Abstract
Objectives
Infants with bronchopulmonary dysplasia (BPD) often undergo gastrostomy tube (GT) placement and/or Nissen fundoplication (Nissen) to improve weight gain and to attenuate chronic respiratory symptoms related to feeding difficulties. After initial hospitalization little is known how these children do with regard to respiratory symptoms when compared to children with BPD who did not receive GTs. This study was done to determine if differences in respiratory outcomes were associated with the presence of a GT or Nissen/GT in children with BPD during the first two years of life.
Methods
Children (n=398) were recruited from the Johns Hopkins BPD Outpatient Clinic. Medical charts were reviewed and acute care usage and respiratory symptoms were assessed by caregiver questionnaires.
Results
Ninety-two children with BPD had GTs, with the majority placed by 6 months of age. Of children with GTs, 64.7% also had Nissen fundoplication. Children with Nissen/GTs were more likely to have birth weights ≤ 10th percentile and to be discharged on supplemental oxygen. After initial hospitalization, children with GTs and Nissen/GTs weaned off supplemental oxygen at significantly older ages than children without GTs. Children with Nissen/GTs also had more hospitalizations than children without GTs. Caregivers of children with GTs and Nissen/GTs reported similar respiratory symptoms as caregivers of children without GTs.
Conclusion
Weaning off supplemental oxygen occurred later in children with GTs and Nissen/GTs compared to children without GTs. Although children with Nissen/GTs had more re-hospitalizations, there were not differences in reported respiratory symptoms between any of the groups by caregiver questionnaire.
Keywords: Prematurity, bronchopulmonary dysplasia, Gastrostomy tube, Nissen Fundoplication, respiratory outcomes
Introduction
Approximately 12% of all infants born in the United States are preterm, with up to 50,000 infants each year weighing less than 1500 grams.1,2 These very low birth weight infants (VLBW) infants are at high risk for developing bronchopulmonary dysplasia (BPD) and other co-morbidities including developmental and feeding-related complications.3
Swallowing dysfunction is a common problem among preterm infants, particularly those with BPD.4 Furthermore, swallowing dysfunction likely contributes to chronic respiratory symptoms in children with BPD. Recently, Mizuno and colleagues reported that infants with BPD on supplemental oxygen had less effective swallowing, higher respiratory rates and more oxygen desaturations compared to preterm infants without BPD.5 They also reported that infants with BPD on supplemental oxygen had lower sucking pressures, shorter sucking frequencies and shorter durations of sucking resulting in lower volume intake.5 This study and others indicate that poor nutrition, swallowing dysfunction and gastroesophageal reflux (GER) may be risk factors for worsening lung disease, poor lung recovery and impaired lung growth in infants with BPD.5,6,7
Limited volume intake and greater energy expenditures in the infant with BPD can increase the risk of growth failure after initial discharge from the hospital.7 Indeed, Wang and colleagues found that VLBW infants with severe BPD had greater rates of growth delay between 2–6 months corrected age. They also found that these infants had significantly lower oxygen saturations with oral feeds when compared to term and VLBW infants with mild BPD.8 Poor weight gain has also been associated with subsequent neurological difficulties in infants with BPD, although BPD severity alone has been correlated with poorer neurological outcomes.9,10,11
The decision to place a gastrostomy tube in an infant with BPD is often influenced by the presence of poor weight gain and/or chronic respiratory symptoms related to swallowing dysfunction. In addition, surgical anti-reflux procedures such as Nissen fundoplication are often performed in children with BPD who are unresponsive to medical treatment for symptomatic GER and when GER contributes to ongoing respiratory symptoms and/or poor weight gain. Anticipated outcomes from these surgical interventions include improved nutrition, better postnatal lung growth, and avoidance of further lung damage in a high risk population.
To determine if respiratory outcomes in the first two years of life differed among BPD children with or without a GT, we reviewed medical charts and assessed acute care usage and respiratory symptoms by caregiver questionnaires from patients recruited from the Johns Hopkins Outpatient BPD clinic. We hypothesized that BPD children with GTs (or Nissen/GTs) would have more respiratory morbidities and respiratory symptoms during the first two years of life compared to BPD children without GTs, in part due to their greater respiratory severity on presentation to the initial outpatient visit. In this study we also sought to determine the age in which children with BPD were weaned off supplemental oxygen and if age of weaning differed with respect to GT or Nissen/GT status. Secondary goals of this study included identifying factors associated with GT or Nissen/GTs placement and the age in which GTs or Nissen/GTs were placed.
Methods
Study Sample
All subjects (n=398) were recruited and consented from the Johns Hopkins Bronchopulmonary Dysplasia (BPD) Clinic between January 2008 and December 2012. Patients with the diagnosis of BPD were referred to the clinic by area neonatal intensive care units or pediatricians. Subjects were recruited if they met the inclusion criteria, which included (1) a diagnosis of BPD by the referring NICU or staffing pediatric pulmonologist, (2) born preterm (≤36 weeks of gestation), and (3) clinical data for review before the age of 2 years. This study was approved by the Johns Hopkins University Institutional Review Board.
Demographics/Socio-economic Factors
Birth weight percentile was derived from U.S. norms for gestational ages.12 Race/ethnicity, primary caregiver education level, and secondhand smoke exposure were self-reported. Insurance coverage (private vs. public) was obtained from billing records. Median household income was derived using residential zip codes and data from 2007–2011 American Community Survey from U.S. Census Bureau.
Clinical Data
Dates of initial discharge and first BPD clinic encounter, and the presence/absence of gastrostomy tubes, Nissen fundoplications, and respiratory support were ascertained at the first BPD clinic encounter through chart review. Inhaled corticosteroid use was defined as any use prior to 2 years of age based on chart review.
Respiratory Morbidities
Respiratory morbidities were assessed using Yes/No questions on questionnaires completed by caregivers. Primary outcomes included emergency department visits, hospital admissions, systemic steroid use, and antibiotic use for respiratory reasons since the last BPD clinic visit (or since initial hospital discharge if assessed at the first BPD clinic visit). Secondary outcomes included the presence/absence of trouble breathing, rescue beta-agonist use, activity limitations, and nighttime symptoms. The secondary outcomes were assessed as occurring 0 days, 1–3 days, and ≥4 days within the past week, but for the purposes of analysis, secondary outcomes were dichotomized into occurring or not occurring in the past week.
Statistical Methods
Demographic frequencies and clinical outcomes stratified by presence of GT or Nissen fundoplication were compared using chi square and t tests. Kaplan-Meier methodology was used to analyze the age at which 92 subjects with GTs had them inserted or removed. With regards to GT removal, 24 subjects have had their GTs removed with the date of removal known, and the data were censored for the remaining 68 subjects with GTs still in place or lost to follow-up. The relationship between the presence of a GT and respiratory morbidities at each clinical visit was assessed using logistic regression adjusted for the age of the subject at the time of questionnaire completion, health insurance status, small for gestational age status, inhaled corticosteroid use, home supplemental oxygen, and home ventilator support. As caregivers may have completed questionnaires at several clinic visits, the logistic regressions accounted for this using Generalized Estimating Equations (GEE) methodology (clustered by subject).13 STATA IC 11 (StataCorp LP, College Station, TX) was used for all statistical analyses. P values <0.05 were considered statistically significant.
Results
Demographics of entire study sample
Three hundred and ninety-eight patients diagnosed with BPD were recruited from the Johns Hopkins Outpatient Bronchopulmonary Dysplasia Clinic between 2008–2012. The average gestational age of subjects was 26.8±2.8 weeks and the average birth weight was 949±472 grams (Table 1). Ninety-two (23.1%) subjects had GTs and of the 92 subjects with GTs, 62 (67.4%) also had Nissen fundoplications. The 153 (38.4%) subjects receiving respiratory support at home included 144 children on supplemental oxygen via nasal cannula and 9 children on home mechanical ventilation (4 with supplemental oxygen entrainment and 5 without).
Table 1.
Study Sample Demographics
| Mean (± SD) [Range] |
Entire Study Sample |
No Gastrostomy Tube |
Gastrostomy Tube without Nissen |
Gastrostomy Tube with Nissen |
P Value* |
||
|---|---|---|---|---|---|---|---|
| N | 398 | 306 | 30 | 62 | - | ||
| Demographics | Sex (% Male) | 59.1 | 60.5 | 63.3 | 50.0 | 0.28 | |
| Gestational Age (weeks) | 26.8 ± 2.8 [22.7 – 36] |
26.9 ± 2.7 [22.7 – 36] |
25.9 ± 2.8 [23 – 36] |
27.0 ± 3.3 [23 – 35] |
0.18 | ||
| Birth Weight (grams) | 949 ± 472 [380 – 3181] (n = 384) |
966 ± 474 [390 – 3181] (n = 297) |
800 ± 314 [380 – 1780] (n = 29) |
939 ± 516 [390 – 3130] (n = 58) |
0.19 | ||
| Birth Weight Percentile (%) | 40 ± 23 [1 – 95] (n = 384) |
41 ± 22 [1 – 95] (n = 297) |
43 ± 26 [4 – 89] (n = 29) |
32 ± 23† [1 – 88] (n = 58) |
0.019 | ||
| Birth Weight ≤ 10th Percentile (%) | 11.7 (n = 384) |
8.8 (n = 297) |
17.2 (n = 29) |
24.1† (n = 58) |
0.002 | ||
| Race/Ethnicity (% Non-white) | 67.6 | 67.7 | 60.0 | 71.0 | 0.57 | ||
| Socio-economic Characteristics | Public Insurance (% Yes) | 61.3 | 57.5 | 71.0 | 75.8† | 0.016 | |
| Estimated Household Income ($’000) | 62.8 ± 21.5 [15.6 – 156.6] |
61.9 ± 20.3 [15.6 – 156.6] |
66.4 ± 27.3 [28.5 – 156.6] |
65.0 ± 23.8 [21.9 – 126.7] |
0.30 | ||
| Primary Caregiver Education Level (%) |
< High School High School Grad. Some College College Graduate Any Post-Grad. Ed. |
9.3 22.2 30.1 21.9 16.5 (n = 279) |
8.0 22.6 30.7 21.2 17.5 (n = 212) |
15.0 20.0 25.0 20.0 20.0 (n = 20) |
12.5 20.8 31.3 25.0 10.4 (n = 48) |
0.85 | |
| Clinical Characteristics | Age at Initial Discharge (months) | 4.1 ± 2.6 [0.1 – 24.5] (n = 395) |
3.4 ± 1.6 [0.1 – 15.6] (n = 303) |
5.7 ± 2.0† [0.4 – 10.3] |
6.9 ± 4.1‡ [0.23 – 24.5] |
<0.001 | |
| Age at First BPD Outpatient Visit (months) | 7.5 ± 5.6 [0.9 – 51.3] |
6.7 ± 5.1 [0.9 – 41.6] |
9.6 ± 5.2† [4.7 – 29.0] |
10.6 ± 6.9† [3.1 – 51.3] |
<0.001 | ||
| Home Ventilator Support (% Yes) | 2.3 | 0.0 | 0.0 | 14.5‡ | <0.001 | ||
| Home Supplemental Oxygen (% Yes) | 36.9 | 32.4 | 46.7 | 56.5† | 0.001 | ||
| Inhaled Corticosteroid Use prior to 2 Years of Age (% Yes) | 79.2 | 74.8 | 90.0 | 95.2† | <0.001 | ||
Chi-square tests were conducted for dichotomous and categorical variables and ANOVA tests for continuous variables for the three groups of subjects (No GT, GT only, and GT with Nissen fundoplication).
Bonferroni p<0.05 compared to group without GTs.
Bonferroni p<0.05 compared to both group without GTs and group with GTs.
With regard to gestational age and absolute birth weight, we found no significant differences between children with or without GTs or Nissen/GTs (Table 1). Infants with Nissen/GTs but not GTs alone, were more likely to have a lower birth weight percentile, be small for gestational age, and to be covered by public insurance compared to infants without GTs. Infants with GTs and Nissen/GTs were discharged later and likewise seen at an older age in BPD clinic compared to those without GTs (ANOVA p values both <0.001). However, there was no difference in the duration of time between initial NICU discharge and first BPD clinic visit (ANOVA p=0.76). All nine infants on home ventilators had Nissen/GTs. Home supplemental oxygen use and any inhaled corticosteroid use were more frequent among infants with Nissen /GTs compared to infants without GTs.
Clinical course of gastrostomy tube placement
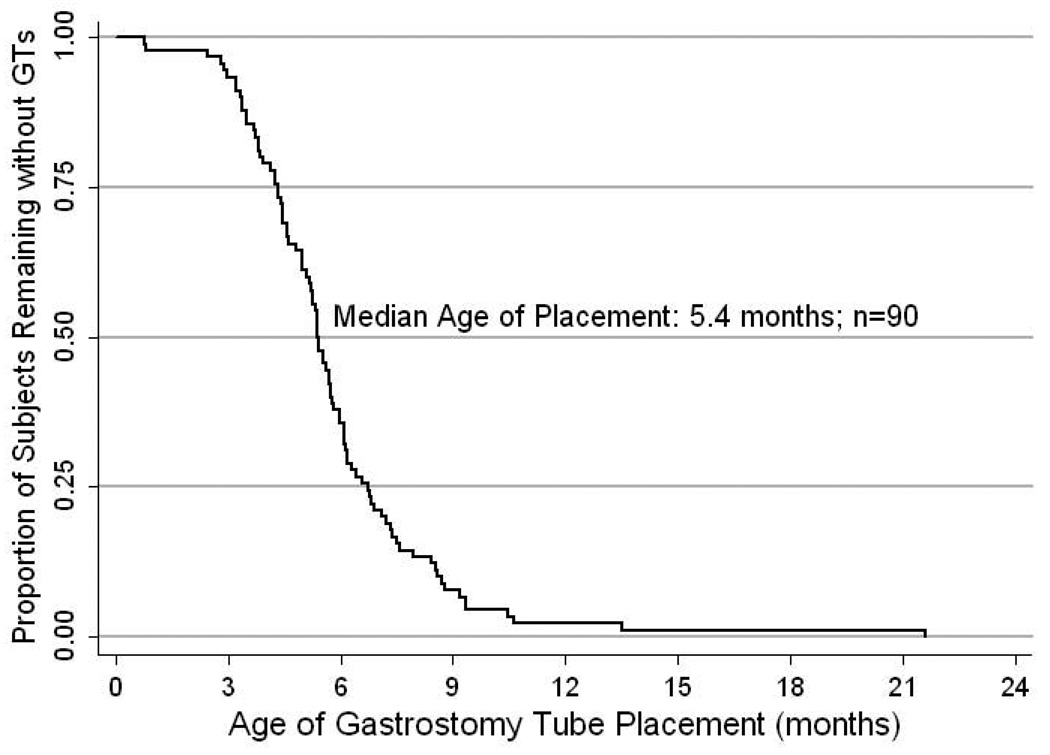
In the two groups of children with GTs or Nissen/GTs, we were interested in knowing the median age of placement and GT removal as well as the median time from GT placement to hospital discharge. In children with GT and Nissen/GTs, the exact date of placement was known for 90 out of 92 subjects. We found the median age of placement was 5.4 months of age with 94% of GTs placed within the first 12 months of life (n=90, Figure 1). Subjects ultimately discharged to home on respiratory support (supplemental oxygen and/or mechanical ventilation) tended to have a later placement of GT (median age of 5.7 months; n=53) compared to subjects discharged without such support (median age of 5.0 months; n=37; log rank p=0.08).
Figure 1.
Age of Gastrostomy Tube Insertion (Months): Kaplan-Meier plot demonstrating the age of GT placement for 90 subjects where the exact date of placement is known.
Of the 90 subjects where the date of GT placement was known, 72 (80%) had the GT placed during the initial NICU hospitalization, and 18 (20%) were placed during a later readmission. For the 72 subjects where the GT was placed during the initial NICU hospitalization, the median time from GT placement to discharge varied by whether a Nissen fundoplication was also performed. The median time from surgery only for GT to discharge was 0.5 months (n=21) compared to a median time from GT placement and Nissen fundoplication to discharge of 1.4 months (n=51; log rank p=0.010). For the 18 subjects where the GT was placed after the initial NICU hospitalization, the GT was placed a median of 4.1 months after discharge from the NICU.
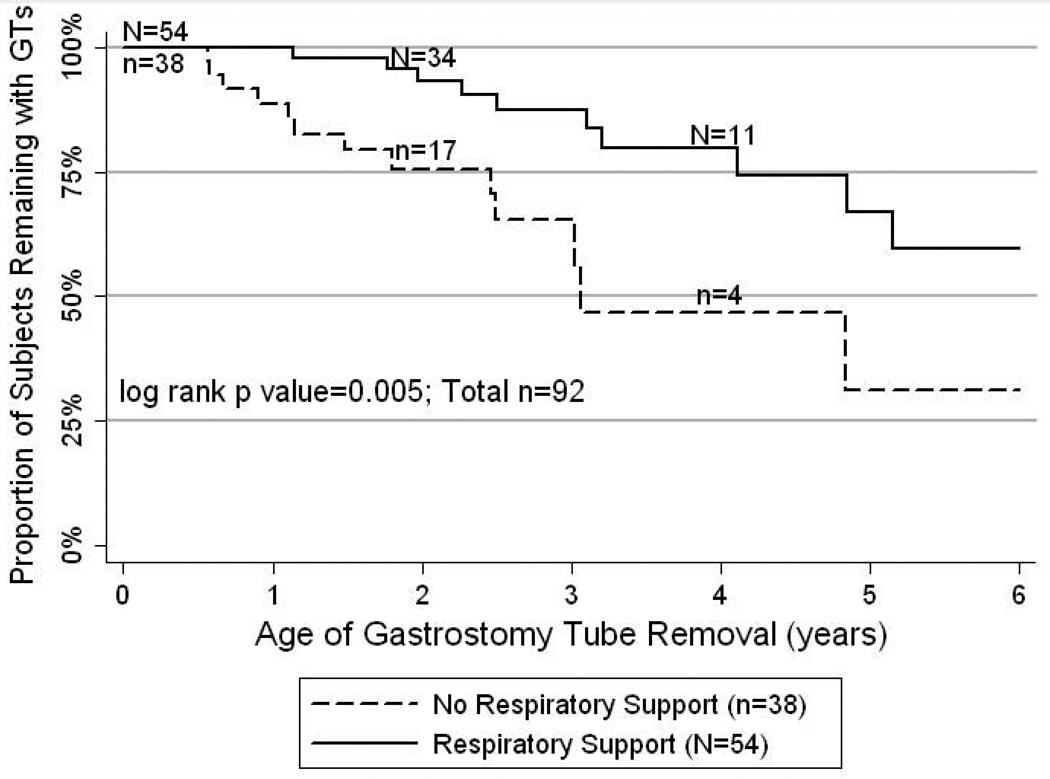
Using censored data, the median age of GT removal was 5.2 years of age (n=92). For the 24 subjects who have had their GT removed, the median age of GT removal was 3.0 years. For the 61 subjects who still had GTs in place and the 7 subjects lost to follow-up prior to GT removal, the median age of follow-up was 2.8 years. However, timing of GT removal may be associated with severity of initial respiratory disease, as children who did not require home supplemental oxygen or home mechanical ventilation had their GTs removed earlier (median age=3.1yo) compared to those who were on respiratory support (median age>6yo; log rank p=0.005; Figure 2).
Figure 2.
Age of Gastrostomy Tube Removal (Years): Kaplan-Meier plot demonstrating the age of GT removal for 92 subjects. For 24 subjects the exact date of removal is known and for the remaining 68, the data has been censored as 61 subjects still have a GT in place and 7 have been lost to follow-up prior to GT removal. The solid line represents 54 subjects who were discharged from the NICU on respiratory support at home (supplemental oxygen and/or mechanical ventilation) and the dashed line represents 38 subjects who were discharged without such support. The lines are statistically different (log rank p=0.005). The numbers above the solid line and below the dashed line represent the subjects with follow-up data at that particular age for each respective line.
Age of oxygen discontinuation by GT status
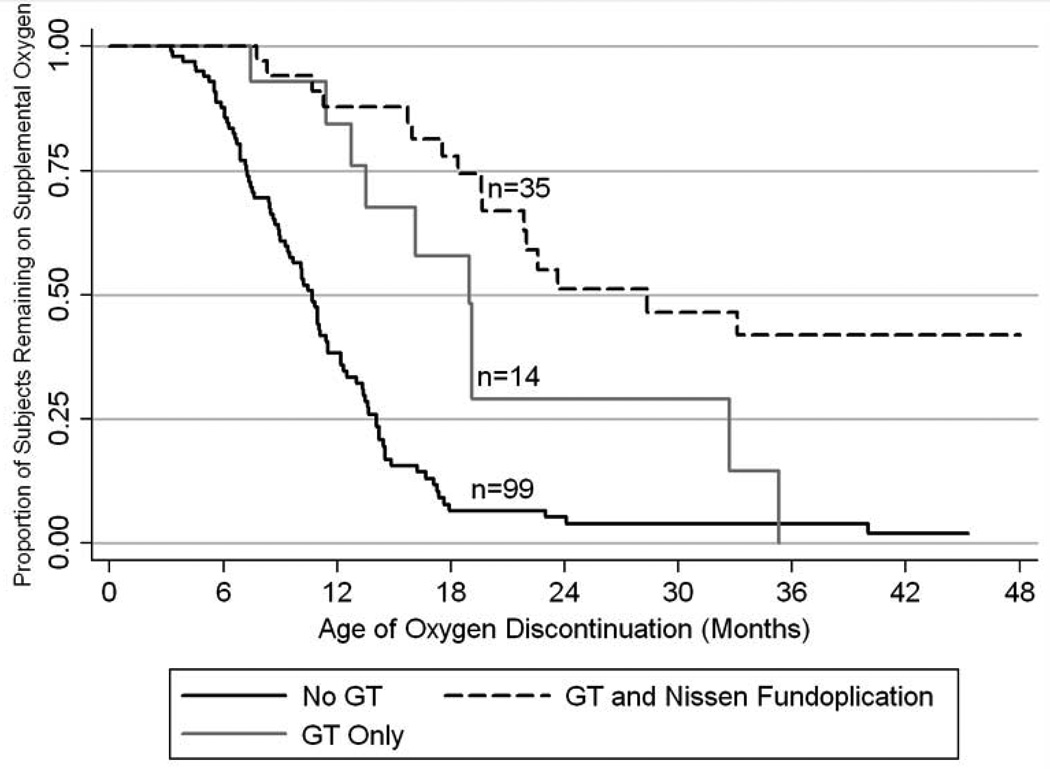
We also sought to determine the age in which BPD children were weaned off supplemental oxygen after initial hospital discharge. Specifically we were interested in determining if GT or Nissen/GT status was associated with the age of weaning from supplemental oxygen. We found that children with BPD without GTs were weaned off oxygen significantly sooner than children with GTs or Nissen/GTs. Furthermore, children with GTs alone were weaned off supplemental oxygen significantly sooner than the children with Nissen/GTs (Figure 3).
Figure 3.
Age of Oxygen Discontinuation (Months): Kaplan-Meier plot demonstrating the age of weaning off supplemental oxygen for 148 subjects. The solid black line represents 99 subjects without GTs who were discharged from the NICU on supplemental oxygen. The solid gray line represents 14 subjects with GTs who were discharged from the NICU on supplemental oxygen. The solid gray line represents 35 subjects with GTs and Nissen fundoplications who were discharged from the NICU on supplemental oxygen (n=31) or oxygen entrainment via ventilator (n=4). All three plotted lines are statistically different from one another, specifically log rank p values are 0.002 for no GT vs. GT only, <0.001 for no GT vs. GT with Nissen, and 0.025 for GT only vs. GT with Nissen.
Respiratory morbidities and respiratory symptoms in children with BPD by GT status
At each outpatient visit, caregivers of subjects who attended the BPD clinic were given questionnaires regarding inter-current illnesses, acute care visits and respiratory symptoms in their children. A total of 736 questionnaires were completed by the caregivers of 338 subjects (84.9%) prior to the age of 2 years with 4.1% of all questions left blank by caregivers; the presence or absence of GT or Nissen/GTs did not impact whether a questionnaire was completed (GT p=0.09; Nissen p=0.52) or how many questionnaires were completed (GT p=0.09; Nissen p=0.07).
After initial hospital discharge, 32.8% of subjects had at least 1 emergency room visit (n=335) and 22.9% had at least 1 hospital admission (n=336) for respiratory symptoms, within the first 2 years of life. We found no significant difference between the children with GTs, Nissen/GTs or no GTs with regard to respiratory morbidities (emergency department visits, systemic steroid use, and antibiotic use for respiratory reasons). However, the presence of a Nissen/GT, was associated with a higher likelihood (1.96 times higher) of being re-admitted to the hospital following initial discharge, after adjusting for age, use of home oxygen, ventilation, and inhaled corticosteroids, insurance status, and small-for-gestational-age status (p=0.043). We found no differences between the groups with regard to respiratory symptoms (trouble breathing, rescue beta-agonist use, activity limitations, and nighttime symptoms) (Table 2).
Table 2.
Odds Ratios of Respiratory Morbidities with GTs and Nissen/GTs
| Number of Forms [Number of Subjects] |
Adjusted Odds Ratio for Risk with GT compared to without GT [95% C.I.]* |
Adjusted OR P value |
Adjusted Odds Ratio for Risk with Nissen compared to without Nissen [95% C.I.]* |
Adjusted OR P value |
||
|---|---|---|---|---|---|---|
| Primary Outcomes For Respiratory Symptoms | Emergency Department | 710 [326] |
1.03 [0.62 – 1.72] |
0.90 | 1.38 [0.78 – 2.42] |
0.27 |
| Hospitalization | 709 [327] |
1.46 [0.81 – 2.61] |
0.21 | 1.96 [1.02 – 3.77] |
0.043 | |
| Systemic Steroid Use | 703 [325] |
1.17 [0.69 – 1.98] |
0.57 | 1.40 [0.79 – 2.49] |
0.25 | |
| Antibiotic Use | 709 [327] |
0.79 [0.47 – 1.32] |
0.37 | 1.22 [0.71 – 2.12] |
0.47 | |
| Secondary Outcomes | Trouble Breathing | 683 [321] |
0.64 [0.38 – 1.09] |
0.10 | 0.69 [0.37 – 1.28] |
0.24 |
| Rescue Medication Use | 663 [317] |
0.88 [0.50 – 1.55] |
0.66 | 1.17 [0.63– 2.21] |
0.61 | |
| Activity Limitations | 662 [314] |
0.63 [0.25 – 1.58] |
0.33 | 0.85 [0.31 – 2.30] |
0.75 | |
| Nighttime Symptoms | 678 [317] |
1.05 [0.55 – 1.99] |
0.89 | 1.34 [0.68 – 2.63] |
0.40 |
Logistic regressions clustered by subject and adjusted for age in months at the time of visit, presence of supplemental oxygen, presence of home ventilation, small for gestational age status, insurance status, and any inhaled corticosteroid use between 0–2 years of age. Only data obtained prior to 24 months of age was utilized.
Discussion
In this study we examined the respiratory outcomes of children with BPD in the outpatient setting during the first two years of life, with respect to GT status. We found that 23.1% of children with BPD had a GT or Nissen/GT with the majority of procedures performed by 6 months of age. The children with GTs and Nissen/GTs were significantly older at initial hospital discharge and were significantly older when weaned from supplemental oxygen compared to the children without GTs. In addition, the BPD children with Nissen/GTs were significantly more likely to require respiratory and/or ventilatory support after initial hospital discharge and were more likely to be re-hospitalized during the first two years of life compared to children without GTs.
Factors associated with having a Nissen/GT included a birth weight of ≤ 10 percentile and a need for supplemental oxygen and/or ventilatory support at the initial clinic visit. These findings suggest that the Nissen/GT group of children may have more severe BPD then the other groups. The children with Nissen/GTs were also more likely to be re-hospitalized and weaned off supplemental oxygen at a later age during the first two years of life. Nevertheless, with the exception of re-hospitalizations, caregivers of children with Nissen/GTs reported similar respiratory morbidities (likelihood of emergency department visits, systemic steroid use, antibiotic use, reports of trouble breathing, need for rescue beta-agonist, activity limitations, and nighttime symptoms) as caregivers of children with GTs alone or without GTs. This suggests that placement of a GT/Nissen in a particularly vulnerable population of children with BPD may help attenuate respiratory morbidity following initial discharge from the hospital. Prospective studies that follow patients longitudinally from birth into the outpatient setting would be useful in identifying other factors associated with long-term respiratory outcomes in this high risk population.
Attenuation of chronic respiratory symptoms by placement of GT or Nissen/GT may be particularly relevant in children with BPD on supplemental oxygen. Several animal studies have reported that hyperoxia exposure in combination with pulmonary aspiration can cause a more severe lung injury than aspiration alone.14,15,16 In addition, Heuer and colleagues found that intra-bronchial instillation of hydrochloric acid can cause injury to multiple organs including the heart, lung, liver and kidney.17 Although these are animal studies, the results suggest that minimizing aspiration and/or GER events, in the presence of supplemental oxygen may help attenuate lung injury and potentially improved respiratory outcomes.
The decision to treat an infant with BPD who has swallowing dysfunction and/or severe GER unresponsive to medical therapy with a surgical intervention can vary among tertiary care centers and involves a risk/benefit analysis. Recent improvements including less invasive procedures have made these interventions more accessible and safer in a high risk group of infants and may help improve respiratory symptoms in preterm infants.18,19 The efficacy of oral motor interventions (OMI) in preterm infants with lung disease, although less invasive is still unclear. Arvedson and colleagues recently conducted a systematic review of the literature to determine the impact of oral motor interventions on pulmonary health of preterm infants. None of the studies in their review reported on pulmonary health, and although some studies showed benefits, the variability and methodological limitations, questioned the utility of their use.20 Large prospective studies that compare outpatient respiratory outcomes between OMI and surgical interventions for children with BPD and with swallowing dysfunction and/or GER are needed.
We also examined timing of GT placement and removal in children with BPD. We found that the median age of removal was 5.2 years of age. Knowing that many children will have GTs removed prior to kindergarten, and that most children will have GTs removed before grade school may help alleviate the concern of caregivers regarding feeding regimens in the school setting.
Our study has several limitations. First, data collection of neonatal history and timing of GT and Nissen fundoplication was retrospective. Morbidity outcomes were also assessed retrospectively via questionnaire and may be subject to recall bias. In addition, the association among groups involving duration of oxygen use after initial hospitalization may be confounded by more severe BPD or poor growth in children with GTs or Nissen/GTs. A prospective study that determines BPD severity at 36 weeks post conception age would help to establish whether a relationship exists between BPD severity, GT or Nissen GT placement and subsequent duration of oxygen use during the first two years of life. Another limitation of this study is that it may not be representative of other children with BPD in the outpatient setting across the United States since the majority of subjects who attended our clinic were non-white and from an urban setting. Nevertheless, minorities are generally at high risk for premature births and studying a population that is disproportionately affected by prematurity is important. All subjects in this study also attended a BPD clinic that focused on the care and needs of preterm children with lung disease. This may not be representative of care given in other areas, and the children referred to this subspecialty clinic may have more severe lung disease and other co-morbidities than the general population of children with BPD since they were specifically referred for the treatment of BPD. Lastly, although we adjusted for measures of severity of BPD, including inhaled corticosteroid use, home supplemental oxygen, and ventilator support in our analysis of respiratory morbidities, it is possible that our results may be confounded by other unmeasured elements of BPD severity.
In summary, we found that 23.1% of BPD children who were followed in an outpatient BPD clinic had GTs or Nissen/GTs. After initial hospital discharge, weaning off supplemental oxygen occurred later in the children with GTs and Nissen/GTs, compared to children without GTs. Re-hospitalizations after initial hospital discharge were higher in the children with Nissen/GTs, however respiratory morbidities as reported by caregivers were similar among the three groups.
Acknowledgements
This work was funded by the Thomas Wilson Foundation, the Flight Attendants Medical Research Institute and the American Academy of Pediatrics Julius B. Richmond Center. The authors would like to thank the patients and families who participated in this study.
Abbreviations
- BPD
bronchopulmonary dysplasia
- GT
gastrostomy tube
- VLBW
very low birth weight
- NICU
neonatal intensive care unit
- Nissen
Nissen Fundoplication
Footnotes
Disclosures: All authors disclose that they have no financial interests in the subject of this manuscript
Reference List
- 1.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60:1–70. [PubMed] [Google Scholar]
- 2.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Chang YS, Yoo HS, Ahn SY, Seo HJ, Choi SH, et al. Swallowing dysfunction in very low birth weight infants with oral feeding desaturation. World J Pediatr. 2011;7:337–343. doi: 10.1007/s12519-011-0281-9. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno K, Nishida Y, Taki M, Hibino S, Murase M, Sakurai M, et al. Infants with bronchopulmonary dysplasia suckle with weak pressures to maintain breathing during feeding. Pediatrics. 2007;120:e1035–e1042. doi: 10.1542/peds.2006-3567. [DOI] [PubMed] [Google Scholar]
- 6.Frank L, Sosenko IR. Undernutrition as a major contributing factor in the pathogenesis of bronchopulmonary dysplasia. Am Rev Respir Dis. 1988;138:725–729. doi: 10.1164/ajrccm/138.3.725. [DOI] [PubMed] [Google Scholar]
- 7.Biniwale MA, Ehrenkranz RA. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:200–208. doi: 10.1053/j.semperi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Wang LY, Luo HJ, Hsieh WS, Hsu CH, Hsu HC, Chen PS, et al. Severity of bronchopulmonary dysplasia and increased risk of feeding desaturation and growth delay in very low birth weight preterm infants. Pediatr Pulmonol. 2010;45:165–173. doi: 10.1002/ppul.21171. [DOI] [PubMed] [Google Scholar]
- 9.Gewolb IH, Vice FL. Abnormalities in the coordination of respiration and swallow in preterm infants with bronchopulmonary dysplasia. Dev Med Child Neurol. 2006;48:595–599. doi: 10.1017/S0012162206001241. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd EG, Knupp AM, Welty SE, Susey KM, Gardner WP, Gest AL. An interdisciplinary bronchopulmonary dysplasia program is associated with improved neurodevelopmental outcomes and fewer rehospitalizations. J Perinatol. 2012;32:33–38. doi: 10.1038/jp.2011.45. [DOI] [PubMed] [Google Scholar]
- 12.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang KY, Zeger SL. Longitudinal Data-Analysis Using Generalized Linear-Models. Biometrika. 1986;73:13–22. [Google Scholar]
- 14.Nader-Djalal N, Knight PR, III, Thusu K, Davidson BA, Holm BA, Johnson KJ, et al. Reactive oxygen species contribute to oxygen-related lung injury after acid aspiration. Anesth Analg. 1998;87:127–133. doi: 10.1097/00000539-199807000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Nader ND, Davidson BA, Tait AR, Holm BA, Knight PR. Serine antiproteinase administration preserves innate superoxide dismutase levels after acid aspiration and hyperoxia but does not decrease lung injury. Anesth Analg. 2005;101:213–219. doi: 10.1213/01.ANE.0000152188.65226.FE. table. [DOI] [PubMed] [Google Scholar]
- 16.Nader-Djalal N, Knight PR, Davidson BA, Johnson K. Hyperoxia exacerbates microvascular lung injury following acid aspiration. Chest. 1997;112:1607–1614. doi: 10.1378/chest.112.6.1607. [DOI] [PubMed] [Google Scholar]
- 17.Heuer JF, Sauter P, Pelosi P, Herrmann P, Bruck W, Perske C, et al. Effects of pulmonary acid aspiration on the lungs and extra-pulmonary organs: a randomized study in pigs. Crit Care. 2012;16:R35. doi: 10.1186/cc11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes N, Robertson N, Lakhoo K. Anti-reflux surgery for the neonatal intensive care-dependent infant. Early Hum Dev. 2003;75:71–78. doi: 10.1016/j.earlhumdev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Thatch KA, Yoo EY, Arthur LG, III, Finck C, Katz D, Moront M, et al. A comparison of laparoscopic and open Nissen fundoplication and gastrostomy placement in the neonatal intensive care unit population. J Pediatr Surg. 2010;45:346–349. doi: 10.1016/j.jpedsurg.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 20.Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. Evidence-based systematic review: effects of oral motor interventions on feeding and swallowing in preterm infants. Am J Speech Lang Pathol. 2010;19:321–340. doi: 10.1044/1058-0360(2010/09-0067). [DOI] [PubMed] [Google Scholar]