SUMMARY
Background
Epithelial invagination is a fundamental morphogenetic behavior that transforms a flat cell sheet into a pit or groove. Previous studies of invagination have focused on the role of actomyosin-dependent apical contraction; other mechanisms remain largely unexplored.
Results
We combined experimental and computational approaches to identify a two-step mechanism for endoderm invagination during ascidian gastrulation. During Step 1, which immediately precedes invagination, endoderm cells constrict their apices due to Rho/Rhokinase-dependent apical enrichment of 1P–myosin. Our data suggest that endoderm invagination itself occurs during Step 2, without further apical shrinkage, via a novel mechanism we call collared rounding: Rho/Rho-kinase-independent lateral enrichment of 1P–myosin drives apico-basal shortening, while Rho/Rho-kinase-dependent enrichment of 1P and 2P myosin in circumapical collars is required to prevent apical expansion and for deep invagination. Simulations show that boundary-specific tension values consistent with these distributions of active myosin can explain the cell shape changes observed during invagination both in normal embryos and in embryos treated with pharmacological inhibitors of either Rho-kinase or Myosin II ATPase. Indeed, we find that the balance of strong circumapical and basolateral tension is the only mechanism based on differential cortical tension that can explain ascidian endoderm invagination. Finally, simulations suggest that mesectoderm cells resist endoderm shape changes during both steps and we confirm this prediction experimentally.
Conclusions
Our findings suggest that early ascidian gastrulation is driven by the coordinated apposition of circumapical and lateral endoderm contraction, working against a resisting mesectoderm. We propose that similar mechanisms may operate during other invaginations.
INTRODUCTION
Invagination, in which a sheet of epithelial cells bends inwards to form a pit or groove, is a fundamental building block of morphogenesis used throughout animal development. Although it is one of the simplest deformations that a sheet of cells can perform, the cellular and mechanical bases for invagination remain poorly understood. Invagination necessarily involves a change in the shape of participating cells from columnar to wedge-shaped, with a reduced apex and an expanded base. The challenge has been to determine the molecular origins and distributions of forces that cause these cell shape changes, and to understand how these forces are integrated to produce global changes in tissue geometry.
Many potential force-generating mechanisms for invagination have been proposed (reviewed in 1, 2). These include localized differences in adhesivity or in local cortical actomyosin contractility, or cell shape changes driven by internal cytoplasmic microtubules. In addition, tissue-extrinsic forces - e.g swelling of an extracellular gel or active spreading (epiboly) of neighboring tissues – could cause a tissue to buckle inward. Computer simulations have shown that under suitable conditions each of these modes of force generation could drive invagination [1,3–7]. However direct experimental support for most of these hypothesized mechanisms remains weak.
The best-studied cellular behavior associated with invagination is apical constriction, in which cells actively shrink their apical surfaces. Studies in Drosophila, sea urchins, Xenopus, and mice support an active role for actomyosin contractility in driving apical constriction [8–12]. Apical enrichment of myosin that has been activated by phosphorylation of the regulatory light chain on serine 19 accompanies apical constriction in all of these cases. Several pathways can control this enrichment, including, but not restricted to, Rho GTPases, and pharmacological or genetic inhibition of apical myosin phosphorylation prevents most invaginations [9,10, 13–15]. The success in documenting the occurrence, necessity, and control of apical constriction in many systems has led to its widespread acceptance as the major cause of invagination.
While apical constriction clearly contributes to many invaginations, it cannot provide a complete explanation of the phenomenon (reviewed in 2, 16). The main phase of apical constriction usually precedes invagination. Invagination itself is often accompanied by a marked apico-basal shortening and it has been suggested that active apico-basal shortening could be an important driving force for invagination, but this hypothesis has not been explored experimentally, nor has a molecular or genetic basis for apico-basal shortening been identified [2, 17]. More generally, the extent to which apical constriction causes a tissue to invaginate will depend on the distributions of other tissue-intrinsic forces and on the extent to which surrounding tissues either help or resist deformation.
Here, we focus on the very simple case of endoderm invagination in ascidians (Urochordata), in which a monolayer plate of just 10 cells invaginates to internalize a primitive gut rudiment in an embryo of ~100 cells. This process is ideally suited for exploring the cytomechanical basis for invagination [18]. Ascidians have small optically clear embryos, making it possible to do comprehensive three-dimensional analysis of cellular shape change from live and fixed embryos [19]. Their stereotyped early development, based on an invariant lineage [20], provides a high-resolution timeline to the c. 45-minute process. Finally, the small cell numbers mean that computer simulations can relate force-generating mechanisms within individual cells to the tissue- and embryo-level deformations that they cause.
Combining 4D microscopy, experimental manipulation of actomyosin contractility and computer simulation, we show that sequential deployment of active myosin to different endoderm cell surfaces is tightly phased with, and could drive, a 2-step sequence of endoderm cell shape changes during invagination. Our results suggest that apical constriction alone cannot explain either step: During Step 1, both apical endoderm constriction and ectoderm epiboly contribute to shaping a tall apically narrow endoderm plate. During Step 2, it is the interplay between circumapical and basolateral endoderm tensions, driving apico-basal shortening around tightly maintained, pre-shrunk apices, that causes invagination itself.
RESULTS
4D morphometric analysis shows that endoderm invagination occurs in two distinct steps
We began by analyzing 3D reconstructions made from serial confocal micrographs of fixed embryos during early gastrulation (between the 64- and 112-cell stages, Figure 1A, Supplementary Figure S1). A comparative analysis of four different species (Ciona intestinalis, C. savignyi, Phallusia mammillata and Boltenia villosa; Figure1 B–D) reveals a core sequence of cell shape changes that are tightly phased with the conserved pattern of cell divisions.
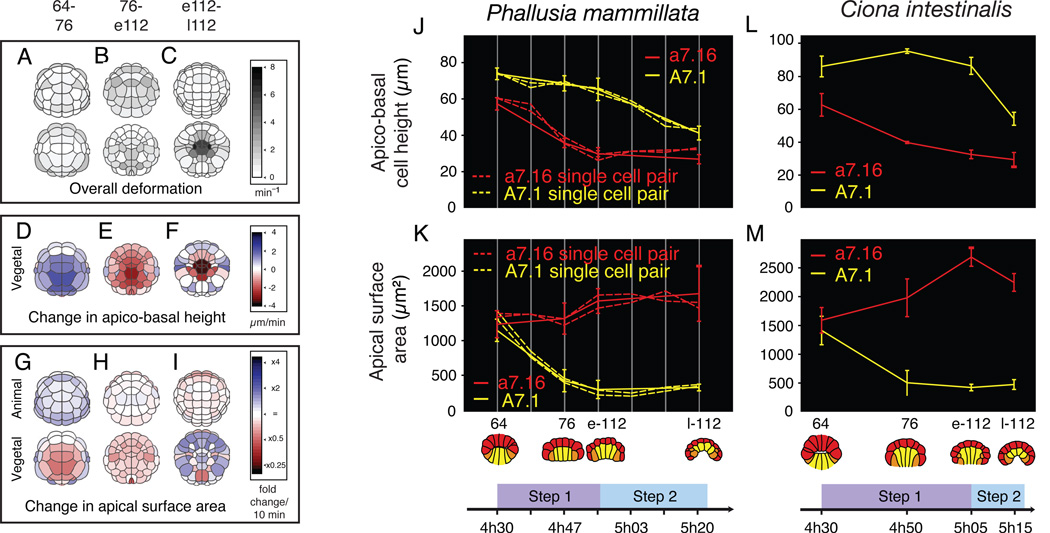
Figure 1. Ascidian invagination occurs in two highly conserved steps.
A : Animal (top) and vegetal (bottom) views of Ciona embryos showing the positions of cell cleavages between the 64- and 76-cell (early Step1, left), 76- and early 112-cell (late Step, center) and early/late 112-cell (Step 2, right) stages. Lines link newly formed sister cell pairs. B-D : Sagittal, vegetal, and frontal views of 3D–reconstructed Ciona intestinalis (B), Phallusia mammillata (C) and Boltenia villosa (D, sagittal only) embryos at the indicated stages. Yellow = endoderm; orange = mesoderm; red = ectoderm. Supplementary Figure S1 shows interactive 3D views of reconstructed embryos.
At the 64-cell stage, embryos are approximately spherical; animal and vegetal cells are equal in height and there is a very small blastocoel. Between the 64- and early 112-cell stages (~30 minutes; left three columns in Figure 1B–D), the vegetal endoderm plate flattens and shrinks its apical surface. The animal hemisphere spreads as cells shorten apico-basally (64- to 76-cell stages), then divide (76- to early 112-cell stage). From early to late 112-cell stage (~15 minutes; rightmost two columns in Figure 1B–D), the presumptive endoderm cells invaginate, as the whole embryo bends inward on the vegetal side to form a pit centered on the vegetal pole. Invagination is followed immediately by endoderm cell cleavage, and then involution of the anterior notochord and lateral muscle primordia (21, and not shown). We observed the same sequence of events in all 4 species with only minor variations (Figure 1B–D and data not shown; see legend for details). The entire sequence occurs without disruption of the epithelial nature of the endodermal plate, as shown by the constant presence of ZO-1-positive tight junctions (Figure S2A–D), and without cell rearrangements. Thus invagination is driven solely by the cumulative effect of individual cell shape changes.
To more precisely characterize the cell shape changes that accompany invagination, we used 3D Virtual Embryo software [19] to reconstruct and quantify the shapes of all cells in fixed Ciona intestinalis embryos (Figure 2A–I). Between the 64- and early 112-cell stages, most blastomeres underwent significant shape changes (Figure 2A–B) that were different for each hemisphere, but common to cells within a hemisphere. The apico-basal heights of vegetal cells first increased up to the 76-cell stage, and then shortened slightly (Figure 2D–E). In parallel, the apices of vegetal cells shrunk, while those of animal cells remained stable or slightly increased (Figure 2G–H). There was also a slight but significant decrease in endoderm cell volume during Step 1 (Figure S2E). The pattern of cell shape changes was dramatically different during the 112-cell stage. During this period, cell deformations were largely restricted to the central part of the vegetal plate (Figure 2C), and were most pronounced in the 10 endodermal precursors, which dramatically shortened apico-basally (Figure 2F), while slightly expanding their apices (Figure 2I), and maintaining constant volume (Figure S2E).
Figure 2. Morphometric analysis of cell shape changes during invagination.
A-I: Evolution of geometric characteristics in whole Ciona intestinalis embryos between the 64-and late 112-cell stages. Color scales indicate both magnitude and direction of changes (n = 3 embryos for 64- 76- and late 112-cell stages; n = 2 for early 112-cell stage). J, K: Measurements of apico-basal height (J) and apical surface area (K) for A7.1 (dashed yellow lines) and a7.16 (dashed red lines) cell pairs in reconstructed Phallusia embryos, imaged live every 5 minutes (each dashed line is an embryo) or fixed prior to imaging (solid lines, n = 7 embryos per data point; error bars are standard deviations). L, M: Measurements for the same cell pairs in fixed Ciona intestinalis embryos (n = 5 embryos per data point). See Figure S2 for additional data on cell volume change and maintenance of epithelial architecture during invagination. Movies S1 and S2 show time lapse sequences of invagination.
To characterize this further, we performed high-resolution 4D live microscopy on the transparent embryos of Phallusia focusing on representative vegetal (A7.1, endoderm precursor) and animal (a7.16, epidermis precursor) blastomeres. Significantly, we observed sharp transitions in the direction and/or magnitude of changes in apical surface area and apico-basal height for both animal and vegetal precursors that coincided with the onset of invagination (Figure 2J, K, dotted lines). Analogous trajectories for A7.1 and a7.16 were observed in Boltenia (Figure 1D, Movie S1) and Ciona (Figure 2L, M, Movie S2) embryos, although the lesser transparency of these embryos precluded a full characterization of cell shape change in 3D.
We conclude that ascidian endoderm invagination occurs in two well-defined and evolutionary conserved steps, characterized by distinct patterns of underlying cell behavior. During Step 1, endoderm cells shrink their apical surface while the vegetal surface flattens, and the mesectoderm spreads and cleaves laterally. During Step 2, the endoderm cells shorten rapidly along their apico-basal axis with no further apical shrinkage while their basal ends expand and the vegetal plate invaginates.
Steps 1 and 2 correlate with distinct patterns of active myosin accumulation
To distinguish potential roles for microtubules and the actin cytoskeleton, we examined gastrulation in ascidian embryos treated with nocodazole or cytochalasin D to depolymerize microtubules and actin, respectively. Embryos treated with 1.3 µM nocodazole at the 64-cell stage stopped dividing, although cycles of nuclear division persisted (data not shown), but underwent a characteristic two-step invagination as deep as controls (Figure S3A and data not shown). Thus neither microtubules, nor cell shape changes associated with mesectoderm cell cleavages, are required for invagination. In contrast, embryos treated with 1 µM cytochalasin did not invaginate (data not shown), pointing to a role for the actin cytoskeleton.
A likely role for filamentous actin in invagination is to support actomyosin contractility. In non-muscle cells, actomyosin contractility is activated by phosphorylation of the myosin II regulatory light chain at either the ser19 position (1P–myosin) or at the ser19 and thr18 positions (2P–myosin) [22]. 1P–myosin is primarily enriched within short-lived and rapidly changing structures such as cleavage furrows [23], while 2P–myosin is restricted to more persistent contractile structures such as stress fibers [24,25].
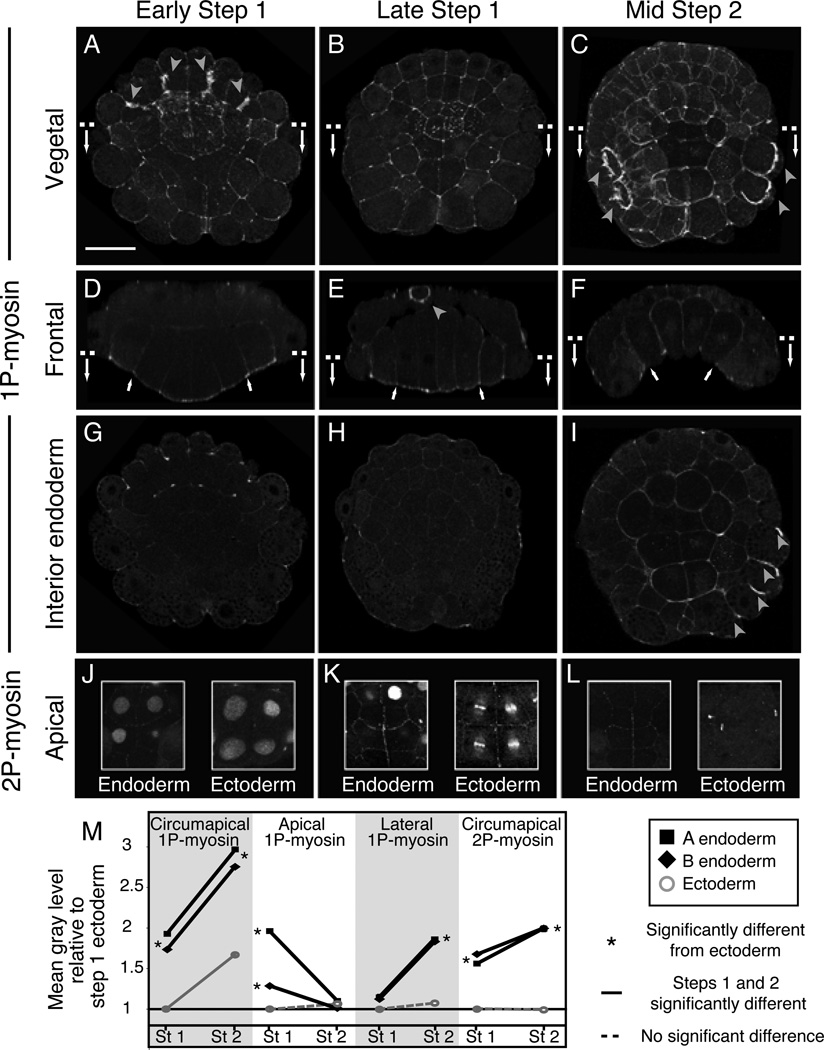
Immunostaining embryos with anti-1P- and 2P–myosin antibodies revealed dynamic patterns of localization (Figures 3–4, Movies S3–4). 1-P myosin was enriched circumapically in all cells during both steps, and also on cleavage furrows (arrowheads in Figure 3). During Step 1, 1P–myosin was weakly detectable on the lateral surfaces of endoderm precursors and strongly enriched on their shrinking apical surfaces (Figure 3A–B, D–E, Movie S3). Strikingly, in early Step 2, when endoderm cells transition from apical shrinkage to apico-basal shortening, 1P–myosin rapidly disappeared from the apical surfaces, and accumulated strongly on the basal and lateral surfaces of all and only the endoderm cells (Figures 3C, F, I). Meanwhile, 2P–myosin gradually accumulated circumapically on the endoderm precursors throughout Step 1 and persisted in Step 2 (Figure 3J–M, Movie S4), while the ectoderm precursors weakly accumulated circumapical 2P–myosin only during late Step 1 (Figure 3K). Quantitative analysis of fluorescence intensities during Steps 1 and 2 supports these observations (Figure 3M). Thus 1P–myosin was always enriched on the endodermal cell surfaces undergoing shrinkage: apical in Step 1; basolateral during Step 2, while circumapical enrichment of 2P myosin was associated with maintenance of small endoderm apices. These distributions are consistent with a direct role for myosin II in causing the forces that drive ascidian invagination.
Figure 3. Patterns of phosphomyosin accumulation correlate with cell shape changes during invagination.
A-I: Vegetal surface views (A-C), frontal sections (D-F), and horizontal sections (G-I) of Boltenia embryos showing 1P–myosin distribution at early and late Step 1 and mid Step 2. Dashed lines in A-C and D-F show positions of frontal and horizontal sections respectively. Gray arrowheads in A-C, I: accumulation of 1P–myosin in cleavage furrows. White arrows in D-F: lateral boundaries of endoderm plate. J-L: Circumapical 2P–myosin distributions on endoderm and ectoderm cells at early (J) and late (K) Step 1 and mid Step 2 (L). The cortical stain was confined to a narrow sub-apical region. Endoderm/ectoderm image pairs come from the same embryo. The antibody to 2P–myosin also labels nuclei and spindle midbodies as seen previously in other cell types [44]. See Movie S4 for 3D views of 2P–myosinM: Quantification of relative pixel intensities (Step 1 vs. Step 2; endoderm vs. ectoderm) for different boundaries from fixed, immunostained Boltenia embryos. Asterisks indicate significant differences (p < 0.05 for 1-tailed Mann-Whitney U-tests; p was usually < 0.0001) between endoderm and ectoderm. Similar results for Ciona are presented in Figure 4). See Movies S3 and S4 for 3D views of 1P and 2P myosin-stained embryos.
Figure 4. Different zones of localized contractility contribute to invagination inC. intestinalis.
A-D: Cross-sectional views of embryos treated with 100µM Blebbistatin during Step 1 (B) or Step 2 (D), fixed at the end of each step and phalloidin-stained. A, C: Controls for Step 1 and Step 2. E-H: Apical surface area (E, G) and apico-basal height (F, H) at early Step1, late Step 1 and late Step 2 in embryos treated with 100µM Blebbistatin (E, F) or 100µM Y-27632 (G, H). Dashed lines link data points at the onset, intermediate time points and end of each treatment. Measurements for paired WT controls shown in solid yellow lines. Asterisks in E-H indicate significant differences (asterisks indicate p < 0.05 for 2-tailed t-test); error bars indicate standard deviations. Numbers of embryos measured were (Blebbistatin: n≥7 for all measurements; Y-27632: n = 4 and n = 5 for Step 2 control and Y-treated embryos, respectively, n ≥ 7 for all other measurements). Embryos in panels G,H and E,F were fixed at a slightly different times during late step 2. I-R: Phalloidin (K,L), 1P–myosin (I,J, M-P) and 2P–Myosin (Q,R) staining in controls and in embryos treated with 100 µM Y-27632 for 30 minutes and fixed at the end of Step 1 (I-L) or Step 2 (M-R). I-N: frontal sections. O, P: horizontal sections along the lines indicated in M,N. Q, R: blow-up of sub-apical horizontal sections across the vegetal pole. Arrows indicate lateral boundaries of the endoderm plate. S: Comparison of phosphomyosin levels on the indicated surfaces of A 7.1 cells in controls (solid black lines) and in embryos treated with 100 µM Y-27632 for 30 minutes prior to fixation at end of either Step 1 or Step 2 (light dashed lines). Figure S3 shows the effects on invagination of microtubule depolymerization and dominant-negative inhibition of RhoA. Movie S5 documents apical expansion during Step 2 in Y-treated embryos.
RhoA-dependent and independent forms of Myosin II activity are required for cell shape changes during both steps of invagination
To further assess the requirement for myosin II activity during invagination, we examined embryos treated at different time points during invagination with blebbistatin, a small molecule inhibitor of myosin II activity [26]. In embryos treated from the 64-cell stage (as in Figure 1B) and fixed at late Step 1, we observed a significant reduction in apical endoderm shrinkage relative to paired controls (Figure 4A,B,E), accompanied by a decrease in both endoderm and mesectoderm cell heights and an overall flattening of the embryo (Figure 4A,B,F). By contrast, in embryos treated with blebbistatin from late Step 1 and fixed near the end of Step 2, we observed a significant reduction in apico-basal shortening and invagination of the endoderm, accompanied by a slight broadening of endoderm cell apices (Figure 4C–F). Thus myosin activity is required both for apical shrinkage during Step 1 and for apico-basal shortening and invagination during Step 2.
In other cases of apical constriction and invagination, myosin is activated by RhoA, in part through Rho-kinase, which phosphorylates the myosin regulatory light chain [13,14]. To test a role for Rho GTPases in ascidian endoderm invagination, we microinjected embryos with point mutated, dominant negative, versions of RhoA, Cdc42 and Rac1. Dominant negative RhoA-injected embryos failed to invaginate (Figure S3B). Surprisingly, in these embryos, apicobasal shortening of endoderm during Step 2 occurred with the same timing as in controls, suggesting that it is under the control of a distinct pathway (data not shown). In contrast, injecting dominant negative forms of Cdc42 and Rac1 had no effect on invagination (not shown).
To test whether RhoA controls invagination via Rho kinase, we examined embryos treated with Y-27632, a specific pharmacological inhibitor of this kinase [27] at different times during invagination (Figure 4G–R). Embryos treated with 100 µm Y-27632 from the early 64-cell stage on (cf Figure 1B) and fixed at the end of Step 1 showed a reduction in endoderm apical shrinkage, a slight increase in endoderm cell heights and incomplete flattening relative to paired controls (Figure 4G–L). In embryos treated with 100 µm Y-27632 from the early 64-cell stage and fixed at the end of Step 2, the endoderm apices expanded during Step 2 (Figure 4G), but apico-basal shortening occurred normally (Figure 4H). To test a specific requirement for Rho-kinase during Step 2, we applied 100µM Y-27632 from the end of Step 1 and examined embryos at the end of Step 2. Again we observed apical expansion and reduced invagination with no effect on apico-basal shortening (Figure 4G–H dark green; M-N; Movie S5). Consistent with these phenotypes, 1P–myosin was lost on the endoderm apices of Y-treated embryos during Step 1 (compare Figure 4K, L, S), as was circumapical 2P–myosin during Step 2 (Figure 4Q–S). However, basolateral accumulation of 1P–myosin during Step 2 was unaffected in Y-treated embryos (Figure 4M–P, S).
We conclude that at least two pathways differentially control myosin activation during ascidian gastrulation: A Rho-dependent pathway controls apical and circumapical accumulation of 1P- and 2P–myosin, and is required for apical constriction and flattening during Step 1 and to prevent apical expansion during Step 2. A second pathway, which is Rho-independent, or at least shows only weak dependence on Rho-signalling, controls basolateral recruitment of activated myosin during Step 2, which is required for apicobasal shortening and invagination.
Design of a tension-based mechanical model for ascidian endoderm invagination
Our experimental observations suggest that boundary-specific cortical tensions, set by levels of activated myosin, could be sufficient to cause the cell shape changes that drive ascidian invagination. To test this idea, and explore general design constraints on a cortical tension-based mechanism for ascidian invagination, we developed computer simulations that predict the dynamics of cell shape change given tension values for different boundaries. Then we randomly sought boundary-specific tension values for which the simulations reproduce shape changes we observed during Step 1 and Step 2.
Details of model construction and analysis are presented in Supplementary Modeling Procedures. Briefly, we modeled a 2D frontal cross section of an ascidian embryo containing a small blastocoel. We considered only the two cell types - endoderm and mesectoderm (mesoderm plus ectoderm) - distinguished by our immunostaining of phosphomyosin. This implies 7 distinct cell boundary types (Figure 5A): apical, lateral and basal boundaries for endoderm and mesectoderm, plus the lateral endoderm-mesectoderm boundary. We represented each cell boundary as a connected set of smaller elements (Figure 5A), and we endowed these elements with two key mechanical properties designed to mimic those of a cortical actomyosin network: Active contractility, characterized by a fixed tension T, and passive resistance to deformation, characterized by an effective internal viscosity µeff. For simplicity, we set µeff to a fixed value for all boundaries and all simulations; relaxing this assumption had little or no effect on the overall results (see Supplementary Modeling Procedures). In contrast, we allowed the tension T to vary for each of the seven boundary types. We assumed that the cytoplasm of all cells was effectively incompressible.
Figure 5. Simulations based on differential cortical tension support a two-step mechanism for endoderm invagination.
A: A model embryo constructed from contractile/viscoelastic elements. Different boundary colors indicate boundary-specific tension values. B: Starting and sample end geometries. C: Criteria used to specify passing geometries for Step 1 and Step 2 simulations of “wild type” ascidian embryos. Symbols are represented on Panel B. D,E: Summary view of how final geometries attained by simulation from initial Step 1 (D) and Step 2 (E) geometries vary as a function of tension ratios. Colored embryos correspond to tension ratios lying nearest the clouds of passing parameter sets shown in F,G. F,G: Position in tension ratio space of successful solutions shown as projections along the Mesecto_L/A (F) or Endo_L/A (G) axes. Values vary logarithmically along both axes and the central color legend applies to both panels. See Supplementary Modeling Procedures for details. Movies S6 and S7 show examples of successful Step 1 and 2 simulations. Figure S4 shows distributions of absolute tensions for Step 1 and 2 parameter space searches, and the results of parameter space searches with: (a) boundary-specific internal viscosities and (b) unconstrained variation of basal tensions.
To compare simulated to measured shape changes, we designed initial embryo geometries to match frontal cross-sections of mid 64-cell (pre-Step 1) or early 112-cell (pre-Step 2) embryos (Figure 5B). We used an independent starting geometry for Step 2 rather than simply continuing successful Step 1 simulations (with Step 2-specific parameters) because the ectoderm cleavage that occurs between Steps 1 and 2 (Figure 1A) creates smaller and more cuboidal ectoderm cells. Then we used quantitative geometric descriptors of cell and embryo shape to define target geometries for simulations to match after a fixed time (Figure 5B–C, 30 simulated minutes for Step 1; 24 simulated minutes for Step 2). For Step 1, target criteria specified an endoderm plate that was tall, apically flat and narrow, and broad basally. For Step 2, target criteria specified a sufficiently deep invagination while maintaining constricted apices, and an apically smooth mesectoderm. For both steps, we also required that there is no excessive apical or basal cell bulging. Movies S6 and S7 show successful examples of Step 1 and 2 simulations.
Some simple considerations simplified our random parameter space searches: First we assumed that the tension on the lateral endoderm-mesectoderm boundary was the average of lateral mesectoderm and lateral endoderm tensions. Second, in initial simulations, we found that to reproduce blastocoel shapes correctly, basal tensions must be similar to lateral tensions for each cell type (Figure S4G), consistent with the similar levels of activated myosin we measured on lateral and basal endoderm surfaces (Figure 3D–F). Therefore we constrained basal tensions to match lateral tensions for each cell. Relaxing this assumption had little overall effect on the outcomes of our parameter space searches (Figure S4E,F). Finally, we constrained the maximum of the remaining four tensions to a fixed value to insure that cell shape changes unfold at similar rates for different parameter sets, and we scaled this fixed value so that simulated cell shapes approached steady state at a rate that is commensurate with what we observe in live embryos (see Figure 2A,B). With these assumptions/constraints, the outcomes of our simulations are determined by only three tension ratios whose values we could sample independently: the ratio of apical endoderm and mesectoderm tensions (Apical_E/M), and the ratios of lateral and apical tensions for each cell type (Endo_L/A and Mesecto_L/A. Figure 5D,E show how varying these tension ratios affects the cell and embryo shapes attained from starting geometries for Steps 1 and 2. Intuitively, the L/A ratios tune apico-basal cell height, while Apical_E/M controls a tug-of-war between endoderm and mesectoderm for apical surface area.
Computer simulations support a two-step mechanism for invagination
Figure 5F,G show the distribution of parameter sets for which simulations satisfied either Step 1 (light and dark blue points) or Step 2 (red points) criteria. Figure S4A,B shows the same data plotted as tension values rather than ratios. For each step, successful parameter sets were clustered within well-defined regions. Moreover, successful regions for Step 1 and Step 2 did not overlap, strongly supporting the hypothesis that ascidian invagination involves two mechanistically distinct steps.
For all Step 1 solutions, the ratio of lateral to apical endoderm tension was low (Endo_L/A << 1), consistent with higher levels of active myosin at the apical surfaces of endoderm cells (Figure 3D,E). For most Step 1 solutions (dark blue points in Figure 5F,G), Apical_E/M values were significantly greater than one, again consistent with observed phosphomyosin distributions. For those solutions in which Apical_E/M < 1 (apical tension higher in the mesectoderm than the endoderm; light blue in Figure 5F,G), the ratio of lateral to apical mesectoderm tensions (Mesecto_L/A) was high enough to cause substantial flattening and spreading of mesectoderm (active epiboly). Moreover, we observed an inverse relationship between Mesecto_L/A and Apical_E/M across all Step 1 solutions (Fig 5G), suggesting that in principle either apical endoderm contraction or mesectoderm epiboly could account for the observed shape changes during Step 1 and that their effects are additive. To assess which of these mechanisms dominates in real embryos, we asked for which parameter sets simulations could also reproduce the cleavage-arrest effect of nocodazole treatment in which embryos invaginate with kinematics almost identical to controls but with fewer, larger mesectoderm cells (Figure S5A). Only a subset of the Step 1 solutions could reproduce both control and nocodazole-like geometries during Step 1, and all of these solutions had Apical_E/M values above 1.5 (Figure S5B & C). Combined with the phosphomyosin distribution, this analysis suggests that Step 1 in live embryos relies on high endoderm apical tensions, rather than on active mesectoderm epiboly.
For all successful Step 2 solutions, boundary tension values were consistent with observed patterns of active myosin accumulation (Figure 5F,G; red points): both apical and lateral endoderm tension were significantly higher than all other tensions (Figure S4A & B), and their ratio was tightly constrained (Figure 5F; 0.6 < Endo_L/A < 1.2), while Mesecto_LA ranged widely (red points in Figure 5G). Interestingly, parameters for which simulations mimicked the effects of nocodazole treatments overlapped with a large fraction of the wild type solutions (yellow points in Figure S5B,C), suggesting that the Step 2 mechanism we identified is robust to variations in cell size and embryo geometry.
Simulations reproduce the effects of myosin inhibition by Blebbistatin and Y-27632
To further test our model, we asked whether it could reproduce the experimental effects of myosin inhibition by Blebbistatin and Y-27632. To simulate Blebbistatin treatment, we reduced cortical tensions on all cell surfaces to a fixed value TB, representing a small and non-specific residual tension, expressed as a percentage of the maximum value allowed in control simulations. Then we sampled values for TB between 5 and 15%, comparing simulated morphologies to those measured for control or Blebbistatin treated embryos. Indeed, predicted morphologies closely matched observed morphologies for both Step 1 and Step 2 (Figure S5D–G).
To simulate Rho/Rho-kinase inhibition, we chose subsets of the successful Step 1 and Step 2 parameter sets which represented ascidian-like invaginations (points colored dark blue and red in Figure 5F,G). Then, we selectively reduced endoderm boundary tensions to percentages of their “control” values (different for each reference parameter set), to mimic the observed reduction of phosphomyosin staining in endoderm precursors of Y-treated embryos (Figure S5H–K). During Step 1, Y treatment reduces the strong apical 1P–myosin staining (Figure 4I,J). Accordingly, reduction of apical endoderm tensions in our simulations reproduced most aspects of Step 1 morphologies in Y-treated embryos. However, simulations only matched the slight increase in endoderm apicobasal height in embryos treated with Y in Step 1 when we additionally reduced lateral endoderm tension. These results suggest that the 1P myosin observed at low levels on lateral endoderm cell surfaces during Step 1 produces active tension that contributes to apical flattening during Step 1 and is RhoA dependent. Unfortunately, the levels of 1P myosin on lateral surfaces of Step 1 wild type embryos were too low to reliably quantify their reduction in fixed Y-treated embryos (Figure 4S). During Step 2, lateral 1P myosin levels are not affected by Y treatment (Figure 4M–P, S). Consistent with this, we found that reducing apical endoderm tension alone produced simulated morphologies that closely matched those measured for Y-treated embryos during Step 2.
The very good fit between our experimental observations and the simulations suggests that the dynamic spatio-temporal control of myosin-driven cortical tensions we identified is a major driver for ascidian invagination (summarized schematically in Figure 7). Importantly, neither apical contraction of the endoderm alone nor its apico-basal shortening alone can explain ascidian invagination; only a balance of the two can do so.
Figure 7. Model for a two-step invagination of ascidian endoderm.
red: 1P-myosin, light blue: 2P-myosin. Black arrows in Step 1 indicate mesectoderm resistance. Magenta arrows indicate apicobasal shortening and lateral spreading of mesectoderm (mechanism unknown), which may lower mesectoderm resistance.
Mesectoderm resists endoderm shape changes during Steps 1 and 2
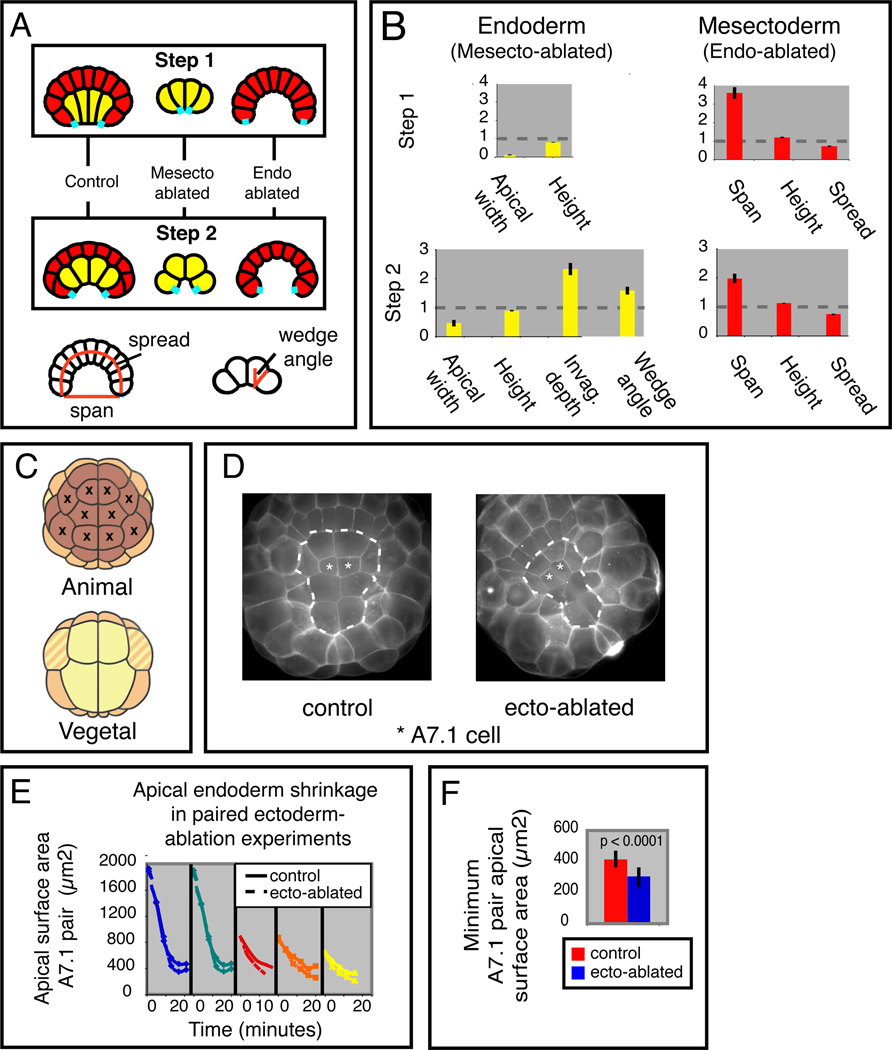
To gain further insights into how the interaction between endoderm and mesectoderm might contribute to cell shape changes during Steps 1 and 2, we simulated tissue ablation and asked how the non-ablated tissue deformed relative to control embryos (Figure 6A). Starting with successful ascidian-like parameter sets for Step 1 (dark blue in Figure 5F,G; Apical_EM > 1) or Step 2 (red in Figure 5F,G), we set all tension values associated with a given tissue to zero, thereby nullifying its mechanical contribution while maintaining appropriate apical, basal, and lateral identity in the remaining cells.
Figure 6. Effect on invagination of virtual and experimental ablations of endoderm or mesectoderm.
A: Schematic overview of the results of virtual ablations of mesectoderm and endoderm. The cyan segments indicate reference points used for measurements in B. The bottom diagrams show how endoderm wedge angle, mesectoderm spread and mesectoderm span were measured. B: Quantification of morphological changes produced by simulated ablation of mesectoderm or endoderm during Step 1 or 2. The height of each bar represents the fractional change for the indicated morphological measure, relative to controls, averaged over 100 runs with different passing parameters. Error bars are standard deviations. Cell height, apical width and invagination depth were measured as in Figure 5B. C: Schematic view of a 32-cell embryo indicating the 10 laser-ablated ectoderm cells. D: Vegetal views of paired control (left) and ectoderm-ablated (right) embryos at late Step 1. Dashed lines mark the endoderm boundary (see Movie S1 for the corresponding time-lapse sequence). E: Evolution of apical A7.1 surface area over time during Step 1 for 5 separate pairs of control vs ectoderm-ablated embryos. F: Minimum apical endoderm surface area achieved during Step 1 in control and ectoderm-ablated embryos. Figure S5 shows additional comparisons of model simulations with experimental perturbations (cleavage arrest by nocodazole, Rho kinase inhibition by Y-27632 and myosin inhibition by blebbistatin).
For successful Step 2 solutions, simulating endoderm ablation increased mesectoderm span and slightly reduced its spread (Figure 6B, bottom right; dotted lines show control measures).
Simulating mesectoderm ablation produced a 2-fold increase in apical constriction and in the depth of endoderm invagination, without affecting endoderm cell height (Figure 6B, bottom left). These results support the hypothesis that endoderm-intrinsic forces are the major drivers for invagination, and that mesectoderm opposes apical constriction, thereby resisting invagination.
When we simulated mesectoderm ablation during Step 1, the endoderm cells hyper-constricted their apices and slightly shortened apico-basally relative to controls (Figure 6B, upper left). Conversely, for simulated endoderm ablations during Step 1, the mesectoderm span increased, and its lateral spread was reduced (Figure 6B, upper right). Thus for tension parameter values which reproduce Step 1, the mesectoderm does not push against endoderm as it flattens and spreads. Instead, our simulations suggest that the mesectoderm resists apical endoderm constriction and that endoderm cells must generate sufficient force to overcome this resistance.
These results predict that ablating mesectoderm in live embryos should lead to an increase in apical endoderm constriction during Step 1. To test this experimentally, we laser-ablated the ten animal-most ectoderm cells at the late 32-cell stage (Figure 6C), then monitored apical endoderm shrinkage during Step 1 relative to paired controls (Figure 6D–F, Movie S8). In 5/5 experiments, we observed an increase in the degree of apical endoderm constriction relative to the paired controls (minimum surface area = 299 +/− 64 µm2 in ablated embryos vs 413 +/‒ 55 µm2 in controls; p < 0.0001; n = 5), confirming the prediction that mesectoderm normally resists apical constriction during Step 1.
DISCUSSION
A fundamental goal for studies of morphogenesis is to understand how embryonic cells organize force generation in space and time to produce characteristic patterns of tissue deformation. The work we present here suggests how spatiotemporal patterns of myosin activation could control cortical tensions to orchestrate the dynamics of ascidian endoderm invagination. First, our reconstructions identify a sequence of cell shape changes, conserved in four ascidian species, in which apical shrinkage and flattening of a columnar endoderm plate plus mesectoderm epiboly (Step 1) precede rapid basolateral shortening around tightly maintained apices (Step 2). Second, analysis of phospho-myosin distributions reveals spatiotemporal patterns of active myosin accumulation consistent with the hypothesis that differential contractility drives cell shape changes during both steps. Third, our computer simulations show that distributions of cortical tension consistent with the phospho-myosin patterns we observe can reproduce the kinematics of invagination observed in normal and experimentally manipulated embryos. Tension parameter sets that reproduce Steps 1 and 2 occupy distinct, and widely separated, regions of parameter space, strongly supporting the idea that each step involves a unique distribution of boundary specific tensions. Although we cannot rule out that additional force-generating mechanisms are involved, our results suggest that differential contractility plays a major role in shaping these boundary-specific tensions.
The systematic exploration of parameter space suggests that the basic mechanism for ascidian invagination is robust with respect to variation in tension parameter values. We observed only minor quantitative variations in otherwise similar patterns of cell shape change across four different species, and these variations can be mimicked in our simulations by small shifts in tension (data not shown). The finding that gastrulation proceeds normally in cleavage arrested embryos and that simulations can reproduce both control and cleavage arrested morphologies for the same choices of tension parameters further attest to this robustness.
Our simulation results suggest that in principle either apical endoderm constriction or active mesectoderm epiboly (or both) could drive endoderm deformation during Step 1. However, only active apical constriction of the endoderm is directly consistent with our 1P–myosin staining, and can simultaneously explain the shape changes observed both in normal and in cleavage arrested embryos. Furthermore, simulated ablations predict – and experimental ablations confirm - that mesectoderm epiboly does not contribute by active pushing, as is often assumed. Instead, contractile forces produced within the endoderm drive both steps of invagination, and mesectoderm resists endoderm shape change – in particular apical constriction - during both steps.
In contrast, both our data and simulations suggest that invagination itself involves the endoderm-intrinsic combination of apico-basal shortening and circumapical contraction – which we call collared rounding (Figure 7). Indeed our simulations suggest that the apposition of strong basolateral and circumapical tension is the only way to reproduce an ascidian-specific invagination based on differential cortical tension. Importantly, inhibiting Rho/Rho kinase during Step 2 in vivo causes excessive apical expansion and reduced invagination without reducing apico-basal shortening, supporting the idea that apico-basal shortening only produces invagination when apical expansion is prevented by sufficient circumapical tension.
Two phase invagination, with apical constriction and columnarization followed by apico-basal shortening, has been observed during invagination in other systems (see [2] for an excellent review). However, a role for myosin-dependent basolateral contraction in driving invagination, and the requirement for a balance between circumapical and basolateral tension, have not previously been documented. Apico-basal shortening is not a universal feature of all invaginating cells (notable examples are bottle cells that form during early gastrulation in Xenopus and dorso-lateral hingepoint cells that form during neurulation in chick and mouse), and thus why it should accompany some cases of invagination and not others remains an interesting puzzle. Perhaps the use of apico-basal shortening coupled to apical/circumapical contraction represents a specialization for rapid invagination as occurs e.g. in ascidians and Drosophila. Alternatively, these differences may reflect the nature and relative contributions of forces acting intrinsic and extrinsic to the invaginating tissues – for example the extent to which surrounding tissues resist invagination as shown here, or aid it, as in Xenopus gastrulation and vertebrate neurulation [reviewed in 2,45].
How are spatiotemporal patterns of myosin activation controlled within the endoderm lineage? As in other systems, we find that apical myosin accumulation plus constriction, and circumapical myosin accumulation plus maintenance of tight apices, depend on the Rho/Rho kinase pathway [10, 13, 28, 29]. The signaling pathways that control Rho during different invaginations are surprisingly diverse, alternately involving folded gastrulation, DPP, Hedgehog, EGF/ERK, Wnts, and ephrin receptors [29–34]. In Ciona, genome-scale ISH surveys identify a small set of candidate signalling molecules, some of which act in pathways that regulate Rho in other systems, and transcription factors that are expressed in a manner consistent with a role in Step 1 [35–37].
The pathways that control Rho-independent basolateral myosin recruitment during Step 2 remain unknown. Key candidates for proximal regulators are protein kinases known to phosphorylate myosin regulatory light chains in other contexts, including Myosin Regulatory Light Chain Kinase (MLCK), Myotonia Dystrophy-related Kinase (MRCK) and P-21 activated Kinase (PAK). Indeed MLCK has been recently shown to control basal myosin activation during otic placode invagination [46]. Our efforts to test these candidate kinases using morpholino-based knock-downs and pharmacological interventions have so far yielded inconclusive results, but this remains an important goal for future studies.
Also unclear is what controls the transition from Step 1 to Step 2. Two-step control during gastrulation might be imposed by cell cycle progression, as Steps 1 and 2 coincide roughly with interphase and M-phase respectively (Sherrard, unpublished data). A similar situation is found during polarization of the C. elegans zygote, where cell cycle progression from interphase to M-phase controls a transition between distinct modes of control over myosin activity (Munro et al, unpublished.). Alternatively, the transition from Step 1 to Step 2 could reflect the temporal dynamics of transcriptional regulation within the endoderm lineage. Indeed, transcriptional profiles in vegetal cells evolve quickly between the mid 64- and the late 112-cell stage, and a small number of secreted factors, including EphrinAc and BMP3, and transcription factors such as lhx3 or TTF1 are expressed specifically in all invaginating cells during the 112-cell stage.
In summary, our results identify spatiotemporal control over myosin activity as a key physiological intermediate between the gene regulatory networks that control endoderm-specific differentiation and the mechanics of cell shape change that drive endoderm invagination. Future studies combining perturbations of key regulatory factors with the analysis of cytoskeletal dynamics and cell shape change in this simple embryo will provide a unique window into the mechanisms that integrate tissue morphogenesis with a global developmental program.
EXPERIMENTAL PROCEDURES
Embryo culture and treatments
Embryos of Ciona intestinalis, Phallusia mammillata, Ciona savignyi and Boltenia villosa were obtained and cultured as previously described [19, 39, 40]. We treated embryos in seawater with 1.3 µM nocodazole, 1 µM cytochalasin, 100 µm blebbistatin, or 100 µM Y-27632 (Sigma), at the 64- or early 112-cell stage.
3D reconstructions and morphometric analysis
Phalloidin-stained embryos were prepared and imaged as described [19]. For recontructions of live Phallusia embryos, we imaged embryos in artificial seawater containing FM4–64 (5mg/ml, Molecular probes), using a two-photon confocal microscope (LSM510 NLO microscope) with a 63× objective (C-Apochromat 1.2 W-corr, Carl Zeiss, Inc.) and 1020nm illumination. For each embryo/timepoint, we collected a complete z-series at 3µm intervals. Raw confocal stacks are available for download at: http://crfb.univ-mrs.fr/aniseed/embryo-collection.php.
We performed morphometric measurements using the 3D Virtual Embryo software [19]. We calculated changes in mean apical surface area for a given cell as a ratio of the final over initial value (n ≥ 3 for each stage). We defined the total shape deformation for a given cell between two stages as the sum over all unit-less normalized shape descriptors (Sphericity, Elongation, Flatness, Squareness, Entropy, Surface/Volume, Convexity) of the absolute value of the difference between initial and final values measured for that descriptor.
Immunostaining and quantitative analysis of phosphomyosin distributions
We fixed embryos for 30 minutes in 100 mM HEPES, pH 7.0, 100 mM EGTA, 10 mM MgSO4, 2% formaldehyde and 0.1% gluteraldehyde, 300 mM Dextrose and 0.2% Triton-X. We treated embryos for 20 minutes in 0.1% sodium borohydride in PBS to reduce unreacted aldehydes, then incubated 24 hours at room temperature with primary antibodies to ser19 phospho-myosin (1:250, Cell Signaling) or thr18/Ser19 phospho-myosin (1:500, Cell Signaling), rinsed three times, then incubated 24 hours in secondary goat anti-rabbit Alexa Fluor 488 or 568 nm (1/600, Invitrogen) and Bodipy FL 488 nm or Alexa 568 nm phallacidin (1:200, Invitrogen). We mounted embryos in Murray’s Clear and confocal imaged them as described previously [41].
We measured fluorescence intensities along cell boundaries in Image J as mean gray levels averaged over 1 µm-thick lines drawn along the boundary of interest. We measured apical 1P–myosin from single cross-sections produced by reslicing raw image stacks parallel to the Animal-Vegetal (AV) axis, and lateral 1P–myosin from single confocal sections taken perpendicular to the AV axis. We measured circumapical 1P- and 2P–myosin from maximum intensity projections of the apical surface. We measured cytoplasmic background levels for each cell as the mean gray-level within a small (5 µm2) box located within the deep cytoplasm.
GFP fusions and dominant negative constructs
We constructed a Gateway compatible Ci-ZO-1 clone by PCR-amplifying the coding sequence from a cDNA clone (Cicl035p23, gift of Yutaka Satou and Nori Satoh) flanked by gateway compatible attR1-attR2 sequences (Forward primer: 5’GGGGACAAGTTTGTACAAAAAAGCAGGCTCAGAAAAAATGATGGATGAGCTAATATGGCAGGAGC3’, Reverse primer: 5’ GGGGACCACTTTGTACAAGAAAGCTGGGTTGAAATGGTCGATAAGAACAGAAACGC 3’). We recombined this fragment into a p221-DONR [42]. A Gateway-compatible RhoA dominant-negative construct T19N [43] was generated by point mutation. Constructs were recombined in pSPE3-RfA-Venus and pSPE3-RfA respectively [42] for RNA synthesis.
Laser ablations
Animal cells of 32-cell stage embryos (see Fig 7C) were ablated using a Micropoint nitrogen-pumped dye laser (Photonics Instruments, 365 nm, 10–20hz, maximum power), mounted on a Nikon Eclipse TE2000-U microscope. A short (< 1 sec) laser pulse focused at the apical surface of a cell was sufficient for lysis. Lysed cell’s remnants were removed manually from the embryos before imaging. Paired control and ablated embryos were labeled with the membrane dye FM4–64, then imaged using a DeltaVision microscope through a 40× water immersion lens. A stack of 15–20 sections spaced at 1µm intervals were collected every 10 seconds. We then measured endoderm apical surface areas from maximal projections using Image-J software.
Computer simulations and searches of tension parameter space
All simulations and analysis were performed using custom software. See Supplementary Modeling Procedures for details.
Supplementary Material
HIGHLIGHTS.
Ascidian endoderm invagination occurs in two distinct steps.
Distinct spatial patterns of Myosin II activation accompany each step.
Coordinated apical and basolateral contraction drives invagination.
Simulations show that differential cortical tensions can explain the dynamics of invagination.
ACKNOWLEDGEMENTS
This work was supported by CNRS (PL), the Marine Genomics Europe FP6EU network of excellence (PL) and by NIGMS Center of Excellence grant P50 GM006605 (EM). FR was funded by the French ministry of research and higher education and by the Association pour la Recherche sur le Cancer (ARC), KS by an NIH-NRSA fellowship. We thank Garry Odell, Victoria Foe, and David McClay for comments on the manuscript. We also acknowledge the staff of the Marine biology Station of Roscoff for collecting animals in France and François Graziani (IBDML) for animal care.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Davidson L, Koehl MAR, Keller R, Oster GF. How do sea urchins invaginate? Using biomechanics to distinguish between mechanisms of primary invagination. Development. 1995;121:2005–2018. doi: 10.1242/dev.121.7.2005. [DOI] [PubMed] [Google Scholar]
- 2.Keller , Davidson RL, Shook D. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 3.Clausi DA, Brodland GW. Mechanical evaluation of theories of neurulation using computer simulations. Development. 1993;118:1013–1023. [Google Scholar]
- 4.Jacobsen AG, Oster GF, Odell GM, Cheng LY. Neurulation and the cortical tractor model for epithelial folding. Journal of embryology and experimental morphology. 1986;96:19–49. [PubMed] [Google Scholar]
- 5.Muñoz JJ, Barrett K, Miodownik M. A deformation gradient decomposition method for the analysis of the mechanics of morphogenesis. Journal of Biomechanics. 2007;40:1372–1380. doi: 10.1016/j.jbiomech.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Odell GM, Oster GF, Alberch P, Burnside B. The mechanical basis of morphogenesis I. Epithelial folding and invagination. Developmental Biology. 1981;85:446–462. doi: 10.1016/0012-1606(81)90276-1. [DOI] [PubMed] [Google Scholar]
- 7.Pouille P-A, Farge E. Hydrodynamic simulation of multicellular embryo invagination. Physical Biology. 2008;5:015005. doi: 10.1088/1478-3975/5/1/015005. (9 pp) [DOI] [PubMed] [Google Scholar]
- 8.Brodu V, Cassanova J. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes and Development. 2006;20:1817–1828. doi: 10.1101/gad.375706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J-Y, Harland RM. Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells. Developmental Biology. 2007;311:40–52. doi: 10.1016/j.ydbio.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolaidou KK, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Current Biology. 2004;14:1822–1826. doi: 10.1016/j.cub.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 11.Simões S, Denholm B, Azevedo D, Sotillos S, Martin P, Skaer H, Hombría JC-G, Jacinto A. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development. 2006;133:4257–4267. doi: 10.1242/dev.02588. [DOI] [PubMed] [Google Scholar]
- 12.Young PE, Pesacreta TC, Kiehart DP. Dynamic changes in the distribution of cytoplasmic myosin during Drosophlia embryogenesis. Development. 1991;111:1–14. doi: 10.1242/dev.111.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Beane WS, Gross JM, McClay DR. RhoA regulates initiation of invagination, but not convergent extension, during sea urchin gastrulation. Developmental Biology. 2006;292:213–225. doi: 10.1016/j.ydbio.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus E. folded gastrulation, cell shape change, and the control of myosin localization. Development. 2005;132:4165–4176. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira MC, Hilfer SR. Calcium regulation of neural fold formation: visulation of the actin cytoskeleton in living chick embryos. Developmental Biology. 1993;159:427–440. doi: 10.1006/dbio.1993.1253. [DOI] [PubMed] [Google Scholar]
- 16.Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Developmental Dynamics. 2005;232:685–694. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- 17.Leptin M. Gastrulation movements: the logic and the nuts and bolts. Developmental Cell. 2005;8:305–320. doi: 10.1016/j.devcel.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Munro EM, Robin F, Lemaire P. Cellular morphogenesis in ascidians: how to shape a simple tadpole. Current Opinion in Genetics and Development. 2006;16:399–405. doi: 10.1016/j.gde.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Tassy O, Daian F, Hudson C, Bertrand V, Lemaire P. A quantitative approach to the study of cell shape changes and interactions during early chordate embryogenesis. Current Biology. 2006;16:345–358. doi: 10.1016/j.cub.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Nishida H. Cell division pattern during gastrulation of the ascidian, Halocynthia roretzi. Development Growth and Differentiation. 1986;28:191–201. doi: 10.1111/j.1440-169X.1986.00191.x. [DOI] [PubMed] [Google Scholar]
- 21.Satoh N. Cellular morphology and architecture during early morphogenesis of the ascidian egg: an SEM study. Biological Bulletin. 1978;155:608–614. doi: 10.2307/1540794. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura F. Regulation of Myosin II during cytokinesis in higher eukaryotes. Trends in Cell Biology. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu S, Ikebe M. ZIP kinase is responsible for the phosphorylation of myosin II and necessary for cell motility in mammalian fibroblasts. Journal of Cell Biology. 2004;165:243–254. doi: 10.1083/jcb.200309056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizutani T, Haga H, Koyama Y, Takahashi M, Kawabata K. Diphosphorylation of the myosin regulatory light chain enhances the tension acting on stress fibers in fibroblasts. Journal of Cellular Physiology. 2006;209:726–731. doi: 10.1002/jcp.20773. [DOI] [PubMed] [Google Scholar]
- 25.Uchimura T, Fumoto K, Yamamoto Y, Ueda K, Hosoya H. Spatial localization of mono- and diphosphorylated myosin II regulatory light chain at the leading edge of motile HeLa cells. Cell Structure and function. 2002;27:479–486. doi: 10.1247/csf.27.479. [DOI] [PubMed] [Google Scholar]
- 26.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellars JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–7. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological Properties of Y-27632, a Specific Inhibitor of Rho-Associated Kinases. Molecular Pharmacology. 2000;57:976–983. [PubMed] [Google Scholar]
- 28.Barrett K, Leptin M. The Rho GTPse and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita N, Sasai N, Misaki K, Yonemura S. Apical Accumulation of Rho in the Neural Plate Is Important for Neural Plate Cell Shape Change and Neural Tube Formation. Molecular Biology of the Cell. 2008;19:2289–2299. doi: 10.1091/mbc.E07-12-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Developmental Cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Developmental Cell. 2007;13:717–729. doi: 10.1016/j.devcel.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Lee J-Y, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Wnt/frizzled signaling controls C. elegans gastrulation by activating acto-myosin contractility. Current Biology. 2006;16:1986–1997. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–4282. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- 34.Xu N, Keung B, Myat MM. Rho GTPase controls invagination and cohesive migration of the Drosophila salivary gland through Crumbs and Rho-kinase. Developmental Biology. 2008;321:88–100. doi: 10.1016/j.ydbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Imai K, Hino K, Yagi K, Satoh N, Satou Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131:4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- 36.Miwata K, Chiba T, Horii R, Yamada L, Kubo A, Miyamura D, Satoh N, Satou Y. Systematic analysis of embryonic expression profiles of zinc finger genes in Ciona intestinalis. Developmental Biology. 2006;292:546–554. doi: 10.1016/j.ydbio.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Shi W, Levine M. Ephrin signaling established asymmetric cell fates in an endomesoderm lineage of the Ciona embryo. Development. 2008;135:931–940. doi: 10.1242/dev.011940. [DOI] [PubMed] [Google Scholar]
- 38.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDougall A, Sardet C. Function and characteristics of repetitive calcium waves associated with meiosis. Current Biology. 1995;3:318–328. doi: 10.1016/s0960-9822(95)00062-5. [DOI] [PubMed] [Google Scholar]
- 40.Munro EM, Odell GM. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Foe V, von Dassow G. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. Journal of Cell Biology. 2008;183(3):457–470. doi: 10.1083/jcb.200807128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roure A, Rothbächer U, Robin F, Kalmar E, Ferone G, Lamy C, Missero C, Mueller F, Lemaire P. A multicassette Gaetway vector set for high throughput and comparative analysis of ciona and vertebrate embryos. PLoS One. 2007;2(9):e916. doi: 10.1371/journal.pone.0000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philips A, Blein M, Robert A, Chambon JP, Baghdiguian S, Weill M, Fort P. Ascidians as a vertebrate-like model organism for physiological studies of Rho GTPase signaling. Biol Cell. 2003;95:295–302. doi: 10.1016/s0248-4900(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 44.Ueda K, Murata-Hori M, Tatsuka M, Hosoya H. Rho kinase contributes to diphosphorylation of myosin II regulatory light chain in non muscle cells. Oncogene. 2002;21:5852–60. doi: 10.1038/sj.onc.1205747. [DOI] [PubMed] [Google Scholar]
- 45.Sai X, Ladher R. FGF signaling regulates cytoskeletal remodeling during epithelil morpohogenesis. Current Biology. 2008;18:976–81. doi: 10.1016/j.cub.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 46.Colas J, Schoenwolf GC. Towards a cellular and molecular understanding of neurulaiton. Developmental Dynamics. 2001;221:177–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.