Abstract
Aim:
Potassium 2-(1-hydroxypentyl)-benzoate (dl-PHPB) is a new drug candidate for ischemic stroke. The aim of this study was to investigate the effects of dl-PHPB on memory deficits and long-term potentiation (LTP) impairment in animal models of Alzheimer's disease.
Methods:
The expression of NMDA receptor subunits GluN1 and GluN2B in the hippocampus and cortex of APP/PS1 transgenic mice were detected using Western blot analysis. Memory deficits of the mice were evaluated with the passive avoidance test. LTP impairment was studied in the dentate region of Aβ1–42-injected rats and APP/PS1 transgenic mice.
Results:
APP/PS1 transgenic mice showed significantly lower levels of GluN1 and p-GluN2B in hippocampus, and chronic administration of dl-PHPB (100 mg·kg−1·d−1, po) reversed the downregulation of p-GluN2B, but did not change GluN1 level in the hippocampus. Furthermore, chronic administration of dl-PHPB reversed the memory deficits in APP/PS1 transgenic mice. In the dentate region of normal rats, injection of dl-PHPB (100 μmol/L, icv) did not change the basal synaptic transmission, but significantly enhanced the high-frequency stimulation (HFS)-induced LTP, which was completely prevented by pre-injection of APV (150 μmol/L, icv). Chronic administration of dl-PHPB (100 mg·kg−1·d−1, po) reversed LTP impairment in Aβ1–42-injected normal rats and APP/PS1 transgenic mice.
Conclusion:
Chronic administration of dl-PHPB improves learning and memory and promotes LTP in the animal models of Alzheimer's disease, possibly via increasing p-GluN2B expression in the hippocampus.
Keywords: dl-PHPB, Alzheimer's disease, APP/PS1 mice, Aβ1–42, hippocampus, learning and memory, long-term potentiation, synaptic plasticity, NMDA receptor
Introduction
Potassium 2-(1-hydroxypentyl)-benzoate (dl-PHPB) is a novel compound synthesized by the Institute of Materia Medica, Chinese Academy of Medical Sciences. Previous studies showed that dl-PHPB ameliorates the effects of ischemic stroke via reducing the infarct volume and improving regional cerebral blood flow in a rat model of transient middle cerebral artery occlusion (tMCAO)1. In 2009, dl-PHPB was approved to undergo phase I clinical trial by the State Food and Drug Administration as a new drug candidate for ischemic stroke. Recently, the phase I clinical trial was completed, and phase II and phase III clinical trials were approved. In addition to the protective effects of dl-PHPB in cerebral ischemia, our preliminary studies showed that dl-PHPB ameliorated the learning and memory deficits in rats that were cerebrally hypoperfused and in rats that were icv infused with β-amyloid (Aβ)2. These results suggest that dl-PHPB may have therapeutic effects against dementia and Alzheimer's disease (AD). However, the ameliorative effects of dl-PHPB in AD animal models in vivo and the possible mechanisms underlying its effects on synaptic plasticity remain to be studied.
Synaptic plasticity is one of the most important properties of the mammalian brain; it refers to the activity-dependent modification of the strength or efficacy of synaptic transmission at preexisting synapses. For more than a century, synaptic plasticity has been proposed to play a central role in the capacity of the brain to incorporate transient experiences into persistent memory traces3. Long-term potentiation (LTP) is a form of synaptic plasticity that has been widely used as a cellular model of learning and memory mechanisms. Various physiological and pathological processes are associated with AD. These processes can influence LTP induction and maintenance, and they include Aβ-induced neuronal toxicity, acetylcholine system dysfunction and changes in NMDA receptor expression. A number of groups have reported that Aβ administration can negatively affect synaptic plasticity. For instance, Aβ peptides have been shown to inhibit LTP in the CA1 region4,5 and dentate gyrus (DG) both in vivo and in vitro region6,7,8,9. Transgenic models of AD such as APP and APP/PS1 mice display early deficits in synaptic plasticity and memory, even before developing typical AD pathology and behavioral deficits10,11,12,13. Moreover, drugs that have been reported to improve memory impairment also show ameliorative effects on LTP14,15,16.
In the present study, to investigate the actions of dl-PHPB on AD models in vivo and the possible electrophysiological mechanisms of dl-PHPB, we investigated the effects of dl-PHPB on memory capability and synaptic plasticity (LTP) under physiological and AD-associated pathological conditions, including in rats that had been icv injected with Aβ1–42 and in APP/PS1 transgenic (Tg) mice. In addition, we examined the effects of dl-PHPB on LTP mediated by NMDA and AMPA/kainate receptors, which are the major excitatory amino-acid receptors, in APP/PS1 mice.
Materials and methods
Animals and treatment
Male Wistar rats (3 months old, 220–250 g) used in LTP recording were obtained from the Experimental Animal Center of the Chinese Academy of Medical Sciences, Beijing. APP/PS1 double-Tg mice were purchased from the Jackson Laboratory (strain name: B6C3-Tg (AβPPswe, PSEN1dE9) 85Dbo/J, stock number: 004462). These mice express a chimeric mouse/human AβPP containing the K595N/M596L Swedish mutations and a mutant human PS1 carrying the exon-9-deleted variant under the control of mouse prion promoter elements, directing transgenic expression predominantly to CNS neurons17. The 2 transgenes cosegregate in these mice. All animals were housed in a temperature- and humidity-controlled room (temperature: 22±1 °C, humidity: 60%) and had access to standard rodent chow and fresh tap water ad libitum. They were kept on a 12 h light/dark cycle and adapted to these conditions for at least 7 d before experiments. All experiments were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of our institute.
dl-PHPB was synthesized (purity>99%) by the Department of Medical Synthetic Chemistry, Institute of Materia Medica, Chinese Academy of Medical Sciences. Male APP/PS1 Tg mice and wild-type (WT) littermates were randomly divided into 4 groups: treated APP/PS1 mice, untreated APP/PS1 mice, treated WT mice, and untreated WT mice. Treated groups received dl-PHPB dissolved in normal saline solution by oral gavage for 5 d per week at a dose of 100 mg/kg body weight. Untreated groups received normal saline solution alone as a vehicle control. Treatment was started when the mice were 13 months old and lasted for 4 weeks. The body weight of each mouse was recorded every 2 weeks. After the treatment, half of the mice in each group were used for behavioral testing, and the other mice were used for LTP recording. The mice were sacrificed after behavioral testing was completed. Their brains were removed, snap-frozen in liquid nitrogen and stored at −80 °C until analysis.
Step-down passive avoidance test
The step-down passive avoidance test was conducted in an apparatus (STT-2, Institute of Materia Medica, Chinese Academy of Medical Sciences) that consisted of a cylindrical rubber platform (4.5 cm in diameter and 4.5 cm in height) fixed at the left end of an acrylic box (33 cm×11 cm×11 cm) with an electrifiable metallic grid floor connected to an electric shock generator. When a mouse was placed on the grid floor of the box, it would suffer an inescapable intermittent electric shock (1 s, 36 V), and the platform provided a shelter for the mouse. The experiments were conducted between 8:00 and 16:00. Before the beginning of the acquisition trial, the mouse was placed in the box to adapt for 3 min without electric shock. Then, the mouse was placed on the platform. The acquisition trial began when the animal stepped down from the platform and placed all 4 paws on the grid floor. On receiving the electric shock, the mouse escaped to the platform. The duration of the training test was 5 min, and the shock was maintained for this period. The error numbers (the times the mouse stepped down from the platform and received a shock) were recorded during this period. The retention test was carried out 24 h after the acquisition trial in a manner similar to the training except that no electric shock was delivered to the grid floor. Step-down latency (duration of staying on the platform) and error numbers were recorded to assess the level of retention of passive avoidance. If the mouse did not step down to the grid floor within 3 min, a ceiling score of 180 s was assigned.
Electrophysiological recording
In vivo preparation
Male Wistar rats (220–250 g) and mice were anesthetized with urethane (1.2 g/kg), placed in a stereotaxic frame and assessed for LTP. Small holes were drilled in the skull at the positions of the stimulating electrodes and recording electrode. For Wistar rats, the stimulating electrode (bipolar stainless steel) was positioned in the perforant path (7.5 mm posterior to bregma, 4.2 mm lateral to midline and 3.0 mm vertical to dura). The recording electrode (mono-polar stainless steel) was placed in the DG region (3.8 mm posterior to bregma, 2.5 mm lateral to midline and 3.5 mm vertical to dura). A separate hole was drilled to introduce a guide cannula for icv injection of drug or vehicle. The cannula was positioned above the lateral ventricle in the opposite hemisphere from that of the recording or stimulating electrodes (0.8 mm posterior, 1.2 mm lateral to bregma and 3.5 mm from the cranial theca). For mice, the stimulating electrode (bipolar stainless steel) was positioned in the perforant path (3.8 mm posterior to bregma, 3.0 mm lateral to midline and 1.5 mm vertical to dura). The recording electrode (mono-polar stainless steel) was placed in the DG region (2.0 mm posterior to bregma, 1.4 mm lateral to midline and 1.5 mm vertical to dura).
Test stimuli were delivered to the perforant path every 30 s (0.033 Hz, 100 μs duration). The depth of the recording and stimulating electrodes was gently adjusted to maximize the amplitude of the extracellular population spike (PS). Baseline population spikes were recorded at 40% of maximal response. The amplitude of PS was used to measure synaptic efficacy.
Induction of LTP in DG in vivo
Baseline PS amplitude was monitored and recorded for at least 30 min prior to the application of a series of high-frequency stimulations (HFS: 10 trains of 10 stimuli at 100 Hz, intertrain interval of 200 ms). This protocol produced a robust LTP response in our previous study (data not shown). Population spikes evoked by low-frequency stimulation (0.033 Hz) were then recorded for a further 60 min after HFS application.
Data collection and data analysis
Extracellular field potentials were amplified, filtered at 5 kHz, digitized and recorded using a TDT RA16PA amplifier and a TDT RX7-5 processor (Tucker-Davis Technologies, Alachua, FL, USA) and observed with OpenEx software (Tucker-Davis Technologies, Alachua, FL, USA). PS amplitudes were collected every 30 s, and the averaged responses of 10 stimuli were measured every 5 min throughout the experiment. The baseline PS amplitude was monitored and recorded for a 30-min period before application of HFS. This value was used as 100% of the PS amplitude baseline, and all subsequent recorded values were normalized to this baseline value. Successful induction of LTP was defined as a change in the amplitude of the PS exceeding 20%. Error bars on the graphs represent the SEM. Control experiments in which vehicle was icv applied were interleafed between test experiments.
Western blotting analysis
APP/PS1 mice were decapitated, and hippocampal samples from the mice were homogenized thoroughly and then lysed in a RIPA lysis buffer (50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate and 0.1% SDS). Protein concentrations were measured with a BCA kit (Pierce Labs, Rockford, IL, USA). Protein samples (40 μg per lane) were separated on polyacrylamide gels, transferred to PVDF membranes, blocked with 5% milk solution (nonfat dry milk in TBST) for 2 h, and subsequently incubated overnight with primary antibodies diluted in blocking solution. The following antibodies were used for Western blotting: monoclonal rabbit anti-GluN1 antibody (1:500, Cell Signaling Technology, Beverly, MA, USA), monoclonal rabbit anti-GluN2B antibody (1:500, Cell Signaling Technology), monoclonal rabbit anti-phosphorylated GluN2B (p-GluN2B) antibody (1:500, Cell Signaling Technology) and monoclonal mouse anti-β-actin antibody (1:10000, Sigma, St Louis, MO, USA). After being washed with TBST for 5 times, the membrane was incubated with secondary antibodies (horseradish peroxidase-conjugated anti-mouse, anti-rabbit or anti-goat IgG) at room temperature for 1 h. The signals were detected using an enhanced chemiluminescence kit, scanned using an LAS4000 Fujifilm imaging system (Fujifilm, Tokyo, Japan) and analyzed by densitometric evaluation using Quantity-One software (Bio-Rad, Hercules, CA, USA). The values were normalized to β-actin intensity levels.
Aβ oligomer preparation and application
Aβ oligomers were prepared according to previously published protocols18. Synthetic Aβ1–42 purchased from Sigma was dissolved in hexafluor-2-propanol (HFIP), aliquoted and kept at -80 °C after evaporation of HFIP. Aβ oligomers were freshly prepared by dissolving the above peptide film in dimethyl sulphoxide and diluting it into cold F12 medium without phenol red to yield a 100 μmol/L stock. This preparation was incubated at 4 °C for 24 h and centrifuged at 14 000×g for 10 min at 4 °C, and the supernatant was further used for electrophysiological experiments.
According to previous reports19,20, the volume of rat cerebrospinal fluid (CSF) varies between 300 and 580 μL, depending on the size of the animal. We estimate that for a rat of 220–250 g, the volume of CSF is approximately 500 μL. Calculation of the concentration of Aβ1–42 was based on this volume.
Statistical analysis
GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA) was used to analyze the data. All data are expressed as means±SEM. Statistical significance was evaluated via Student's t-test or one-way analysis of variance followed by appropriate post tests using SPSS software (SPSS Inc, Chicago, IL, USA). A value of P<0.05 was considered statistically significant.
Results
dl-PHPB ameliorated memory deficits in APP/PS1 Tg mice
The effects of dl-PHPB on the impairment of behavioral performance in aged AD model Tg mice were evaluated. APP/PS1 Tg mice, which express both mutant human APP and PS1, were chosen for the following experiments. After oral treatment with dl-PHPB for 1 month, the behavior of the APP/PS1 mice at the age of 14 months was tested with the step-down passive avoidance test.
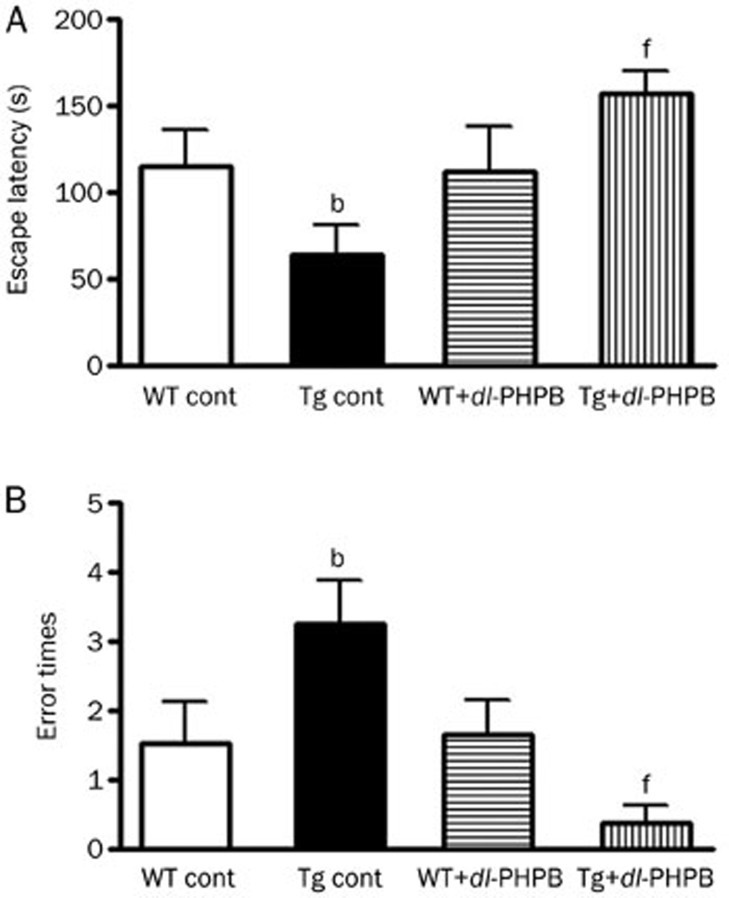
Escape latency was recorded in the retention test session, representing the time spent by animals before stepping down from a platform onto an electrified grid floor. APP/PS1 Tg control mice spent significantly less time on the platform than the WT control group (Tg control: 64.5±16.7 s, n=15; WT control: 115.5±32.0 s, n=13; bP<0.05 vs WT control group; Figure 1A), indicating obvious memory deficits in Tg mice. However, dl-PHPB-treated APP/PS1 Tg mice (100 mg/kg for 1 month, po) spent significantly more time (Tg dl-PHPB: 157.3±43.6 s, n=15) on the platform compared with untreated Tg mice (fP<0.01 vs Tg control group, Figure 1A). The frequency of animals stepping down from the platform to the electrified grid floor (called errors) was recorded. Compared with the WT control group, animals in the Tg control group scored more errors (Tg control: 3.3±0.8, n=15; WT control: 1.5±0.4, n=13; bP<0.05 vs WT control group; Figure 1B), and treatment with dl-PHPB reduced the error frequency (Tg dl-PHPB: 0.4±0.1, fP<0.01 vs Tg control group, n=15; Figure 1B). Therefore, these results suggest that oral treatment with dl-PHPB ameliorated the impairment of behavioral performance in aged AD Tg mice.
Figure 1.
Long-term oral treatment with dl-PHPB ameliorated memory deficits in APP/PS1 Tg mice. Escape latency and error frequency were evaluated via step-down passive avoidance test. (A) Escape latency was recorded for the retention test 24 h after training, representing the time spent by animals before stepping down from the platform onto the grid floor. Animals in the Tg control group spent significantly less time on the platform compared with WT control group (bP<0.05 vs WT control group, n=13–15). dl-PHPB treatment (100 mg·kg−1·d−1 for 1 month, po) ameliorated this effect and significantly prolonged escape latency (fP<0.01 vs Tg control group, n=13–15). (B) Compared with the WT control group, animals in the Tg control group showed higher error frequencies (bP<0.05 vs WT control group, n=13–15), and treatment with dl-PHPB reduced the error frequency (fP<0.01 vs Tg control group, n=13–15). All data are presented as means±SEM.
Effects of dl-PHPB on basal synaptic transmission and LTP in the DG region of normal rats
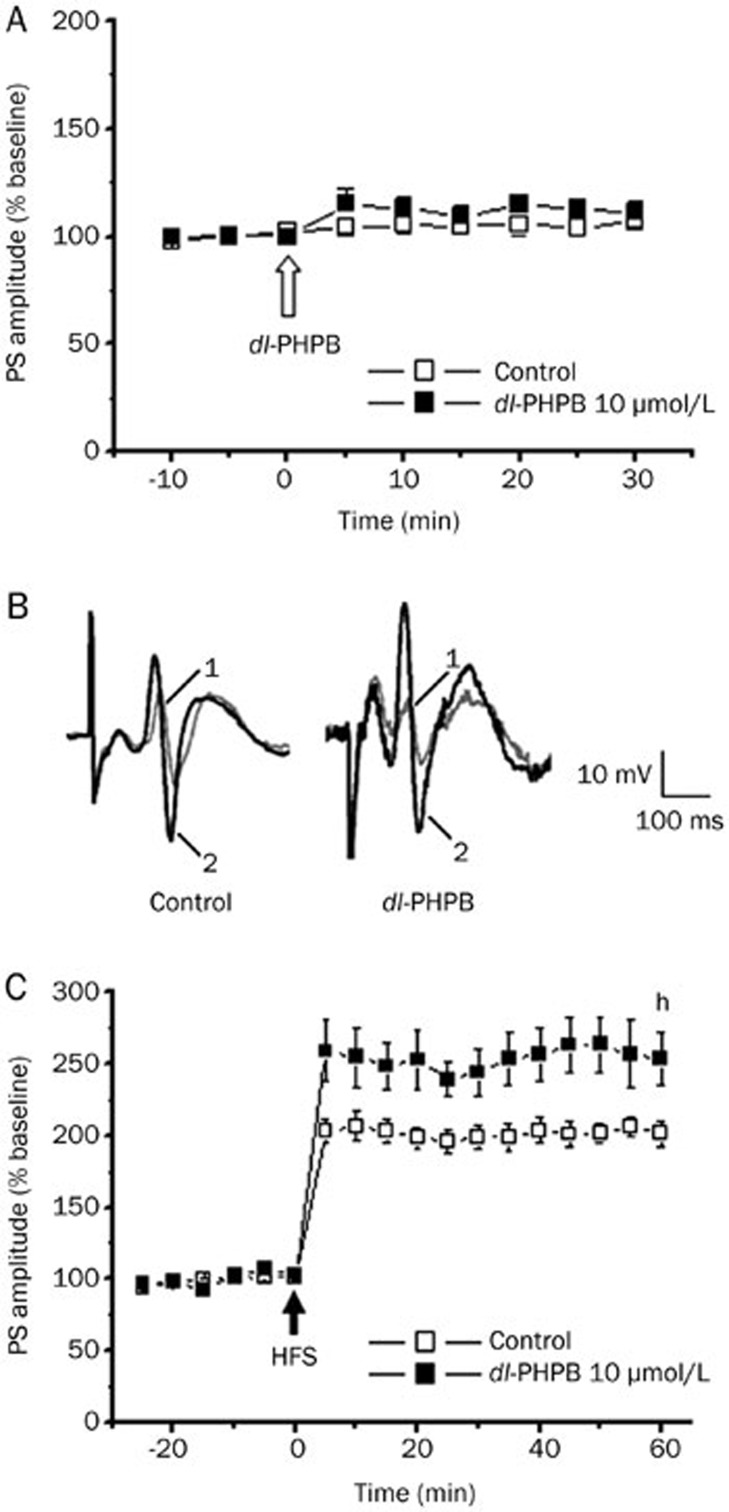
The effect of dl-PHPB on basal excitatory synaptic transmission in the hippocampal DG region of anesthetized rats was evaluated. After a stable baseline for 15 min was established, rats were icv injected with 5 μL vehicle (0.9% saline) or 1 mmol/L dl-PHPB in 5 μL CSF (final concentration, approximately 10 μmol/L). The PS amplitude was recorded for the following 30 min. There were no significant changes in the PS amplitude after dl-PHPB or vehicle injection (dl-PHPB: 111.1%±5.3%, n=7; control group: 106.6%±2.4%, n=7; P>0.05; Figure 2A and 2B), suggesting that dl-PHPB did not affect basal synaptic transmission. To assess whether dl-PHPB affected synaptic plasticity in normal rats, the effect of dl-PHPB on HFS-induced LTP in the hippocampal DG region was examined. As shown in Figure 2C, pre-injection with dl-PHPB (1 mmol/L in 5 μL, approximately 10 μmol/L in CSF) for 30 min significantly enhanced HFS-induced LTP, and the potentiation persisted for at least 60 min (dl-PHPB: 252.9%±18.5%, n=8; control group: 201.2%±9.3%, n=9; hP<0.05). These results suggest that dl-PHPB directly promoted synaptic plasticity in the hippocampus.
Figure 2.
Effects of dl-PHPB on basal synaptic transmission and LTP in the DG of normal rats. dl-PHPB was applied intracerebroventricularly. (A) dl-PHPB had no effect on basal synaptic transmission. After a baseline recording of 15 min, rats were icv injected (white arrow) with dl-PHPB (10 μmol/L) or vehicle. The time course of changes in the PS amplitude is expressed as the percentage of the mean baseline value. Statistical significance was evaluated at 30 min after injection of dl-PHPB. There was no significant change after drug application (n=7). (B) Typical PS waveforms recorded in different treatments. (C) Rats were icv injected with dl-PHPB (10 μmol/L) or vehicle followed by a series of HFS (black arrow) 30 min later. dl-PHPB (10 μmol/L) can significantly increase LTP induced by HFS compared with the control group. Example data traces in the upper panel are responses recorded at the times prior to HFS and 60 min after HFS. Statistical significance was evaluated at 60 min after induction of LTP. All data were presented as means±SEM. hP<0.05 vs control, n=8–9.
dl-PHPB reversed the Aβ1–42 induced LTP impairment in hippocampus in vivo
A number of groups have reported that acute treatment with Aβ1–42 inhibits LTP induction in the DG region both in vitro and in vivo15. As we previously reported, dl-PHPB ameliorates behavioral deficits induced by icv infusion of Aβ thus, it is important to investigate the effects of dl-PHPB on Aβ-induced LTP impairment in vivo to evaluate the possibility of applying dl-PHPB in AD therapy.
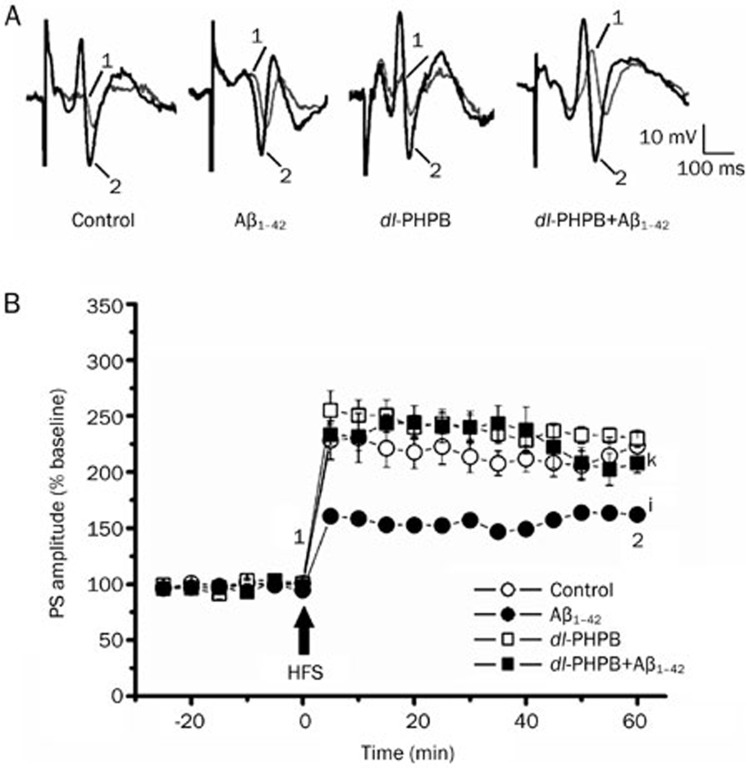
In the current study, based on a previous report21, Aβ1–42 (1 nmol/L in 5 μL sterile water) was icv injected 60 min before the application of HFS in the LTP experiment. PS amplitudes in the Aβ1–42 group were strongly decreased compared with the control group (Aβ1–42: 161.9%±3.1%, n=5; control group: 223.4%±14.2%, n=5; iP<0.01; Figure 3), indicating a decay of the synaptic transmission caused by Aβ1–42 injection. However, in rats that had been pretreated with dl-PHPB (100 mg/kg for 1 month, po), acute injection of Aβ1–42 did not suppress PS amplitudes on HFS application (dl-PHPB+Aβ1–42: 208.0%±8.6%, n=5; kP<0.05 vs Aβ1–42 group; Figure 3). These results suggested that oral treatment with dl-PHPB prevents the LTP impairment caused by Aβ1–42 injection.
Figure 3.
Protective effects of oral treatment with dl-PHPB on Aβ-mediated inhibition of LTP in vivo. dl-PHPB was applied orally. (A) Typical PS waveforms recorded in different treatments. (B) In the control group, HFS induced robust LTP, whereas injection of soluble Aβ1–42 (1 nmol, icv) 60 min prior to HFS strongly inhibited the induction of LTP. In animals orally pretreated for 1 week with 100 mg/kg dl-PHPB, the injection of Aβ1–42 (1 nmol, icv) did not impair the induction of LTP compared with the Aβ1–42 injected group. Statistical significance was evaluated at 60 min after induction of LTP. All data are presented as means±SEM. iP<0.01 vs control group, n=5. kP<0.05 vs Aβ1–42 group, n=5.
dl-PHPB reversed the LTP impairment in APP/PS1 Tg mice in vivo
To investigate whether the dl-PHPB-mediated improvement in memory deficits correlated with synaptic plasticity in a Tg AD model, the effect of long-term treatment with dl-PHPB on HFS-induced LTP in the hippocampal DG region was examined in APP/PS1 mice in vivo.
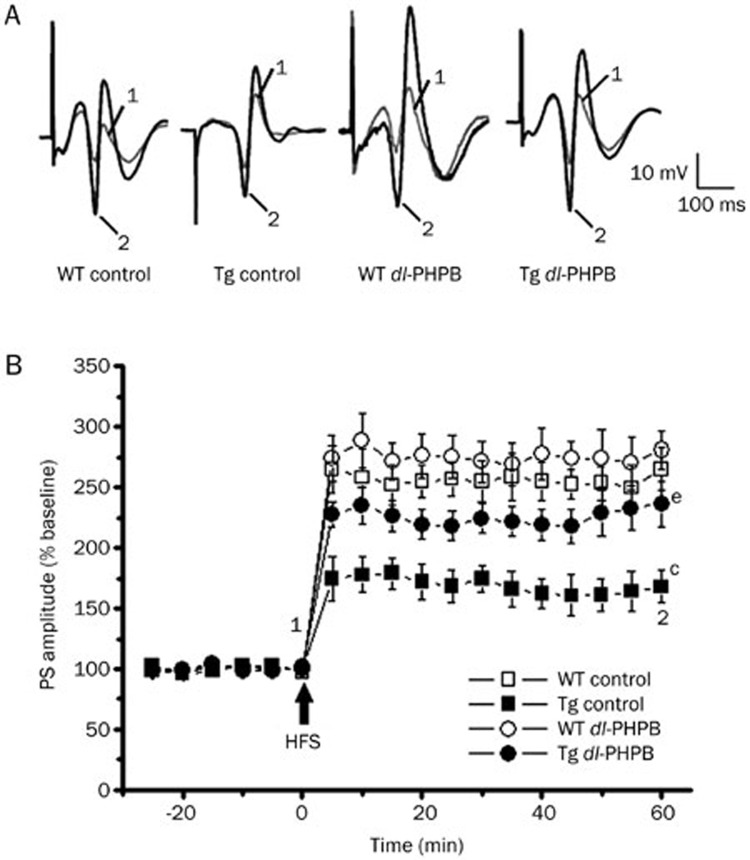
Strong LTP was induced in the DG region in WT control mice aged 14 months, whereas only weak LTP was observed in APP/PS1 Tg mice. Statistical analysis showed a significant difference between these 2 groups (Tg control: 167.9%±13.5%, n=5; WT control: 265.2%±17.7%, n=5; cP<0.01; Figure 4). Treating APP/PS1 Tg mice with dl-PHPB at 100 mg/kg orally for 1 month enhanced LTP (Tg dl-PHPB: 236.5%±19.0%, n=5) compared with the APP/PS1 control group (eP<0.05; Figure 4). However, treating WT mice with dl-PHPB did not significantly affect LTP (Figure 4).
Figure 4.
dl-PHPB reversed the LTP impairment in APP/PS1 Tg mice in vivo. (A) Typical PS waveforms recorded from different treatments. (B) LTP was decreased in APP/PS1 mice compared with non-Tg mice, but this reduction was ameliorated by dl-PHPB treatment (100 mg·kg−1·d−1 for 1 month, po). Data are expressed as the mean percentage change in PS. Analysis of the mean percentage changes in PS at 60 min after HFS. This result revealed a significant decrease in the LTP level in the APP/PS1 control group compared with non-Tg mice (cP<0.01 vs WT control group, n=5); dl-PHBP treatment significantly attenuated the LTP reduction. eP<0.05 vs Tg control group, n=5.
Effects of dl-PHPB on LTP were associated with NMDA receptors
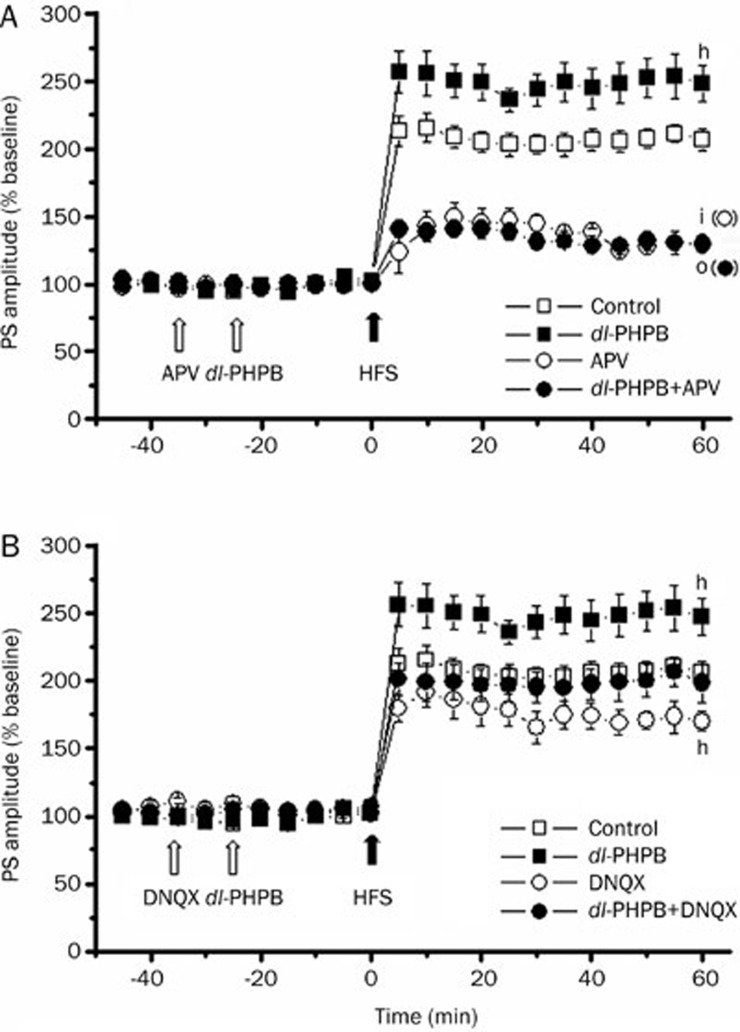
With the exception of mossy fiber-CA3 synapses, the induction of LTP in all subfields of the hippocampus is NMDA-dependent. This potentiation is prevented by blockade of postsynaptic NMDA receptors. Application of the NMDA-receptor-specific antagonist D-APV (150 μmol/L in CSF, icv) 40 min before HFS significantly suppressed LTP (D-APV: 128.7%±4.2%, n=5; control group: 206.5%±8.4%, n=5; iP<0.01; Figure 5). Pre-injection of D-APV completely prevented the LTP improvement induced by dl-PHPB (1 mmol/L in 5 μL, approximately 10 μmol/L in CSF) (D-APV+dl-PHPB: 129.6%±4.8%, n=5; dl-PHPB group: 247.8%±13.5%, n=9; oP<0.01; Figure 5A). To determine whether the dl-PHPB-induced LTP improvement was also mediated by other receptors, the effects of DNQX, an antagonist of AMPA and Kainate receptors, on dl-PHPB-induced LTP improvement were observed. The results indicated that the application of DNQX (100 μmol/L in CSF, icv) 40 min before HFS suppressed LTP (Figure 5B). dl-PHPB-induced LTP improvement was also attenuated when DNQX was injected 10 min before application of dl-PHPB (1 mmol/L in 5 μL, approximately 10 μmol/L in CSF, Figure 5B). However, the suppression by DNQX of dl-PHPB-induced LTP improvement was far weaker than that of the NMDA-specific antagonist D-APV. These results suggest that dl-PHPB enhances LTP mainly through NMDA receptor modulation.
Figure 5.
The effects of dl-PHPB on LTP were related to NMDA receptors. (A) Example data traces recorded at the times prior to HFS and 60 min after HFS. Injection of D-APV (icv, white arrow) 40 min prior to HFS significantly inhibited LTP induction. Application of 10 μmol/L dl-PHPB (white arrow) 10 min after D-APV injection could not reverse the inhibition of LTP levels even though 10 μmol/L dl-PHPB could facilitate LTP induction when pretreated alone. (B) Changes of PS amplitude following HFS in the presence and absence of 10 μmol/L dl-PHPB and DNQX. Icv injection of DNQX 40 min prior to HFS significantly inhibited LTP induction. Application of 10 μmol/L dl-PHPB 10 min after DNQX injection slightly reversed the inhibition of LTP levels. Statistical significance was evaluated at 60 min after induction of LTP. All data are presented as means±SEM. hP<0.05, iP<0.01 vs control group, oP<0.01 vs dl-PHPB, n=5–8.
dl-PHPB restored the phosphorylation of GluN2B in the hippocampus of APP/PS1 Tg mice
NMDARs are thought to be highly important in the mechanisms of learning and memory. In this study, to further explore the possible mechanisms of the behavioral and LTP improvements upon dl-PHPB treatment, the protein expression levels of GluN1 and GluN2B in APP/PS1 Tg mice were measured by Western blotting. The mice and drug administration protocols used for this experiment were the same as those in the behavioral and LTP experiments.
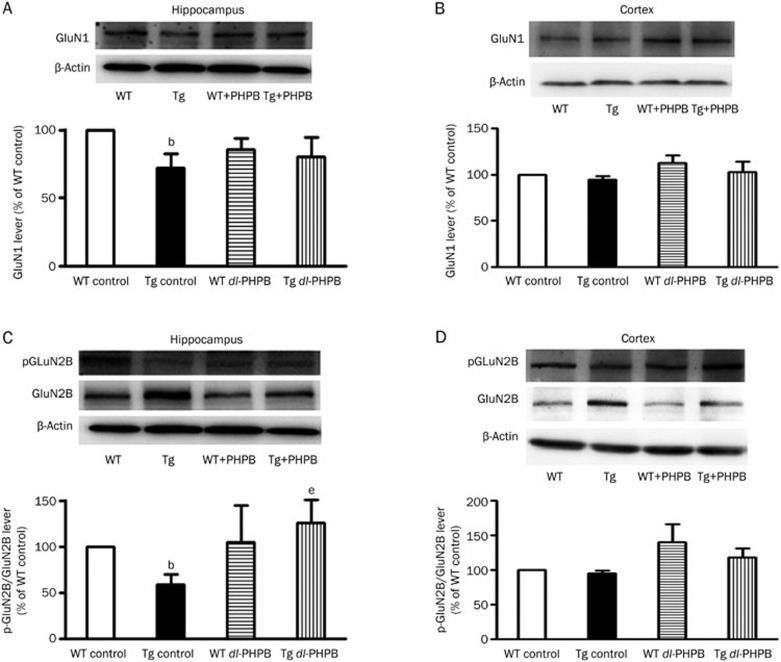
As shown in Figure 6, GluN1 and p-GluN2B levels in APP/PS1 Tg mice were reduced to 72.2% and 59.1%, respectively, of WT control mice in hippocampus (n=4, bP<0.05 vs WT control), but there was no significant change in the cortex. Furthermore, oral dl-PHPB treatment significantly increased the p-GluN2B level in the hippocampus (Tg dl-PHPB: 126.9%±24.8% of WT control group, n=4; eP<0.05 vs Tg control group, Figure 6C). The GluN1 level showed no change after dl-PHPB treatment in either the hippocampus or the cortex of the rat brain.
Figure 6.
dl-PHPB restored the phosphorylation level of NMDARs in the hippocampus of APP/PS1 Tg mice. (A–B) Quantitative analysis of GluN1 in cortex (A) and hippocampus (B) of WT or APP/PS1 mice treated with vehicle or dl-PHPB. Representative Western blots for GluN1 are shown in the upper panel. Quantified results were normalized to β-actin expression. (C–D) Quantitative analysis of p-GluN2B in the cortex (C) and hippocampus (D) of WT or APP/PS1 mice treated with vehicle or dl-PHPB. Representative Western blots of p-GluN2B are shown in the upper panel. Quantified results were normalized to GluN2B expression. All values are expressed as percentages compared to vehicle-treated WT control mice and represented as the group mean±SEM (n=4). bP<0.05 vs WT control group. eP<0.05 vs Tg control group.
Discussion
dl-PHPB, a novel drug candidate for treating cerebral ischemia, may be useful for the treatment of AD, based on our preliminary studies showing that dl-PHPB ameliorated behavioral deficits induced by cerebral hypoperfusion or Aβ icv infusion2. However, the effects of dl-PHPB on memory loss amelioration and the possible underlying mechanism remain to be investigated.
AD is a progressive neurological disorder. One of the most important aspects of its pathogenesis is the accumulation of Aβ peptide. Aβ, a peptide of 40 or 42 amino acids in length, is derived from the proteolytic processing of APP22. Many studies have used a mouse model overexpressing mutant human APP and PS1; this mouse exhibits age-dependent increases in the levels of Aβ peptides and develops robust amyloid plaque pathology like that in AD23. In the present study, to investigate the possibility of treating AD with dl-PHPB, the effects of dl-PHPB on LTP were investigated using 2 animal models: rats icv injected with Aβ1–42 and APP/PS1 Tg mice.
It is well known that passive avoidance learning is based on hippocampus-related memory24. Notably, the Tg mice aged 14 months spent significantly less time on the experimental platform and had higher error frequencies than their WT littermates, showing profound memory impairment. Long-term oral treatment with dl-PHPB increased the escape latency and decreased the error frequency in Tg mice. These results indicated that oral treatment with dl-PHPB ameliorated behavioral deficits in APP/PS1 Tg mice.
In the experiments shown in Figure 2, dl-PHPB was applied intracerebroventricularly, whereas it was administered orally in the experiments shown in Figure 3. The experimental conditions were different in these 2 sets of experiments, and both types of administration have advantages. As explained above, in this study, oral treatment with dl-PHPB rescued LTP and memory deficits in rats acutely injected with Aβ1–42 and in APP/PS1 mice. It is thought that the results of oral administration may be similar to the clinical application of dl-PHPB. By contrast, icv injection allowed us to study the direct effects of drug action on targets in the brain. In our study, the LTP levels after oral treatment and icv injection in WT rats are not consistent. This discrepancy may be caused by the difference in pharmacokinetics and in the drug distribution of dl-PHPB in vivo. Our study showed that dl-PHPB promoted LTP when it was icv injected, indicating that dl-PHPB acted directly on the hippocampus. However, the mechanisms underlying this effect remain to be studied.
Increasingly, investigations are demonstrating that a deficit in synapses rather than in neurons might be the primary problem in the early phases of AD25. LTP, a form of synaptic plasticity, is widely used as the primary experimental method for exploring the synaptic basis of learning and memory in the brain26, particularly in the hippocampus, which is thought to play a major role in memory formation and consolidation processes. There is substantial evidence indicating that Aβ peptides or amyloid-derived diffusible ligands elicit neuronal toxicity through selective actions on synaptic plasticity such as on LTP6,27,28. In the current study, we show that long-term oral treatment with dl-PHPB may rescue the LTP inhibition induced by Aβ1–42. The treatment dosage used was also therapeutically beneficial for improving behavior in our previous study. Our results indicated that dl-PHPB blocked Aβ1–42-induced synaptic dysfunction. However, the mechanisms of this process are not clear and possibly include changes in the NMDA receptors29, K+ channel activity30,31 and voltage-dependent calcium channel activity32,33,34. In addition, the function of the acetylcholine system is also important for LTP induction. Our previous studies have demonstrated that dl-PHPB had almost no effect on delayed rectifier and A-type K+ channel currents, nor did it affect the function of the acetylcholine system (data not shown). Therefore, the present study focused on the effects of dl-PHPB on Aβ and on NMDA-receptor-related activity of LTP.
Increasingly, the impairment of synaptic plasticity with age has been observed in AD Tg mice compared to their WT littermates. Previous studies have indicated that synaptic plasticity is strongly impaired in APP/PS1 Tg mice as well as in other APP Tg mice35. Consistent with these results, we found that LTP was attenuated in 14-month-old APP/PS1 Tg mice. Oral treatment with dl-PHPB led to an increase in LTP in APP/PS1 Tg mice. In combination with the beneficial effects of dl-PHPB on synaptic dysfunction and behavioral deficits, these results indicated that dl-PHPB has a therapeutic effect in animal models with APP and PS1 mutations.
Increasingly, studies are indicating that modifications of excitatory amino-acid receptors, such as NMDA, AMPA and kainate receptors, are important mechanisms in the process of learning and memory. In particular, the NMDA receptors are important for activating LTP. Previous studies have shown that LTP induction by HFS in the hippocampal DG requires the activation of postsynaptic NMDA receptors36,37. Consistent with these reports, the LTP we induced in this study was strongly inhibited by the NMDA antagonist D-APV. It was also inhibited by DNQX, an AMPA/kainate receptor antagonist, which is consistent with a previous report38. Although the IC50 of DNQX for inhibiting AMPA/kainate receptors is in the 1–10 μmol/L range, the concentration reported for inhibiting LTP might be in the 10–50 μmol/L range39. In addition, previous studies have shown that DNQX (100–200 μmol/L) is insufficient to block the baseline of glutamate-evoked postsynaptic potentials40; therefore, in our study, 100 μmol/L DNQX was chosen to fully inhibit LTP with no obvious effects on basal synaptic transmission. We also found that the protein expression levels of the NMDA receptors GluN1 and p-GluN2B were decreased in the hippocampus of APP/PS1 Tg mice. Long-term oral treatment with dl-PHPB significantly restored the p-GluN2B level in APP/PS1 Tg mice, but it did not affect the GluN1 level. Our results indicated that NMDA receptors, especially GluN2B, played an important role in the beneficial effects of dl-PHPB on LTP deficits. Interestingly, some previous studies41,42 have shown that overexpression of GluN2B in the forebrains of Tg mice leads to enhanced activation of NMDA receptors, facilitating synaptic potentiation. These mice exhibited superior ability in learning and memory in various behavioral tasks. Some groups have also shown that the phosphorylation level of GluN2B is lower in AD Tg mice than in their WT littermates43. In summary, our results suggest that the GluN2B receptor might be the target of dl-PHPB that drives the observed effects on LTP and memory loss amelioration.
Previous experiments have suggested that LTP involves the NR2A receptors44,45 and that long-term depression is dependent on the NR2B receptors46. Some groups have also shown that the phosphorylation level of NR2B is lower in AD Tg mice than in WT littermates. For example, papers from Tang YP's group have shown that Tg mice overexpressing NR2B exhibit superior ability in learning and memory in various behavioral tasks41. These studies demonstrate that NR2B is the predominant tyrosine-phosphorylated protein in the PSD and that Tyr 1472 is the major phosphorylation site. The phosphorylation of NR2B at Tyr 1472 is thought to stabilize NMDA receptors on the cell surface and make them functionally active. Therefore, we tested NR2B expression and phosphorylation as an important factor reflecting the process of learning and memory. NR2B expression was relatively high in the Tg control group. The reasons for this phenomenon are unclear. The most likely explanation is that in adult APP/PS1 mice, synaptic excitation is abnormal, and receptor activities are imbalanced, which might in turn lead to neuronal impairment and deficits in LTP. However, the exact mechanisms remain to be further investigated.
NR1 is an essential NMDA receptor subunit and is expressed throughout the brain. NR1 subunits bind the co-agonist glycine, which is critical for activation of the NMDA receptors. In the current study, we assessed NR1 expression as a reflection of the total NMDA level, including the NR2A- and NR2B-NMDA receptors. Several phosphorylation sites on NR1 are required for increasing NMDA receptor surface expression. We will test the phosphorylation level of GluN1 in further studies.
In our previous studies, dl-PHPB was shown to ameliorate ischemic stroke by increasing cerebral blood flow, reducing infarct volume and improving neurobehavioral deficits in a rat model of tMCAO1. Recently, dl-PHPB was found to play a role in neuronal apoptosis through mediating the expression of genes involved in apoptosis47. All of these findings indicate that there may be multiple mechanisms involved in the effects of dl-PHPB, and these effects of dl-PHPB might support neuronal protection and the treatment of AD in a nonspecific manner, in addition to the effects on NMDA receptors.
In summary, we demonstrated for the first time that dl-PHPB ameliorated the learning and memory deficits and restored the impairment of LTP in AD Tg mice in vivo. Furthermore, the beneficial effects of dl-PHPB might occur through the various receptor subunits of NMDA, especially GluN2B, which is closely linked to learning and memory. Our results indicated that dl-PHPB might promote synaptic plasticity and improve cognition under pathological conditions. Therefore, dl-PHPB might be a promising drug candidate for the treatment of AD.
Author contribution
Xiao-liang WANG designed the research and revised the paper; Ping-ping LI performed the research; Ping-ping LI and Xiao-liang WANG wrote the paper; Wei-ping WANG, Zhi-hui LIU, Shao-feng XU, Wen-wen LU and Ling WANG helped to analyze data.
Acknowledgments
This work was supported by the National Major Special Project on New Drug Innovation of China (No 2012ZX09301002-004) and the National Natural Science Foundation of China (No 81373387).
References
- Zhang Y, Wang L, Li J, Wang XL. 2-(1-Hydroxypentyl)-benzoate increases cerebral blood flow and reduces infarct volume in rats model of transient focal cerebral ischemia. J Pharmacol Exp Ther. 2006;317:973–9. doi: 10.1124/jpet.105.098517. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xu S, Peng Y, Ji X, Cao D, Li J. Potassium 2-(1-hydroxypentyl)-benzoate improves learning and memory deficits in chronic cerebral hypoperfused rats. Neurosci Lett. 2013;541:155–60. doi: 10.1016/j.neulet.2013.01.053. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–7. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- Freir DB, Holscher C, Herron CE. Blockade of long-term potentiation by beta-amyloid peptides in the CA1 region of the rat hippocampus in vivo. J Neurophysiol. 2001;85:708–13. doi: 10.1152/jn.2001.85.2.708. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM. Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci. 2007;27:7648–53. doi: 10.1523/JNEUROSCI.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QS, Kagan BL, Hirakura Y, Xie CW. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J Neurosci Res. 2000;60:65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Saleshando G, O'Connor JJ. SB203580, the p38 mitogen-activated protein kinase inhibitor blocks the inhibitory effect of beta-amyloid on long-term potentiation in the rat hippocampus. Neurosci Lett. 2000;288:119–22. doi: 10.1016/s0304-3940(00)01210-6. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–40. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–6. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Reverse D, Caluwaerts N, Ris L, Kuiperi C, Van den Haute C. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J Neurosci. 2002;22:3445–53. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–92. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Frankiewicz T, Parsons CG. Memantine restores long term potentiation impaired by tonic N-methyl-D-aspartate (NMDA) receptor activation following reduction of Mg2+ in hippocampal slices. Neuropharmacology. 1999;38:1253–9. doi: 10.1016/s0028-3908(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Wang Q, Reed MN, Irving EA, Upton N, Hofmeister J. Protection against Abeta-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol Aging. 2011;32:614–23. doi: 10.1016/j.neurobiolaging.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Shioda N, Han F, Yeh JZ, Narahashi T, Fukunaga K. Galantamine enhancement of long-term potentiation is mediated by calcium/calmodulin-dependent protein kinase II and protein kinase C activation. Hippocampus. 2009;19:844–54. doi: 10.1002/hipo.20572. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17:157–65. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek JL, Neff NH. Is cerebrospinal fluid the major avenue for the removal of 5-hydroxyindoleacetic acid from the brain. Neuropharmacology. 1973;12:497–9. doi: 10.1016/0028-3908(73)90067-1. [DOI] [PubMed] [Google Scholar]
- Lai YL, Smith PM, Lamm WJ, Hildebrandt J. Sampling and analysis of cerebrospinal fluid for chronic studies in awake rats. J Appl Physiol. 1983;54:1754–7. doi: 10.1152/jappl.1983.54.6.1754. [DOI] [PubMed] [Google Scholar]
- Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: a role for interleukin-1beta. J Biol Chem. 2003;278:27971–80. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–24. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93:487–94. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78:151–64. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, El Agnaf O. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J Neurosci. 2005;25:2455–62. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texido L, Martin-Satue M, Alberdi E, Solsona C, Matute C. Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49:184–90. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Jalonen TO, Charniga CJ, Wielt DB. beta-Amyloid peptide-induced morphological changes coincide with increased K+ and Cl– channel activity in rat cortical astrocytes. Brain Res. 1997;746:85–97. doi: 10.1016/s0006-8993(96)01189-4. [DOI] [PubMed] [Google Scholar]
- Plant LD, Webster NJ, Boyle JP, Ramsden M, Freir DB, Peers C. Amyloid beta peptide as a physiological modulator of neuronal 'A'-type K+ current. Neurobiol Aging. 2006;27:1673–83. doi: 10.1016/j.neurobiolaging.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Abe K, Kimura H. Amyloid beta toxicity consists of a Ca2+-independent early phase and a Ca2+-dependent late phase. J Neurochem. 1996;67:2074–8. [PubMed] [Google Scholar]
- Freir DB, Herron CE. Inhibition of L-type voltage dependent calcium channels causes impairment of long-term potentiation in the hippocampal CA1 region in vivo. Brain Res. 2003;967:27–36. doi: 10.1016/s0006-8993(02)04190-2. [DOI] [PubMed] [Google Scholar]
- Kim J, Hoffman DA. Potassium channels: newly found players in synaptic plasticity. Neuroscientist. 2008;14:276–86. doi: 10.1177/1073858408315041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Niidome T, Hongo H, Akaike A, Kihara T, Sugimoto H. Impaired muscarinic regulation of excitatory synaptic transmission in the APPswe/PS1dE9 mouse model of Alzheimer's disease. Eur J Pharmacol. 2008;583:84–91. doi: 10.1016/j.ejphar.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BL, Kennelly JJ, Colley PA, Overstreet LS, Slater NT, Pasternak JF. AP5 blocks LTP in developing rat dentate gyrus and unmasks LTD. Exp Neurol. 1995;131:83–92. doi: 10.1016/0014-4886(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Aroniadou VA, Teyler TJ. The role of NMDA receptors in long-term potentiation (LTP) and depression (LTD) in rat visual cortex. Brain Res. 1991;562:136–43. doi: 10.1016/0006-8993(91)91197-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen G, Gao W, Ebner T. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neuroscience. 2009;162:713–22. doi: 10.1016/j.neuroscience.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood RA, Li Q, Glanzman DL. Serotonin facilitates AMPA-type responses in isolated siphon motor neurons of Aplysia in culture. J Physiol. 2001;534:501–10. doi: 10.1111/j.1469-7793.2001.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–9. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–95. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike Y, Kimura T, Yamashita S, Furudate H, Mizoroki T, Murayama M. GABA(A) receptor-mediated acceleration of aging-associated memory decline in APP/PS1 mice and its pharmacological treatment by picrotoxin. PLoS One. 2008;3:e3029. doi: 10.1371/journal.pone.0003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–5. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–89. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–44. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Hu Y, Peng Y, Long Y, Xu S, Feng N, Wang L. Potassium 2-(1-hydroxypentyl)-benzoate attenuated hydrogen peroxide-induced apoptosis in neuroblastoma SK-N-SH cells. Eur J Pharmacol. 2012;680:49–54. doi: 10.1016/j.ejphar.2012.01.031. [DOI] [PubMed] [Google Scholar]