome
Prdx6 could serve as a biomarker of TBI outcome and of therapeutic efficacy
Acknowledgments
Authors thank P. Darwin Bell, Ph.D. and Ms. S. Steele (Department of Nephrology of the Medical University of South Carolina) for their help with collective duct epithelial cells culture, Ms. R. J. Navarro, MSN/MHA (Division of Emergency Medicine of the Medical University of South Carolina) for CSF and plasma samples collection and Elizabeth Yeh, Ph.D. (Department of Cell and Molecular Pharmacology and Experimental Therapeutics of the Medical University of South Carolina) for helpful discussions prior to submission of our manuscript.
Funding.
This publication was supported by grants from the NIH (CA08660, CA117259, NCRR P20RR024485 - COBRE in Oxidants, Redox Balance and Stress Signaling) and support from the South Carolina Centers of Excellence program and was conducted in a facility constructed with the support from the NIH, Grant # C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. This publication was also supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant # UL1 TR000062 and Dr. K Borg was supported through NIH Grants # KL2 RR029880 and KL2 TR0.
Abbreviations
- TBI
traumatic brain injury
- CSF
cerebrospinal fluid
- LMWT
low molecular weight thiol
- SPT
surface protein thiol
- BPT
buried protein thiol
- Prdx6
peroxiredoxin VI
- LP
lipid peroxidation
- PLPCOOH
1-palmitoyl-2-linolenoyl-hydroperoxy-sn-glycero-3-phosphoryl choline
- CP
choroid plexus
Footnotes
Competing interests:
Authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schneier AJ, et al. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118(2):483–92. doi: 10.1542/peds.2005-2588. [DOI] [PubMed] [Google Scholar]
- 2.Irani DN. Cerebrospinal Fluid in Clinical Practice. Saunders (an imprint of Elsevier Inc.); 2009. [Google Scholar]
- 3.Bayir H, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatric research. 2002;51(5):571–8. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(2):422–7. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leichert LI, Jakob U. Global methods to monitor the thiol-disulfide state of proteins in vivo. Antioxidants & redox signaling. 2006;8(5–6):763–72. doi: 10.1089/ars.2006.8.763. [DOI] [PubMed] [Google Scholar]
- 6.Tyurin VA, et al. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. Journal of neurochemistry. 2000;75(5):2178–89. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- 7.Cserr HF. Physiology of the choroid plexus. Physiological reviews. 1971;51(2):273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- 8.Sultana R, Perluigi M, Allan Butterfield D. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free radical biology & medicine. 2013;62:157–69. doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subczynski WK, Hyde JS. Concentration of oxygen in lipid bilayers using a spin-label method. Biophysical journal. 1983;41(3):283–6. doi: 10.1016/S0006-3495(83)84439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed TT. Lipid peroxidation and neurodegenerative disease. Free radical biology & medicine. 2011;51(7):1302–19. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Manevich Y, et al. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11599–604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free radical biology & medicine. 2005;38(11):1422–32. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Power JH, et al. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta neuropathologica. 2008;115(6):611–22. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen A, et al. Proteomic and phosphoproteomic analyses of the soluble fraction following acute spinal cord contusion in rats. Journal of neurotrauma. 2010;27(1):263–74. doi: 10.1089/neu.2009.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(11):3780–5. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manevich Y, et al. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. Journal of lipid research. 2007;48(10):2306–18. doi: 10.1194/jlr.M700299-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, et al. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. The Biochemical journal. 2009;419(3):669–79. doi: 10.1042/BJ20082061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg ME, et al. The lipid whisker model of the structure of oxidized cell membranes. The Journal of biological chemistry. 2008;283(4):2385–96. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 19.Saigusa T, et al. Collecting duct cells that lack normal cilia have mislocalized vasopressin-2 receptors. Am J Physiol Renal Physiol. 2012;302(7):F801–8. doi: 10.1152/ajprenal.00253.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resnick A, Hopfer U. Force-response considerations in ciliary mechanosensation. Biophysical journal. 2007;93(4):1380–90. doi: 10.1529/biophysj.107.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetzl L, et al. Maternal and fetal oxidative stress and intrapartum term fever. American journal of obstetrics and gynecology. 2010;202(4):363, e1–5. doi: 10.1016/j.ajog.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uys JD, et al. Cocaine-induced adaptations in cellular redox balance contributes to enduring behavioral plasticity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36(12):2551–60. doi: 10.1038/npp.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuhlmeier KM, et al. Antioxidant protein 2 prevents methemoglobin formation in erythrocyte hemolysates. European journal of biochemistry/FEBS. 2003;270(2):334–41. doi: 10.1046/j.1432-1033.2003.03393.x. [DOI] [PubMed] [Google Scholar]
- 24.Esposito A, et al. FRET imaging of hemoglobin concentration in Plasmodium falciparum-infected red cells. PLoS One. 2008;3(11):e3780. doi: 10.1371/journal.pone.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochimica et biophysica acta. 1985;839(1):62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 26.Bayir H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Developmental neuroscience. 2006;28(4–5):420–31. doi: 10.1159/000094168. [DOI] [PubMed] [Google Scholar]
- 27.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free radical biology & medicine. 2008;45(5):549–61. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free radical biology & medicine. 1999;27(3–4):322–8. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 29.Jeong J, et al. Novel oxidative modifications in redox-active cysteine residues. Molecular & cellular proteomics: MCP. 2011;10(3):M110 000513. doi: 10.1074/mcp.M110.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi HJ, et al. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat Struct Biol. 1998;5(5):400–6. doi: 10.1038/nsb0598-400. [DOI] [PubMed] [Google Scholar]
- 31.Schulte J, et al. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock. 2011;35(5):460–5. doi: 10.1097/SHK.0b013e3182115f40. [DOI] [PubMed] [Google Scholar]
- 32.Manevich Y, et al. Allelic variants of glutathione S-transferase P1-1 differentially mediate the peroxidase function of peroxiredoxin VI and alter membrane lipid peroxidation. Free radical biology & medicine. 2013;54:62–70. doi: 10.1016/j.freeradbiomed.2012.10.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirst RA, Rutman A, O’Callaghan C. Hydrogen peroxide at a concentration used during neurosurgery disrupts ciliary function and causes extensive damage to the ciliated ependyma of the brain. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2009;25(5):559–61. doi: 10.1007/s00381-008-0768-4. [DOI] [PubMed] [Google Scholar]
- 34.Hunter MI, Nlemadim BC, Davidson DL. Lipid peroxidation products and antioxidant proteins in plasma and cerebrospinal fluid from multiple sclerosis patients. Neurochem Res. 1985;10(12):1645–52. doi: 10.1007/BF00988606. [DOI] [PubMed] [Google Scholar]
- 35.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56(12):1746–8. doi: 10.1212/wnl.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 36.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(8):989–91. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
- 37.Basuroy S, et al. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. American journal of physiology Cell physiology. 2009;296(3):C422–32. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takata K, et al. Aquaporins: water channel proteins of the cell membrane. Progress in Histochemistry and Cytochemistry. 2004:1–83. doi: 10.1016/j.proghi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Bienert GP, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. The Journal of biological chemistry. 2007;282(2):1183–92. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 40.Laterra JK, Betz RL, Goldstein G. Blood—Cerebrospinal Fluid Barrier. In: Siegel ABGJ, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6. Lippincott-Raven; Philadelphia: 1999. [Google Scholar]
- 41.Wright DW, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49(4):391–402. 402 e1–2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.