Abstract
Heat shock proteins (HSP) are rapidly induced after stresses such as heat shock and accumulate at high concentrations in cells. HSP induction involves a family of heat shock transcription factors (HSF) that bind the heat shock elements of the HSP genes and mediate transcription in trans. We discuss methods for the study of HSP binding to HSP promoters and the consequent increases in HSP gene expression in vitro and in vivo.
Keywords: heat, shock, factor, binding, transcription
(1) Introduction
Heat shock factor (HSF) was first discovered in yeast as a sequence specific transcription factor that binds to the promoters of heat shock protein (HSP) genes1. HSF was shown to bind as a trimer to three inverted repeats of the sequence nGAAn at high affinity, an activity that was later shown in Drosophila HSF and human HSF12–5. In more complex organisms, there at least 4 members of the family in avian and mammalian species and multiple members in higher plants6–11. The current consensus in mammalian cells is that HSF1 is the most potent regulator of the heat shock response with the remaining factors playing supplementary roles in stress and perhaps more significant roles in development6, 12, 13. The mechanisms by which HSF1 is triggered by stress are not entirely clear. HSF1 is thought to be repressed by its products- the heat shock proteins through a feedback inhibition mechanism14, 15. Activation is thus envisaged as a reversal of such inhibition as denatured proteins sequester HSPs during heat shock and HSF1 liberated to bind to HSE elements in HSP genes. However, alternative hypotheses have been proposed involving stress-mediated HSF1 phosphorylation and binding to large non-coding RNA16, 17. HSF1 and HSF2 are predicted to encode at least two splicing variants, with HSF2A and HSF2b showing differential expression during eryrthroid differentiation6. HSF2A appears to be active in transcriptional regulation while HSF2B appears to be inactive6.
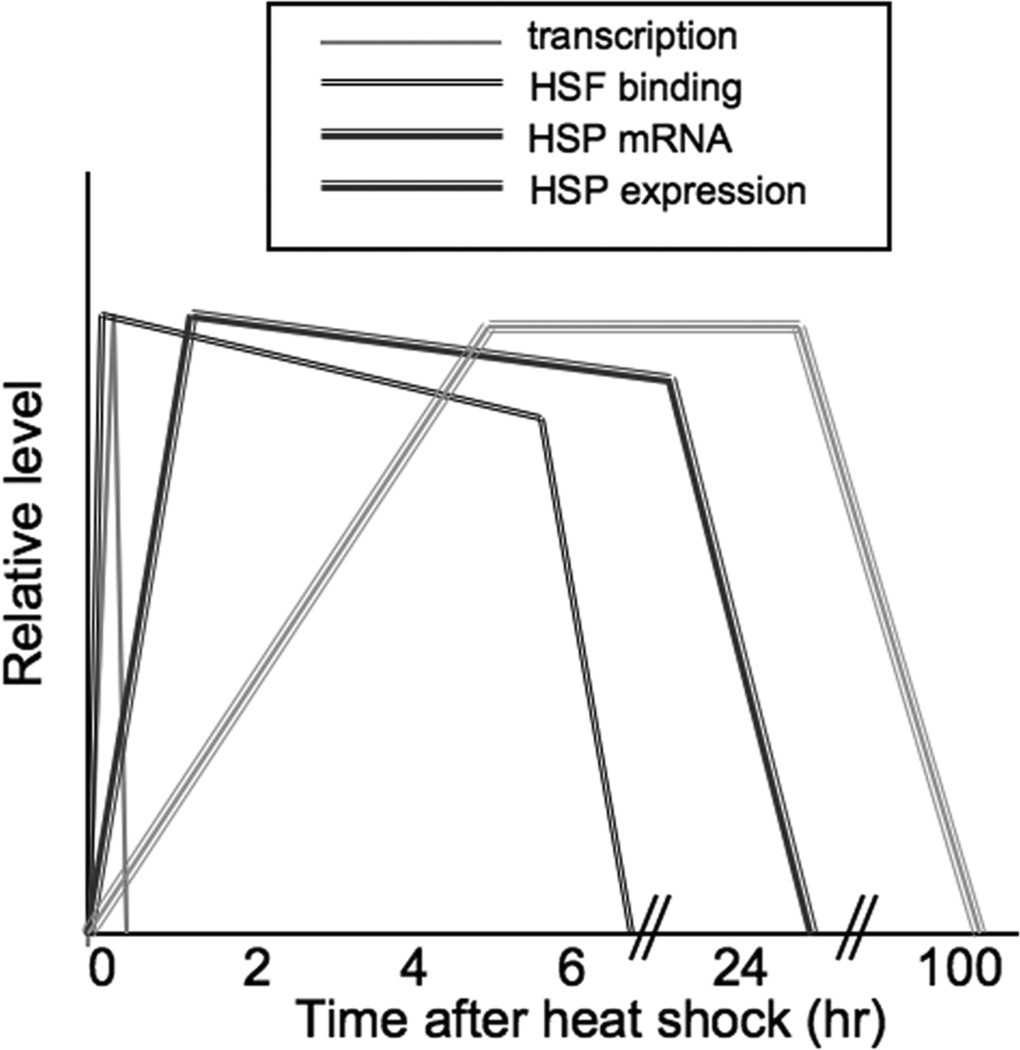
Heat shock causes a rapid increase in HSF1 binding to HSP promoters and an acute elevation in the transcription of HSP genes (relative rates are indicated in Figure 1). Transcription decays rapidly after initiation, while HSF continues to bind to HSE for several hours after transcription is ceased18, 19. HSP mRNA is then observed within 1 hr of activation and is maintained at these high levels for up to 24 hr due to enhanced stabilization after stress20. After acute stress, HSP protein expression is delayed due to initial translational inhibition but Hsp70, Hsp90 and Hsp110 are observed by 2–6 hr after a 43°C heat shock and can persist in cells for up to 100 hr21 (Figure 1). In this report, we are concerned with the early phase of HSP gene expression, involving HSF1 binding to HSP genes and activation of transcription.
Figure 1.
Relative kinetics of hsp gene transcription, HSF binding, HSP mRNA expression and heat shock protein expression after heat shock.
(2) Materials
Purification of HSF2 and Electrophoretic Mobility Shift Assay (EMSA)
Primers
For human HSF2:
Forward primer was 5' -3’ GC[GAATCC]ATGAAGCAGAGTTCGA,
Reverse primer 5'-3' AAA[GTCGAC]TTCCTGGGGATTTAGCTA.
For murine HSF2:
Forward primer 5'-3' GG[GAATCC]ATGAAGCAGAGTTCGAACG,
Reverse primer 5'-3' AGT[GTCGAC]TTGGGAGTTTAACTATCT.
EMSA oligonucleotides
Hsp70 HSE top strand: 5'-CACCTCGGCTGGAATATTCCCGACCTGGCAGCCGA-3'.
Mutant oligonucleotides
5'-CACCTCGGCTGCAATAATCCCGACCTGGCAGCCGA-3'.
Cells: BL21 (DE3) E. coli, Human HeLa
Columns and filters
20 ml glutathione-sepharose (Pierce Chemicals)
Mono-Q HR 5/5 (Pharmacia)
Centricon 10 ultrafilter
Buffers
E. coli Lysis buffer: 7M guanidine-HCl in 0.1 M potassium phosphate buffer pH 7.4 containing 50 mM DTT and 0.05% NP-40
Dialysis buffer : 50 mM potassium phosphate buffer containing 0.1 M KCl and 2 mM DTT
BSA Solution: 0.1 mg/ml bovine serum albumin
EMSA lysis buffer : 10 mM (HEPES), 10 mM NaCl, 0.1 mM EDTA, 1.0 mM dithiothreitol (DTT), 1.0 mM phenylmethylsulfonyl fluoride (PMSF), 2.0 mg/ml aprotinin, leupeptin, 20 mM NaF and 2.0 mM Na3VO4 (pH 7.9)
HSF extraction buffer: aprotinin, leupeptin, 20 mM NaF and 2.0 mM Na3VO4 (pH 7.9) on ice. Cells are then lysed by addition of Nonidet P-40 to 0.6% and lysates clarified by spinning at 12,000 g. Nuclear pellets
EMSA incubation buffer: (12 ul) contained 2.0 ul nuclear extract or recombinant protein, 2.0 mg /ml bovine serum albumin, 2.0 mg /ml poly dI-dC, 0.5–1.0 ng 32-P- labeled, double stranded oligonucleotide probe, 12 mM Hepes, 12% glycerol, 0.12 mM EDTA, 0.9 mM MgCl2, 0.6 mM DTT, 0.6 mM PMSF and 2.0 mg/ml aprotinin and leupeptin (pH 7.9).
ChIP Assay
ChIP Dilution Buffer (1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris–HCl, pH 8.0, and 167 mM NaCl).
Protein A Agarose Slurry (Sigma Chemicals, St Lous, Mo)
ChIP Washing buffers:
washing buffer 1 (20 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, and 0.1% SDS),
washing buffer 2 (20 mM Tris–HCl, pH 8.0, 500 mM NaCl, 1% Triton X-100, 2 mM EDTA, and 0.1% SDS),
washing buffer 3 (10 mM Tris–HCl, pH 8.0, 250 mM LiCl, 1% NP-40, 1% sodium deoxycholate, and 1 mM EDTA), TE (10 mM Tris–HCl, pH 7.5, 1 mM EDTA).
ChIP elution buffer (1% SDS, 0.1 M NaHCO3).
5M NaCl
ChIP Uncrosslinking Buffer: 0.5 M EDTA, 10 microliter of 1 M Tris–HCl, pH 6.5, and 2 microliter Proteinase K
ChIP hsp70.1 primers: exon region forward primers: {hsp70.1 exn Forward:
5’ ggacatcagccagaacaagc 3’ hsp70.1 exn Reverse: 5’ aagtcgatgccctca aac ag 3’
hsp70.1 HSE Reverse:5’ cggcttttataagtcgtcgt 3’ hsp70.1 HSE Forward: 5’ aggcgaaacccctggaata 3’}
Run-on transcription
Run-on Lysis Buffer: 10 mM Tris.HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2 and 0.5% nonidet-P40.
Run-On Storage Buffer: 50 mM Tris (pH 8.30), 40 % glycerol, 5 mM MgCl2 and 40 units of RNAsin (Roche Molecular Biochemicals).
Run-on Reaction Buffer: 10 mM Tris-HCL (pH 8.0), 5mM MgCl2, 0.3 M KCl, 5 mM DTT, 1mM ATP, 1mM CTP, 1mM GTP, and 50 uCi [α-32P] UTP (3000 Ci/mmol).
Hybridization Solution: UltraHyb solution (Ambion).
Hybridization Washing Buffers: (1) 2 × SSC, 0.1% SDS, (2) high stringency solution (1 × SSC, 0.1% SDS), (3) 2 × SSC, 0.1% SDS with 10 ug RNase A.
(3) Purification of heat shock factors and in vitro EMSA
In order to study the properties of HSF family members in vitro we have prepared purified, recombinant HSF1 and HSF2.
(i) Complementary DNA cloning of human and mouse HSF2A and HSF2B
RNA was isolated from NIH-3T3 (mouse) or HeLa (human cells) and messenger RNA prepared by poly-T affinity chromatography (PolyAtract system, Promega, Madison, WI). cDNA was then prepared from the mRNA using the AMV reverse transcriptase system (Promega) and HSF2 cDNAs amplified using Taq polymerase and the polymerase chain reaction using the following primer sets mentioned above6:
Forward primers contain Eco-R1 restriction site consensus sequences [marked in boxes] and the reverse primers contain Sal-1 sequences for subsequent cloning of amplified DNAs into the PGEX5 prokaryotic expression vector (Pharmacia). After transformation and growth of competent bacteria, colonies are screened for either total HSF2 using oligonucleotides (17642013;1785; CAGGAGCAAGTTCACATAAATA and 1786–1807; GGCATATCACTATCCAGAGGTG) predicted to detect all forms of HSF2 or for the larger form (HSF2A) using oligonucleotides predicted to hybridize specifically with this species (1420–1440; TTGTATTATTGATGTAATCT and (1392–1412; CATCTGCACAGAACTAG TGA). Oligonucleotides are then end-labeled with 32-P ATP and T4 polynucleotide kinase. Plasmids detected using these probes are isolated, screened for the presence of inserts and for the production of HSF2-glutathione transferase fusion proteins from representative cDNAs in bacteria exposed to the inducing agent IPTG (Pharmacia). After induction, bacterial lysates are prepared and screened by immunoblot with anti-GST antibodies (St Cruz Antibodies) and anti-HSF2 antibody Ab-3158 prepared in the Calderwood lab. Representative clones from human HSF2A and HSF2B and murine HSF2A and HSF2B are then further analyzed by dideoxynucleotide sequencing.
(ii) Purification of HSF2 proteins
HSF2 variants are cloned into the pGEX-5 expression vector, between the Eco RI and Sal I sites and the resulting plasmids are used to transform BL21 (DE3) E. coli bacteria. HSF2 was thus expressed as a fusion protein with glutathione-S transferase. All purification steps are carried out at 4°C. Briefly, IPTG-induced bacteria are pelleted and dissolved in E. Coli Lysis Buffer and dialyzed against E. coli Lysis Buffer. The samples are centrifuged at 2500 g for 5 min and the supernatant loaded on a 20 ml volume glutathione-Sepharose column at a flow rate of 0.5 ml/min, washed extensively with Dialysis Buffer and eluted with this buffer A containing 10 mM reduced glutathione. The eluate was loaded onto a Mono-Q HR 5/5 ion-exchange chromatography column at a flow rate of 0.8 ml/min and eluted with a 24 ml linear gradient from 0.1 M to 1.0 M KCl final concentration of KCl in buffer A. Absorbance was monitored at 280 nm and the fractions corresponding to HSF2 assayed for binding to HSE, pooled and concentrated with a Centricon 10 ultrafilter in the presence of 0.1 mg/ml bovine serum albumin. Relative concentrations of active HSF2 are estimated by quantitative EMSA (Fig. 1).
GST-HSF1 is purified using a similar protocol22. Alternatively, we have described a detailed method for purifying recombinant HSF1 after expression in E. coli from the pET7.1 vector23. Recombinant HSF1 without a GST tag is prepared by ammonium sulfate precipitation, heparin-agarose affinity and ion exchange chromatography in pure form as assessed by SDS-PAGE and reverse-phase HPLC23.
Activity of purified GST-HSF2A, GST-HSF2B or GST-HSF1 is estimated by EMSA. Proteins are incubated with 32P-labeled HSE at a range of dilutions and then subjected to EMSA analysis as described below. GST-HSF2A and GST-HSF2B are serially diluted 1/2200, 1/660, 1/220, 1 / 66, 1/ 22 and 3/ 22 prior to EMSA.
(4) Nuclear extraction from tissue culture cells and EMSA
EMSA is carried out using purified recombinant HSF or after the extraction of intracellular HSF complexes from either whole cell or nuclear extracts from heat shocked cells and incubation of complexes with double stranded oligonucleotides encoding heat shock elements in HSP genes (HSE). To prepare HSF from cells growing in vitro, nuclear extracts are prepared according to Schreiber24. In our standard assay, cells are incubated for 15 min in 200–800 ul of EMSA Lysis Buffer on ice. Cells are then lysed by addition of Nonidet P-40 to 0.6% and lysates clarified by spinning at 12,000 g. Nuclear pellets are then resuspended in 25 ul ice-cold EMSA extraction buffer. Extracts containing HSF are then aliquoted and stored at −80°C.
For incubation with oligonucleotide probe, each binding mixture (12 ul) contained 2.0 ul nuclear extract or recombinant protein, 2.0 mg /ml bovine serum albumin in EMSA Incubation Buffer. Samples are incubated at room temperature for 15 min, then fractionated by electrophoresis on 4.0% polyacrylamide, 1 × TBE gels. Oligonucleotide hHSE was synthesized, annealed and labeled by end filling with 32P-dCTP at 6,000Ci/mmol (DuPont, NEN) to an activity of 100, 000 cpm/ng. hHSE contains the heat shock element (HSE) from the top strand of the human HSP70.1 promoter25. (We have found that double stranded oligonucleotide end filling with Klenow fragment or end labeling of single stranded oligonucleotides with T4 kinase to be equally effective) The oligonucleotide shown in Materials (575 ng) and the complementary oligonucleotide (2300ng) (resulting in 1150 ng double stranded oligo) are made up to 25 ng/ul in 46 ul of TE buffer, annealed by incubation at 100°C for 5 min and cooled overnight. As a control, we carried out the EMSA procedure with a similar oligonucleotide containing mutations in the HSE elements, indicated in bold in the sequence shown in Materials.
For experiments on cell extracts, a number of controls are used standardly. To determine specific binding of HSF1 to labeled HSE, we examine ability to inhibit HSE-HSF association with a ten-fold excess of unlabeled wild type oligonucleotide included in the incubation. In addition, specific binding is further indicated by failure of a tenfold excess of the mutant HSE shown above to inhibit binding. The protein (HSF) in the HSF-HSE complex can be identified by the addition of specific anti-HSF1 or anti-HSF2 antibodies to the reaction mix. We used a 1:100 dilution of anti-HSF1 antibody 68-3 prepared in our laboratory to positively identify HSF1 in the complexes26, 27. As a control we use pre-immune antiserum obtained from the same rabbit. For commercially obtained antibodies, a serial dilution approach was used to determine optimal antibody concentrations.
HSF1 from heat shocked cells is contained in large complexes of at least 600 kD and is fractionated on 4% tris-borate non-denaturing gels28, 29. For most purposes we found that the mini-gel (Bio-Rad, CA) format was quite adequate for separation, although for supershift assay and higher resolution a larger format was used30.
(5) Measuring HSF1 binding to HSP promoters in vivo by the chromatin immunoprecipitation (ChIP) assay
ChIP (Chromatin Immunoprecipitation) offers an attractive solution to transcription analysis by combining the specificity of immunoprecipitation and the sensitivity of PCR. The method allows monitoring of the interactions between DNA and transcription factors and/or components of chromatin remodeling complexes but it is technically challenging due to the low abundance and/or only temporary interactions of these proteins31.
Our ChIP assays were performed as described in previous publication Run-on transcription with some modifications32, 33. For each ChIP assay, heat shocked or control 106 Hela cells were formaldehyde (1% final) fixed for 10 min (Note that the optimal cross-linking concentration and duration need be determined empirically with different tissue or cell type, state and even intensity. Other protocols could rely either on other chemical reagents or UV mediated physical cross-linking to preserve native nuclear structures for subsequent biochemical and molecular analysis). After neutralization with 0.125 M glycine, cell pellets were lyzed in 200 micoliter ChIP Lysis Buffer with protease inhibitors. Samples were sonicated and then diluted into1800 ul ChIP Dilution Buffer. ChIP was carried out with precipitating antibodies either anti-HSF1, or anti-IgG as control from Stressgen (Vancouver, CA, USA) added to pre-cleared chromatin with protein A agarose slurry at 4C overnight. (Note that the optimal concentration of the primary antibody for ChIP must be determined empirically even with different lot of “same name “antibody”). Then four sequential washings were performed by adding the washing buffer 1, washing buffer 2 and washing buffer finally elution in 500 ul elution buffer (1% SDS, 0.1 M NaHCO3). To reverse the cross-linking fixation, 20 ul of 5 M NaCl were added and the mixture incubated at 65 °C for 4 h. Afterwards, we added 10 ul of Uncrosslinking Buffer and incubated at 45 °C for 1 h, then purified the DNAs. The immunoprecipitated DNA is now analyzed by PCR amplification using appropriate primer pairs for the HSE consensus region and the control region in the hsp70.1 exon. A total of 27–30 cycles of PCR were carried out with 2 microliter of eluted DNA and primers to amplify amplify the exon region (from +752 to +878) and the HSE containing region from −334 to −233 (Figure 2). Amplified PCR products were analyzed by agarose gel/ethidium bromide. The input was used as positive control and anti-IgG mock ChIP as negative control. Alternatively, the PCR products were quantified by using ABI 7300 real time PCR system and 2−ΔΔCt method for the fold increase in ChIP PCR products compared with the control (anti-IgG) was plotted for the respective region of hsp70.1. (Note for some transcription factors, if a specific antibody unavailable, a tagged construct could be made and transfect the cells and obtain its over - expression in cells. Then ChIP assay could be performed by using commercially available antibody, which is against such tag
(6) Measuring the contribution of HSF1-HSE binding to transcription
(i) Luciferase reporter assays for HSF activity
To construct an intracellular reporter of HSF activity (pGL.hsp70B), we used 1.44 kB of the human HSP70B gene inserted into the pGL.Basic plasmid (Promega). The HSP70B gene is almost entirely silent at physiological temperatures but powerfully activated by heat shock34. pGL.hsp70B was constructed by digestion with BglII and HindIII and cloning into pGL.Basic. We have also used the human Hsp27 gene by a similar process, inserting the 730 kB BglII and HindIII digest of a HSP27 promoter fragment into pGL.Basic. For overexpression of HSF1, human HSF1 cDNA35 is inserted into the pcDNA3.1 (−) expression vector (Invitrogen) at the XhoI and EcoRI sites36. Human HSF2A was inserted into the pcDNA3.1(+) vector at the XhoI and EcoRI sites to produce pHSF2A37.
To assay of HSF1 transcriptional activity in HeLa cells, the cells are maintained in HAM’s F-12 (Mediatech) with 10% heat inactivated fetal bovine serum (FBS). HeLa cells (2.5 × 105 cells/well) in 6-well plates are transfected with the pGL.hsp70B or pGL.hsp27 plasmids38. pCMV-β-lacZ plasmid is co-transfected as an internal control for transfection efficiency. pHM6 empty vector is used as a blank plasmid to balance the amount of DNA transfected in transient transfection. Luciferase and β-galactosidase activity assays are performed after 24hr of transfection according to the Promega protocol. Luciferase activity is normalized to β-galactosidase activity. Results are expressed as relative luciferase (relative light units) activity of the appropriate control.
(ii) Nuclear run on assay of rate of Hsp70 gene transcription
To determine HSP70 gene transcriptional rate, cells are treated according to experiment then quenched in ice-cold phosphate buffered saline, pH 7.4 (PBS) on ice. Cells are next washed in PBS and lysed in Run-On Lysis Buffer Nuclei are collected by centrifuge (500 × g, 5 min) at 4°C and resuspended in storage buffer.
To assay rate of transcription, 100 ul of nuclei and 100 ul of Run-on Reaction Buffer are added and samples incubated 30 min at 30°C with shaking. RNA is then extracted from the reaction mix using Trizol (invitrogen) according to the manufacturer’s protocol.
The hsp70 DNA containing cDNA probe39 or control β-actin probe are linearized and purified by phenol/chloroform extraction and ethanol precipitation. Probes are then denatured and slot-blotted onto Hybond N+ membrane. (Membranes are first pre-hybridized with UltraHyb solution (Ambion) for 2 hr at 42°C, before equivalent counts of newly transcribed RNA (106 cpm) are added to the solution). Hybridization is then carried out for 24 hr at 42°C. Membranes are then washed twice for 20 min at 42°C in low stringency solution (2 × SSC, 0.1% SDS), twice for 20 min in high stringency solution (1 × SSC, 0.1% SDS) and once for 30 min at 37°C in low stringency solution containing 10 ug RNase A. Membranes are then rinsed in low stringency solution and analyzed by incubation with X-ray film. We have successfully used this protocol for assay of transcription of the mouse hsp70.1, c-fms, IL-1β and TNF-α genes40.
Acknowledgments
This work was supported by NIH research grants RO-1CA047407, R0-1CA119045 and RO-1CA094397
Footnotes
The EMSA technique has the advantages that it is rapid, sensitive and straightforward to carry out. For assessing the significance of the transcription factor- response element interaction, it is however lacking in that response elements in chromatin are wound along nucleaomes and may not be available for binding. The EMSA reaction is carried out using naked DNA. In addition as ChIP on CHIP and ChIP-seq studies begin to accumulate it is evident that response elements for particular factors are more flexible than suspected from early studies.
Some of these problems can be avoided using the ChIP assay that measures HSF binding to chromosomal DNA in vivo. This technique is highly dependent on availability of high affinity and specific antibodies for transcription factors. This can be overcome by overexpression of the factor with a seuqence tag and carrying out ChIP with anti-TAG antibody. However this can introduce potential artifacts involved with protein overexpression.
To assess the results of HSF-DNA binding we have used two approaches. We have used transfection of reporter constructs containing either HSP promoters or HSE coupled to reporter genes CAT or luciferase. The assays have the advantages of being rapid and permitting accumulation of plentiful data. The promoter portion of the construct can be tailored to assess the activity of a single transcription factor such as HSF1. The assay is however indirect and does not measure the transcription of the native, chromosomally-embedded gene. There are other potential complications, as reporters require to be translated and yield enzymatically active proteins.
Transcriptional rate of HSP genes such can be assess directly by run-on assay. This assay indicates that joint activities of all response elements in the HSP gene promoters. Although genes such as HSP70B respond only to HSF1 or heat shock, others such as HSP70A have more complex promoters.
References
- 1.Sorger PK, Pelham HRB. Purification and characterization of a heat-shock element binding protein from yeast. EMBO Journal. 1987;6:3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorger PK, Nelson HCM. Trimerization of a yeast transcriptional activator via a coiled-coil motif. Cell. 1989;59:807–813. doi: 10.1016/0092-8674(89)90604-1. [DOI] [PubMed] [Google Scholar]
- 3.Sorger PK, Pelham HRB. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- 4.Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993;259:230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- 5.Wu C. Heat shock transcription factors: structure and regulation. Ann Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 6.He H, Soncin F, Grammatikakis N, et al. Elevated expression of heat shock factor (HSF) 2A stimulates HSF1-induced transcription during stress. J Biol Chem. 2003;278(37):35465–35475. doi: 10.1074/jbc.M304663200. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto M, Hayashida N, Katoh T, et al. A Novel Mouse HSF3 Has the Potential to Activate Non-classical Heat Shock Genes during Heat Shock. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-07-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanabe M, Kawazoe Y, Takeda S, Morimoto RI, Nagata K, Nakai A. Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. Embo J. 1998;17(6):1750–1758. doi: 10.1093/emboj/17.6.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanabe M, Sasai N, Nagata K, et al. The mammalian HSF4 gene generates both an activator and a repressor of heat shock genes by alternative splicing. J Biol Chem. 1999;274(39):27845–27856. doi: 10.1074/jbc.274.39.27845. [DOI] [PubMed] [Google Scholar]
- 10.Kumar M, Busch W, Birke H, Kemmerling B, Nurnberger T, Schoffl F. Heat Shock Factors HsfB1 and HsfB2b Are Involved in the Regulation of Pdf1.2 Expression and Pathogen Resistance in Arabidopsis. Mol Plant. 2009;2(1):152–165. doi: 10.1093/mp/ssn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharf KD, Rose S, Zott W, Schoffl F, Nover L. Three tomato genes code for heat stress transcription factors with a region of remarkable homology to the DNA-binding domain of the yeast HSF. Embo J. 1990;9(13):4495–4501. doi: 10.1002/j.1460-2075.1990.tb07900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 13.Morange M. HSFs in development. Handb Exp Pharmacol. 2006;(172):153–169. doi: 10.1007/3-540-29717-0_7. [DOI] [PubMed] [Google Scholar]
- 14.Abravaya K, Myers MP, Murphy SP, Morimoto RI. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock protein expression. Genes Dev. 1992;6:1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- 15.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94(4):471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 16.Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6(1):4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440(7083):556–560. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 18.Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress- induced apoptosis. Mol Cell Biol. 1997;17(9):5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price BD, Calderwood SK. Heat-induced transcription from RNA polymerases II and III and HSF binding are co-ordinately regulated by the products of the heat shock genes. J Cell Physiol. 1992;153:392–401. doi: 10.1002/jcp.1041530219. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Tang D, Lechpammer S, et al. Double-stranded RNA-dependent protein kinase (pkr) is essential for thermotolerance, accumulation of HSP70, and stabilization of ARE-containing HSP70 mRNA during stress. J Biol Chem. 2002;277(46):44539–44547. doi: 10.1074/jbc.M208408200. [DOI] [PubMed] [Google Scholar]
- 21.Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982;55(656):579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Khaleque MA, Zhao MJ, Zhong R, Gaestel M, Calderwood SK. Phosphorylation of HSF1 by MAPK-activated protein kinase 2 on serine 121, inhibits transcriptional activity and promotes HSP90 binding. J Biol Chem. 2006;281(2):782–791. doi: 10.1074/jbc.M505822200. [DOI] [PubMed] [Google Scholar]
- 23.Soncin F, Prevelige R, Calderwood SK. Expression and purification of human heat-shock transcription factor 1. Protein Expr Purif. 1997;9(1):27–32. doi: 10.1006/prep.1996.0672. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with "mini-extracts" prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu B, Hunt C, Morimoto RI. Structure and expression of the human gene encoding the major heat shock protein HSP70. Molecular and Cellular Biology. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce JL, Chen C, Xie Y, et al. Activation of heat shock transcription factor 1 to a DNA binding form during the G(1)phase of the cell cycle. Cell Stress Chaperones. 1999;4(1):36–45. doi: 10.1054/csac.1999.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cahill CM, Waterman WR, Xie Y, Auron PE, Calderwood SK. Transcriptional repression of the prointerleukin 1beta gene by heat shock factor 1. J Biol Chem. 1996;271(40):24874–24879. [PubMed] [Google Scholar]
- 28.Nunes SL, Calderwood SK. Heat shock factor-1 and the heat shock cognate 70 protein associate in high molecular weight complexes in the cytoplasm of NIH-3T3 cells. Biochem Biophys Res Commun. 1995;213(1):1–6. doi: 10.1006/bbrc.1995.2090. [DOI] [PubMed] [Google Scholar]
- 29.Westwood T, Wu C. Activation of drosophila heat shock factor: conformational changes associated with monomer-to-trimer transition. Mol Cell Biol. 1993;13:3481–3486. doi: 10.1128/mcb.13.6.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y, Zhong R, Chen C, Calderwood SK. Heat shock factor 1 contains two functional domains that mediate transcriptional repression of the c-fos and c-fms genes. J Biol Chem. 2003;278(7):4687–4698. doi: 10.1074/jbc.M210189200. [DOI] [PubMed] [Google Scholar]
- 31.Solomon MJ, Varshavsky A. Formaldehyde-mediated DNA-protein crosslinking: a probe for in vivo chromatin structures. Proc Natl Acad Sci U S A. 1985;82(19):6470–6474. doi: 10.1073/pnas.82.19.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takacs-Vellai K, Vellai T, Chen EB, et al. Transcriptional control of Notch signaling by a HOX and a PBX/EXD protein during vulval development in C. elegans. Dev Biol. 2007;302(2):661–669. doi: 10.1016/j.ydbio.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 33.Khaleque MA, Bharti A, Gong J, et al. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27(13):1886–1893. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]
- 34.Tang D, Khaleque MA, Jones EL, et al. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10(1):46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabindran SK, Gioorgi G, Clos J, Wu C. Molecular Cloning and expression of a human heat shock factor, HSF1. Proceedings of the National Academy of Sciences (USA) 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oesterreich S, Hickey E, Weber L, Fuqua SA. Basal regulatory promoter elements in the hsp27 gene in human breast carcinoma cells. Biochem Biophys Res Commun. 1996;222:155–163. doi: 10.1006/bbrc.1996.0714. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Xie Y, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses Ras-induced transcriptional activation of the c-fos gene. J Biol Chem. 1997;272(43):26803–26806. doi: 10.1074/jbc.272.43.26803. [DOI] [PubMed] [Google Scholar]
- 38.Wang XZ, Asea A, Xie Y, Kabingu E, Stevenson MA, Calderwood SK. RSK2 represses HSF1 activation during heat shock. Cell Stress & Chaperones. 2000;5:432–437. doi: 10.1379/1466-1268(2000)005<0432:rrhadh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt C, Calderwood SK. Characterization and sequence of a mouse HSP70 gene and its expression in mouse cell lines. Gene. 1990;87:199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- 40.Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J Biol Chem. 2002;277(14):11802–11810. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]