Summary
We have developed an enhanced molecular chaperone-based vaccine through rapid isolation of Hsp70 peptide complexes after the fusion of tumor and dendritic cells (Hsp70.PC-F). In this approach, the tumor antigens are introduced into the antigen processing machinery of dendritic cells through the cell fusion process and thus we can obtain antigenic tumor peptides or their intermediates that have been processed by dendritic cells. Our results show that Hsp70.PC-F has increased immunogenicity compared to preparations from tumor cells alone and therefore constitutes an improved formulation of chaperone protein-based tumor vaccine.
Keywords: Heat shock proteins70 (Hsp70), Dendritic cells (DC), Cell fusion, Extraction of Hsp70 peptide complexes (Hsp70.PC), Tumor vaccine
1. Introduction
The heat shock proteins 70 (Hsp70) family is intrinsic to cellular life, permitting proteins to perform essential enzymatic, signaling and structural functions within the tightly crowded milieu of the cell and working to avert the catastrophe of protein aggregation during stress [1, 2]. There are at least 12 members of the human Hsp70 family, including proteins expressed in the cytoplasm, endoplasmic reticulum and mitochondria [1, 3, 4]. For molecular chaperone function, Hsp70 family members are equipped with two major functional domains, including a carboxy-terminal region that binds peptides and denatured proteins and an N-terminal ATPase domain that controls the opening and closing of the peptide binding domain [3]. These two domains play important roles in the functions of Hsp70 in tumor immunity, mediating the acquisition of cellular antigens and their delivery to immune effector cells [5, 6]. Hsp70 expression becomes dysregulated in many types of cancer leading to elevated Hsp70 levels under non-stress conditions that protect emerging cancer cells from the apoptosis that accompanies many steps in transformation, but also create an opportunity for vaccine design [4, 7–9].
The molecular chaperone-based tumor vaccine has been pioneered by Pramod Srivastava who has prepared autologous vaccines in mice and in human patients with the direct aim of targeting the unique antigens that characterize each individual neoplasm [10–14]. In this approach, Hsp70 peptide complexes (Hsp70.PC) are isolated from the patients’ tumors by affinity chromatography using ATP-agarose and formulations of Hsp70 applied in a multi-dose regimen. The aim is for Hsp70.PC to facilitate antigen cross-presentation to the patient’s T cells through host APC and for the unique mixture of peptides from the individual tumor to induce antitumor immunity. Despite immunologic and clinical responses obtained in a subset of patients with malignant tumors in the early phase I and/or II trials with molecular chaperone GP96.PC (vitespen) purified from patient-derived tumors [14–17], the randomized phase III trials, however, showed mixed results [18, 19].
We have attempted to produce an enhanced molecular chaperone-based vaccine through rapid isolation of Hsp70 peptide complexes from fusions of tumor and dendritic cells (Hsp70.PC-F). In our animal studies, Hsp70.PC-F vaccines show superior immunological properties such as enhanced induction of CTL against tumor cells and stimulation of DC maturation over counterparts from tumor cells [20]. More importantly, immunization of mice with Hsp70.PC-F resulted in a T-cell-mediated immune response including significant increase of CD8 T cells and induction of effector and memory T cells able to break T cell unresponsiveness to a non-mutated tumor antigen and provide protection of mice against challenge with tumor cells. By contrast, the immune response to vaccination with Hsp70.PC derived from tumor cells alone is muted against such non-mutated tumor antigen. Hsp70.PC-F complexes differed from those derived from tumor cells in a number of key manners, most notably, enhanced association with immunologic peptides. In addition, the molecular chaperone Hsp90 was found to be associated with Hsp70.PC-F as indicated by co-immunoprecipitation, suggesting ability to carry an increased repertoire of antigenic peptides by the two chaperones. These experiments indicate that Hsp70.PC derived from DC-tumor fusion cells have increased their immunogenicity and therefore constitute an improved formulation of chaperone protein-based tumor vaccine.
The rationale for the extraction of Hsp70.PC from DC-tumor fusion cells is based on the observation that DC are the most potent antigen-presenting cells [21, 22]. The fusion of DC and tumor cells through chemical [23–40], physical [26, 41–50] or biological [51, 52] means creates a heterokaryon which combines DC-derived costimulatory molecules, efficient antigen-processing and -presentation machinery, and an abundance of tumor-derived antigens. The DC and tumor cells become one hybrid cell sharing a unified cytoplasm. The integration of cytoplasm from DC and tumor cells renders the tumor antigens endogenous to the DC heterokaryon and, therefore, facilitates the entry of tumor antigens into the DC endogenous pathway of antigen-processing and -presentation machinery [29, 53, 54]. It is likely that the antigen-processing machinery from DC can sort or select the immunogenic peptides to be processed and presented and work much more efficiently than that from tumor cells, thus increasing the quality and quantity of the Hsp-associated complexes.
2. Materials
(2-1) Isolation of tumor cells from patient-derived solid sample or malignant fluid
DNase (0.1 mg/ml, Sigma-Aldrich, Saint Louis, MO)
Collagenase (1 mg/ml, Worthington Biochemical Corporation, Lakewood, NJ)
Ca2+/Mg2+-free Hanks balanced salt solution (HBSS medium, Mediatech Inc., Manassas, VA)
A sterile 50-µm nylon mesher (Sigma-Aldrich, Saint Louis, MO)
Heat-inactivated human AB serum (Sigma-Aldrich, Saint Louis, MO)
RPMI 1640 medium (Mediatech, Manassas, VA)
L-glutamine (2 mM, Mediatech, Manassas, VA)
Penicillin and Streptomycin (100 units/ml and 100 µg/ml) (Mediatech Inc., Manassas, VA)
(2-2) Generation of DC from human peripheral blood monocytes
Ficoll density gradient centrifugation (Ficoll-Paque™ plus, GE healthcare Bio-Sciences AB, Sweden)
Granulocyte–macrophage colony-stimulating factor (hGM-CSF, 1000 units/ml) (Genzyme, Framingham, MA)
Interleukin-4 (hIL-4, 500 units/ml) (R&D Systems, Minneapolis, MN)
(2-3) Preparation of DC-tumor fusions
Polyethylene glycol (PEG, 50% MW1450) (Sigma-Aldrich, Saint Louis, MO)
(2-4) Preparation of Hsp70.PC extraction from DC-tumor fusions
Tris-HCl (pH7.4, 50mM) (Boston Bioproduct, Ashland, MA)
NaCl (50mM) (Sigma-Aldrich, Saint Louis, MO)
Nonidet P-40 (NP40, 1%, Sigma-Aldrich, Saint Louis, MO)
Protease inhibitor cocktail tablets, Complete Mini (Roche, Mannheim, Germany)
Sodium Orthovanadate (NaVO4, 1mM) (Boston Bioproduct, Ashland, MA)
Antibody against human Hsp70 (5C1A12, Developed by ProMab Biotechnologies, Inc., Albany, CA)
Dye Reagent Concentrate for protein assay (Bio-Rad, Hercules, CA)
Protein A Sepharose (GE Healthcare, Waukesha, WI)
Protein G Sepharose (GE Healthcare, Waukesha, WI)
(2-5) Measurement of levels of endotoxin
Limulus amebocyte lysate (LAL kit, Cambrex Bio Science Inc., Walkersville, MD)
3. Methods
Cell fusion between DC and tumor cells can be achieved through chemical, physical or biological means. In our laboratory, we use PEG to fuse DC and tumor cells. We have used the following protocol to prepare Hsp70.PC extracts from DC-tumor fusion cells.
(3-1) Generation of DC from human peripheral blood monocytes
DC can be generated from human peripheral blood monocytes (PBMC) derived from patients or from healthy donors. We usually use Ficoll to separate PBMC and culture these cells in medium containing hGM-CSF. The protocol is based on a previously described method [55–57] with modifications:
Peripheral blood mononuclear cells (PBMC) obtained from patients or leucopacks are transferred into 50ml centrifuge tube and sedimented at low speed.
The serum on the top of tube is collected into a clear tube as serum for cell culture. The blood cells at the bottom of the tube are re-suspended with RPMI 1640 medium without serum (1:2 dilution).
The blood cells are gently laid on top of the tube containing a Ficoll density gradient.
Tubes are centrifuged at 1,800 rpm for 20 min at room temperature.
After Ficoll density gradient centrifugation, the cells in the interface layer are collected into another tube with RPMI 1640 and 2% serum.
Cells are washed twice with serum-free medium and the numbers of cells are counted.
Culture 1 × 106 cells /ml in RPMI 1640 containing 5% human serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin for 1 hour in a humidified CO2 incubator.
After 1 hour culture, gently wash and remove the non-adherent cells. The adherent fraction is cultured in RPMI/AIM-V (1:1) medium with 1% of human serum and 1000U/ml hGM-CSF and 500 U/ml hIL-4 for 5 days.
On day 3 of culture, cell clusters appear. Fresh medium with 1000 U/ml of hGM-CSF is added if the color of medium becomes yellow.
On day 5 of culture, the loosely adherent cell or cell clusters are collected by gently dislodging the cells by pipetting and then counted the cells (most cells are immature DC)
(3-2) Preparation of tumor cells
Tumor cells can be either freshly isolated from tumor samples or obtained from vials of frozen cell lines. The method described here is used to isolate and culture tumor cells from patient-derived breast or ovarian cancer sample under sterile condition.
The resected human tumor sample is weighted, minced to small pieces (1–3 mm) and digested in HBSS solution containing 1 mg/ml collagenase, 0.1 mg/ml DNase, 100 U/ml penicillin and 100 µg/ml streptomycin.
The digested tumor tissue is then mashed through a sterile 50-µm nylon mesher under sterile conditions in a tissue culture hood.
Cells are washed twice with cold HBSS solution.
Single-tumor cell suspensions are obtained by passing through a cell strainer and the numbers of tumor cells counted.
Culture the tumor cells in high glucose DMEM medium containing 10% human serum and antibiotics. Remove the non-adherent dead cells.
Incubate the cells at 37°C for 2 to 3 days. Cells are ready for fusion when they are in the logarithmic phase of growth.
(3-3) Cell fusion
DC generated from PBMC are cultured in 1000 U/ml hGM-CSF medium for 5 days.
Tumor cells are maintained in DMEM supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 g/ml streptomycin.
The DC are mixed with tumor cells at a 10:1 ratio and the mixture is washed once with serum-free medium followed by low speed spin (500 rpm) to obtain cell pellets.
The mixed cell pellets are gently resuspended in pre-warmed 50% PEG solution (1 ml per 1–5 × 108 cells) for 5 min at room temperature.
The PEG solution is diluted by slow addition and mixing of 1, 2, 4, 8 and 16 ml warm serum-free medium within 10 mins.
The cell pellets obtained after centrifugation at 1,350 rpm are resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2mM L-glutamine, 10mM nonessential amino acids, 1 mM sodium pyruvate, 10% NCTC 109, 100 U/ml penicillin, 100 g/ml streptomycin, and 500 U/ml hGM-CSF, and further cultured for 5 days.
After 5 days, DC-tumor fusion cells are loosely adherent to the culture dish, whereas tumor–tumor fusions and unfused tumor cells are attached firmly to the dish. The loosely adherent fusion cells are obtained first by the gentle pipetting.
The fusion efficiency is determined by dual expression of tumor antigens such as MUC1 and DC markers (MHC class II molecules or co-stimulatory molecules).
(3-4) Extraction of Hsp70 peptide complexes (Hsp70.PC) from DC-tumor fusion cell products
DC-tumor fusion cells are collected and counted.
Resuspend the cell pellets with lysis buffer (50mM Tris-HCl, pH 8.0, containing 50mM NaCl, 1% Nonidet P-40, 1mM PMSF) (1 ml lysis buffer for 2 × 107 cells) on ice for 30 min.
Centrifuge the cell lysate at 13,000 rpm for 15 minutes at 4°C.
After centrifuge, collect the supernatant into a clear tube.
- Check protein concentration with standard procedure (Bio-Rad Bradford Protein Assay Kit):
- Prepare dye reagent by diluting 1 part Dye Reagent Concentrate with 4 parts distilled, deionized water.
- Prepare three to five dilutions of a protein standard, which is representative of the protein solution to be tested.
- Pipette 100 µl of each standard and sample solutions into 5.0 ml of diluted dye reagent.
- Incubate the test samples at room temperature for at least 5 minutes. Absorbance will increase over time. Samples should be incubated at room temperature for no more than 15 minutes.
- Measure absorbance at 595 nm. Calculate the protein concentration based on the standard curve.
The lysates are clarified by centrifugation, and the aqueous phase is collected and incubated with mAb against human Hsp70 at concentration of 1:100, rotating through overnight at 4°C.
-
For protein analysis, the immunoprecipitates are dissolved in Laemmli SDS sample buffer (0.1 Tris-Cl, 4% SDS, 20% glycerol, 0.05% bromphenol blue, 5% 2-ME) and analyzed by immunoblotting.
For Binding of the Immune Complex:- Mix Protein A Sepharose and Protein G Sepharose at a 1:1 ratio followed by wash with lysis buffer once.
- Spin down at 13,000 rpm for 1 minute at 4°C, discard the supernatant and resuspend the beads with 250 µl lysis buffer.
- Pipette 100 µl A/G mixture beads into sample tubes and incubate for 2 hours at 4°C.
- After incubation, spin down at 13,000 rpm for 1 minute at 4°C. Remove supernatant.
- Wash beads with 0.5 ml of lysis buffer for 5 minutes (rotate at 4°C) followed by centrifugation at 13,000 rpm for 1 minute at 4°C.
- Wash beads with 0.5 ml of sterile PBS for 5 minutes (rotate at 4°C) followed by centrifugation at 13,000 rpm for 1 minute at 4°C.
-
After extensive wash with lysis buffer, the immunoprecipitates are eluted with sterile high salt elution buffer.
Elute the proteins with (500 mM NaCl, 100 µl) at RT for 2 hour.
Centrifuge at 13,000 rpm for 1 minute at 4°C.
Collect the supernatant and measure the protein concentration by Bradford protein assay.
The Hsp70.PC preparations are checked by limulus amebocyte lysate assay to ensure minimal contamination with endotoxins, aliquoted into 1.5 ml eppendorf tubes and stored at −80°C.
4. Notes
(4-1) Cell Fusion
The PEG solution is diluted by gradual addition and progressive mixing of 1, 2, 4, 8 and 16 ml warm serum-free medium. The cell pellets obtained after centrifugation at 1,350 rpm are resuspended in medium containing 10% heat-inactivated FCS and GM-CSF. The variable factor for cell fusion is the length of time the cells are exposed to PEG. We have found that there is some difference in the sensitivity of cells to PEG. It is desirable to perform a dose–response test to evaluate the conditions of PEG fusion for each type of tumor cell and to determine the optimal exposure time. Unlike electrofusion, DC–tumor fusion by PEG is an active and evolving process, and it is thus likely that the larger the initial contact surface between cells, the faster the integration of these cells. Fusion efficiency is lowest immediately after the fusion process is initiated, and one-week culture results in more than a 10-fold increase in efficiency (Gong J., unpublished data). In addition, short-term culture will give the fusion cells sufficient time to integrate and display the antigen in the context of MHC molecules.
(4-2) Extraction of Hsp70 peptide complexes
For protein concentration measurement, Bradford dye reagent absorbance will increase over time. Samples should incubate at room temperature for at least 5 minutes, but no more than 15 minutes. After protein A/G sepharose binding with Hsp70 immunoglobulin, the beads should be gently mixed with lysis buffer to wash off non-specific interactions. However, the use of vortex should be avoided since it may break the binding of sepharose beads and immunoglobulins. Background caused by actin contamination can be avoided by adding 10 mM ATP to lysis buffer. All steps should be performed at 4°C to reduce proteolysis and denaturation of antigens. This is especially important for the binding step which is typically incubated overnight (or at least 2 hours) at 4°C.
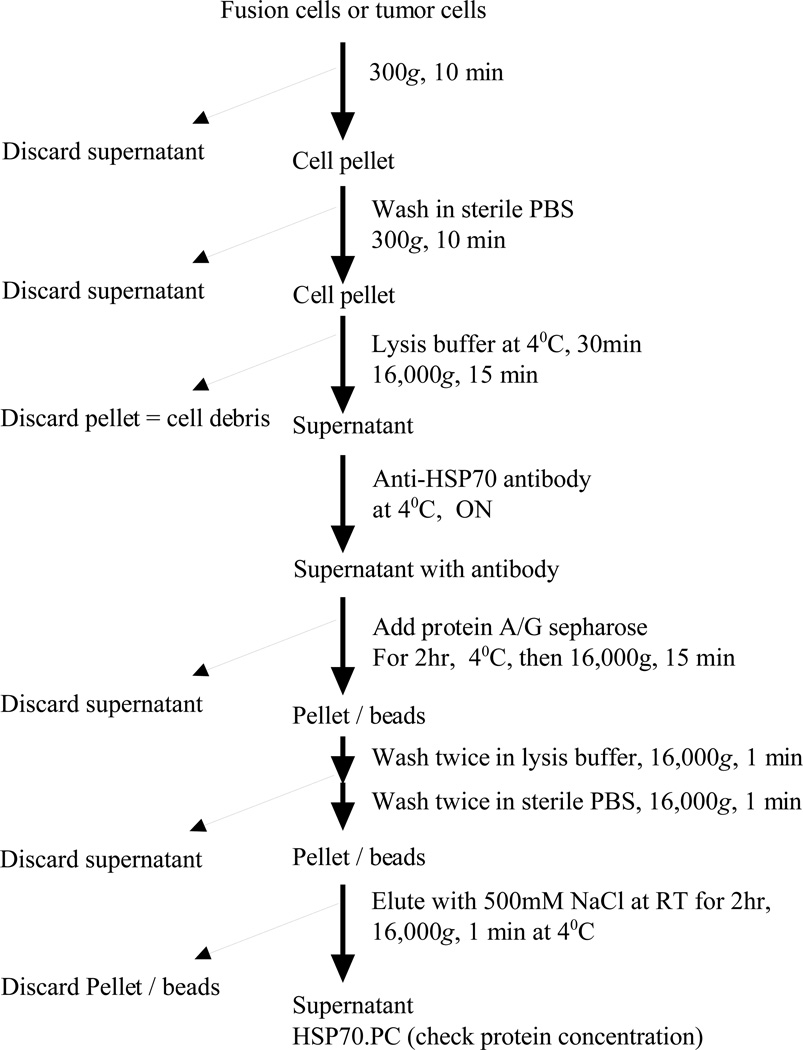
Figure 1.
Flow chart for HSP70.PC purification
References
- 1.Lindquist S, Craig EA. The heat shock proteins. Ann. Rev. Genet. 1988;22:631–637. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 2.Georgopolis C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Ann. Rev. Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 3.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 4.Tang D, Khaleque AA, Jones ER, Theriault JR, Li C, Wong WH, Stevenson MA, et al. Expression of heat shock proteins and HSP messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10 doi: 10.1379/CSC-44R.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169:5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava PK, Amato RJ. Heat shock proteins: the 'Swiss Army Knife' vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–2597. doi: 10.1016/s0264-410x(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 7.Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000;926:122–125. doi: 10.1111/j.1749-6632.2000.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 8.Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, et al. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- 9.Clark PR, Menoret A. The inducible Hsp70 as a marker of tumor immunogenicity. Cell Stress Chaperones. 2001;6:121–125. doi: 10.1379/1466-1268(2001)006<0121:tihaam>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava P. Hypothesis: controlled necrosis as a tool for immunotherapy of human cancer. Cancer Immun. 2003;3:4. [PubMed] [Google Scholar]
- 12.Srivastava PK. Immunotherapy of human cancer: lessons from mice. Nat Immunol. 2000;1:363–366. doi: 10.1038/808795. [DOI] [PubMed] [Google Scholar]
- 13.Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino G, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 2002;20:4169–4180. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235–3245. [PubMed] [Google Scholar]
- 15.Parmiani G, De Filippo A, Pilla L, Castelli C, Rivoltini L. Heat shock proteins gp96 as immunogens in cancer patients. Int J Hyperthermia. 2006;22:223–227. doi: 10.1080/02656730600647957. [DOI] [PubMed] [Google Scholar]
- 16.Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino G, et al. Vaccination of metastatic melanoma patients with autologous tumor- derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 2002;20:4169–4180. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 17.Pilla L, Patuzzo R, Rivoltini L, Maio M, Pennacchioli E, Lamaj E, Maurichi A, et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol Immunother. 2006;55:958–968. doi: 10.1007/s00262-005-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 19.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, Xing PX, et al. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. J Immunol. 2006;177:5946–5955. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- 21.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 22.Steinman RM. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001;68:106–166. [PubMed] [Google Scholar]
- 23.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 24.Gong J, Koido S, Chen D, Tanaka Y, Huang L, Avigan D, Anderson K, et al. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002;99:2512–2517. doi: 10.1182/blood.v99.7.2512. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Zhang W, Chan T, Saxena A, Xiang J. Engineered fusion hybrid vaccine of IL-4 gene-modified myeloma and relative mature dendritic cells enhances antitumor immunity. Leuk Res. 2002;26:757–763. doi: 10.1016/s0145-2126(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 26.Lindner M, Schirrmacher V. Tumour cell-dendritic cell fusion for cancer immunotherapy: comparison of therapeutic efficiency of polyethylen-glycol versus electro-fusion protocols. Eur J Clin Invest. 2002;32:207–217. doi: 10.1046/j.1365-2362.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 27.Homma S, Toda G, Gong J, Kufe D, Ohno T. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol. 2001;36:764–771. doi: 10.1007/s005350170019. [DOI] [PubMed] [Google Scholar]
- 28.Cao X, Zhang W, Wang J, Zhang M, Huang X, Hamada H, Chen W. Therapy of established tumour with a hybrid cellular vaccine generated by using granulocyte-macrophage colony-stimulating factor genetically modified dendritic cells. Immunology. 1999;97:616–625. doi: 10.1046/j.1365-2567.1999.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516–5524. [PubMed] [Google Scholar]
- 30.Hayashi T, Tanaka H, Tanaka J, Wang R, Averbook BJ, Cohen PA, Shu S. Immunogenicity and therapeutic efficacy of dendritic-tumor hybrid cells generated by electrofusion. Clin Immunol. 2002;104:14–20. doi: 10.1006/clim.2002.5224. [DOI] [PubMed] [Google Scholar]
- 31.Xia J, Tanaka Y, Koido S, Liu C, Mukherjee P, Gendler SJ, Gong J. Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells. J Immunol. 2003;170:1980–1986. doi: 10.4049/jimmunol.170.4.1980. [DOI] [PubMed] [Google Scholar]
- 32.Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol. 2003;170:3806–3811. doi: 10.4049/jimmunol.170.7.3806. [DOI] [PubMed] [Google Scholar]
- 33.Takeda A, Homma S, Okamoto T, Kufe D, Ohno T. Immature dendritic cell/tumor cell fusions induce potent antitumour immunity. Eur J Clin Invest. 2003;33:897–904. doi: 10.1046/j.1365-2362.2003.01194.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JK, Li J, Zhang J, Chen HB, Chen SB. Antitumor immunopreventive and immunotherapeutic effect in mice induced by hybrid vaccine of dendritic cells and hepatocarcinoma in vivo. World J Gastroenterol. 2003;9:479–484. doi: 10.3748/wjg.v9.i3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Holmes LM, Franek KJ, Burgin KE, Wagner TE, Wei Y. Purified hybrid cells from dendritic cell and tumor cell fusions are superior activators of antitumor immunity. Cancer Immunol Immunother. 2001;50:456–462. doi: 10.1007/s002620100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia D, Chan T, Xiang J. Dendritic cell/myeloma hybrid vaccine. Methods Mol Med. 2005;113:225–233. doi: 10.1385/1-59259-916-8:225. [DOI] [PubMed] [Google Scholar]
- 37.Homma S, Kikuchi T, Ishiji N, Ochiai K, Takeyama H, Saotome H, Sagawa Y, et al. Cancer immunotherapy by fusions of dendritic and tumour cells and rh-IL-12. Eur J Clin Invest. 2005;35:279–286. doi: 10.1111/j.1365-2362.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 38.Kao JY, Zhang M, Chen CM, Chen JJ. Superior efficacy of dendritic cell-tumor fusion vaccine compared with tumor lysate-pulsed dendritic cell vaccine in colon cancer. Immunol Lett. 2005;101:154–159. doi: 10.1016/j.imlet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa F, Iinuma H, Okinaga K. Dendritic cell vaccine therapy by immunization with fusion cells of interleukin-2 gene-transduced, spleen-derived dendritic cells and tumour cells. Scand J Immunol. 2004;59:432–439. doi: 10.1111/j.0300-9475.2004.01411.x. [DOI] [PubMed] [Google Scholar]
- 40.Akasaki Y, Kikuchi T, Homma S, Abe T, Kofe D, Ohno T. Antitumor Effect of Immunizations With Fusions of Dendritic and Glioma Cells in a Mouse Brain Tumor Model. J Immunother. 2001;24:106–113. [PubMed] [Google Scholar]
- 41.Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, Dalgleish AG. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta. 2000;1500:265–279. doi: 10.1016/s0925-4439(99)00108-8. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka H, Shimizu K, Hayashi T, Shu S. Therapeutic immune response induced by electrofusion of dendritic and tumor cells. Cell Immunol. 2002;220:1–12. doi: 10.1016/s0008-8749(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 43.Siders WM, Vergilis KL, Johnson C, Shields J, Kaplan JM. Induction of specific antitumor immunity in the mouse with the electrofusion product of tumor cells and dendritic cells. Mol Ther. 2003;7:498–505. doi: 10.1016/s1525-0016(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 44.Jantscheff P, Spagnoli G, Zajac P, Rochlitz CF. Cell fusion: an approach to generating constitutively proliferating human tumor antigen-presenting cells. Cancer Immunol Immunother. 2002;51:367–375. doi: 10.1007/s00262-002-0295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goddard RV, Prentice AG, Copplestone JA, Kaminski ER. In vitro dendritic cell-induced T cell responses to B cell chronic lymphocytic leukaemia enhanced by IL-15 and dendritic cell-B-CLL electrofusion hybrids. Clin Exp Immunol. 2003;131:82–89. doi: 10.1046/j.1365-2249.2003.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marten A, Renoth S, Heinicke T, Albers P, Pauli A, Mey U, Caspari R, et al. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Hum Gene Ther. 2003;14:483–494. doi: 10.1089/104303403321467243. [DOI] [PubMed] [Google Scholar]
- 47.Trevor KT, Cover C, Ruiz YW, Akporiaye ET, Hersh EM, Landais D, Taylor RR, et al. Generation of dendritic cell-tumor cell hybrids by electrofusion for clinical vaccine application. Cancer Immunol Immunother. 2004;53:705–714. doi: 10.1007/s00262-004-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki T, Fukuhara T, Tanaka M, Nakamura A, Akiyama K, Sakakibara T, Koinuma D, et al. Vaccination of dendritic cells loaded with interleukin-12-secreting cancer cells augments in vivo antitumor immunity: characteristics of syngeneic and allogeneic antigen-presenting cell cancer hybrid cells. Clin Cancer Res. 2005;11:58–66. [PubMed] [Google Scholar]
- 49.Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sharav T, Sparbier K. Tumour-dendritic hybrid cell vaccination for the treatment of patients with malignant melanoma: immunological effects and clinical results. Vaccine. 2005;23:2367–2373. doi: 10.1016/j.vaccine.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu K, Kuriyama H, Kjaergaard J, Lee W, Tanaka H, Shu S. Comparative analysis of antigen loading strategies of dendritic cells for tumor immunotherapy. J Immunother. 2004;27:265–272. doi: 10.1097/00002371-200407000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Phan V, Errington F, Cheong SC, Kottke T, Gough M, Altmann S, Brandenburger A. A new genetic method to generate and isolate small, short-lived but highly potent dendritic cell-tumor cell hybrid vaccines. Nat Med. 2003;9:1215–1219. doi: 10.1038/nm923. [DOI] [PubMed] [Google Scholar]
- 52.Hiraoka K, Yamamoto S, Otsuru S, Nakai S, Tamai K, Morishita R, Ogihara T, et al. Enhanced tumor-specific long-term immunity of hemagglutinating [correction of hemaggluttinating] virus of Japan-mediated dendritic cell-tumor fused cell vaccination by coadministration with CpG oligodeoxynucleotides. J Immunol. 2004;173:4297–4307. doi: 10.4049/jimmunol.173.7.4297. [DOI] [PubMed] [Google Scholar]
- 53.Koido S, Ohana M, Liu C, Nikrui N, Durfee J, Lerner A, Gong J. Dendritic cells fused with human cancer cells: morphology, antigen expression, and T cell stimulation. Clin Immunol. 2004;113:261–269. doi: 10.1016/j.clim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia- derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002;51:299–310. doi: 10.1007/s00262-002-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gong J, Nikrui N, Chen D, Koido S, Wu Z, Tanaka Y, Cannistra S, et al. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J Immunol. 2000;165:1705–1711. doi: 10.4049/jimmunol.165.3.1705. [DOI] [PubMed] [Google Scholar]
- 56.Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci U S A. 2000;97:2715–2718. doi: 10.1073/pnas.050587197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koido S, Nikrui N, Ohana M, Xia J, Tanaka Y, Liu C, Durfee JK, et al. Assessment of fusion cells from patient-derived ovarian carcinoma cells and dendritic cells as a vaccine for clinical use. Gynecol Oncol. 2005;99:462–471. doi: 10.1016/j.ygyno.2005.07.019. [DOI] [PubMed] [Google Scholar]