Peribronchial inflammation (1–3) and exaggerated bronchospastic responses to antigen-specific and nonspecific stimuli (airways hyperresponsiveness [AHR]) are the pathologic and physiologic cornerstones of the asthmatic syndrome. Eosinophils are believed to play an important role in these responses, as tissue eosinophilia is a common feature in asthmatic airways, and eosinophils have been demonstrated to cause mucosal injury and likely contribute to the generation of altered lung physiology. The initiation and maintenance of allergic airway inflammation is mediated by poorly defined mechanisms. Even more confounding is the appreciation that different immunologic processes mediate different facets of asthma and that various types of inflammatory responses differentially contribute to the multiple features manifested by asthmatic patients. This is reflected in studies that suggest that, in addition to eosinophils, other cells — including mast cells, lymphocytes, and neutrophils — contribute to asthma pathogenesis (4–6). As a result, it has been difficult to design specific, inflammation-based therapeutic interventions for this disorder. Recent studies, however, have added to our understanding of the role of chemokines and chemokine receptors in the generation of eosinophilic and noneosinophilic airway inflammatory responses. This has begun to provide an appreciation of the complex levels of organization of airway inflammatory responses, and may help to identify novel therapeutic options.
Over the past several years, a family of chemotactic cytokines has been identified that appears to have specificity for the type of leukocytes that are recruited to the site of inflammation. Chemokines have been primarily divided into 2 main subfamilies, CXC (α) and CC (β), based upon their sequence homology and the position of the first 2 cysteine residues (7). This group of chemoattractants continues to grow at a staggering pace through broad-based searches for sequence homology in expressed sequence tag databases (Table 1). In vitro characterization is straightforward and would suggest that most of these novel chemokines have redundant or similar functions with other known chemokines. However, careful examination in animal models of allergic airway responses shows that the production of these chemokines is organized in a coordinated manner and that the chemokines function at distinct stages of disease evolution. In addition to the continuously growing family of chemokines, their receptors are only now being identified through the cloning and characterization of numerous “orphan” receptors. The importance of chemokines and their receptors during immune responses is nicely illustrated in viral biology. Several chemokines and/or soluble receptor-like molecules are encoded by viral genomes and released during infection to alter leukocyte recruitment/activation pathways (8). In addition, HIV has clearly been shown to utilize multiple chemokine receptors (especially CCR5 and CXCR4), along with CD4, to enter immune cell populations during infection. The chemokine-chemokine receptor system will likely play similarly important roles in asthma and atopy. Thus, it will be imperative to continue to identify the specific chemokines and their receptors that are expressed in these diseases and determine their function through the use of appropriate animal models.
Table 1.
Chemokine receptors and their ligands
Chemokines and asthma.
A number of chemokines have been identified in human asthma whose production appears to be related to the severity of asthmatic inflammation and reactive airway responses. Some chemokines have been localized to specific cell populations within the allergic lung (9–11). In particular, epithelial cells and macrophages produce significant levels of chemokines. These cell populations can have an immediate impact on the environment of the airway and surrounding lung tissue. Initial investigations in human populations have centered on eosinophil-specific chemokines, as the eosinophil appears to be an important cell population in the pathology of asthma. Over the past several years, a number of chemokines, including IL-8, RANTES, monocyte chemotactic protein-3 (MCP-3), and MCP-4, have been identified that induce eosinophil recruitment. It is still not clear, however, whether all of these chemokines contribute in a major way to in vivo eosinophil chemotaxis, or whether a specific chemokine mediates the bulk of the chemotactic activity and can be targeted for therapy. In addition, a single-nucleotide polymorphism in the proximal RANTES promoter has recently been identified (12), and polymorphisms are likely to occur in other chemokine genes. To date, however, there is no information about the ability of the RANTES polymorphism or other polymorphisms to alter chemokine production and/or effector function and, by so doing, alter asthma phenotype.
The identification of eotaxin as the first chemokine with preferential ability to recruit eosinophils has focused researchers of allergic responses on this molecule. Eotaxin was first discovered in the BAL fluid of guinea pigs after an allergen challenge (13). It has since been cloned in humans and mice and appears to have similar functions, although a paucity of data still exists in inflammatory disorders. Unlike other eosinophil chemotactic chemokines, eotaxin binds to a single receptor, CCR3, that is highly expressed on eosinophils, explaining the specificity of the recruitment of these cells (14–16). Interestingly, eotaxin can induce increased binding of eosinophils under shear force to endothelium via β1 and β2 integrin–mediated mechanisms, suggesting that eotaxin can affect several aspects of eosinophil migratory functions (17). In human asthma, eotaxin is produced at high levels and localized to the airway epithelium. This concentrated expression may preferentially target eosinophils to the epithelium and induce degranulation leading to the release of epithelium-damaging proteins. In addition to recruiting and activating eosinophils, eotaxin can effect other cell populations. Basophils degranulate in the presence of eotaxin, while Th2-type cells can migrate toward eotaxin. Thus, eotaxin may be a primary target for therapeutic intervention. A number of other chemokines and their receptors may also provide significant targets for therapeutic intervention because of their ability to recruit and activate other important cell populations into and around the airway. These chemokines and their receptors are discussed below.
Chemokines in animal models of allergic airway inflammation and hyperreactivity.
Chemokines contribute to tissue responses through their ability to recruit and activate leukocyte populations, induce degranulation, and cause the release of inflammatory mediators from effector cells such as basophils, mast cells, neutrophils, and eosinophils. It is this last aspect of chemokine biology that may be the most devastating during asthmatic responses. Several laboratories have explored the activational functions of specific chemokines and determined their mechanistic relationship to inflammation and physiologic dysregulation. Data from these labs have indicated that CC family chemokines work in a coordinated manner and that different moieties exacerbate airway hyperreactivity responses at specific stages of the evolving response (18–20). For example, the ability of a particular chemokine to activate and cause mast cells and basophils to degranulate may play an important role in the initiation of responses in human asthma. In particular, MCP-1 appears to play a significant role during the early stages of allergic responses because of its ability to induce mast cell activation and LTC4 release into the airway, which directly induces AHR (21). A similar coordinated response appears to be operative in the generation of tissue eosinophilia. A number of CC family chemokines have the ability to recruit eosinophils. Animal studies have identified macrophage inflammatory protein-1α (MIP-1α), eotaxin, MCP-3, and RANTES as important recruitment factors during allergic airway responses (22–24). The movement of eosinophils through the vessel wall into the lung interstitium, and subsequently into the airway, during the early stages of asthma is dependent upon RANTES and MIP-1α, whereas eotaxin is necessary for eosinophil accumulation during chronic stages of the response (18, 21). Thus, the coordinated expression and ligation of these chemokines and receptors appears to be differentially regulated at specific stages of disease.
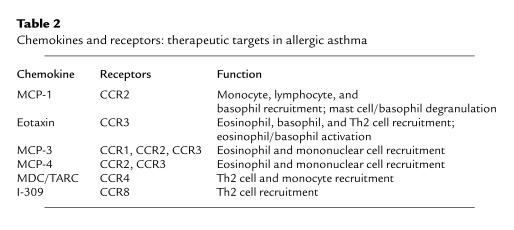
Other chemokines impact on the intensity of the asthmatic response through their ability to induce the recruitment and/or activation of additional cell populations within the lung (Table 2). In particular, a number of chemokines may have the ability to preferentially recruit Th2-type cells, including monocyte-derived chemokine (MDC) and I-309. The ability to preferentially recruit Th2-type cells would further exacerbate asthmatic responses and significantly alter the airway environment. Two other members of the MCP-1 family, MCP-3 and MCP-4, not only can recruit monocytes, but also have been shown to be potent eosinophil chemoattractants. Thus, the induction and evolution of allergic airway inflammation is the result of a coordinated effort that is dependent upon multiple chemokines for the recruitment and activation of different cell populations at specific stages of the disease. It is also evident that a single cell population or mechanism does not mediate the altered airway physiology seen in the disorder. Rather, a number of different pathways are activated in an integrated fashion to generate the inflammatory changes and physiologic dysfunction observed in asthma and models of the asthmatic response.
Table 2.
Chemokines and receptors: therapeutic targets in allergic asthma
In addition to the initiation and maintenance of leukocyte accumulation, CC chemokine members may have the capacity to augment or directionally differentiate T lymphocytes toward Th1- or Th2-type response (25). In particular, MCP-1 can drive undifferentiated T-lymphocyte populations toward an IL-4–producing Th2-type cell, whereas MIP-1α appears to promote a Th1-type response by enhancing IFN-γ and decreasing IL-4 production. Chemokines can also regulate antibody isotype switching to IgE production by B cells (26). Although the exact lymphocyte signal-transduction pathways have not been elucidated for chemokines, a couple of relevant activational pathways have been observed. It appears that MCP-1 can directly induce the phosphorylation of p42/44 mitogen-activated protein kinases, while RANTES has been shown to induce IL-2 receptor expression through activation of phospholipase D. Thus, various chemokines may differentially signal the T lymphocyte by multiple related pathways. These properties suggest that chemokines may play important roles in asthmatic disease progression and aid in determining the overall direction of the developing response. The role of chemokines in lymphocyte activation will be an interesting and controversial area for future studies.
Chemokine receptors and asthma.
The responses induced by the CC chemokines are initiated via specific G protein–coupled receptors on the surface of leukocytes. Although not entirely characterized, it appears that there are no less than 9 different CC family chemokine receptors (27). Unfortunately, limited information has been gained about the expression or function of specific receptors during human disease, or in animal models, because of the lack of appropriate reagents. The limited understanding of the function of chemokine receptors during homeostatic or disease conditions makes it extremely difficult to develop specific therapeutics. In general, multiple CC chemokines have promiscuous binding to multiple receptors (Table 1). Several receptors appear to be prime targets for therapeutic intervention, owing to their relationship with allergic and specific immune responses. In particular, CCR2, which binds members of the MCP-1 family, and CCR3, which specifically is bound by eotaxin, have been characterized and presently represent some of the best therapeutic targets in asthma. Although the distribution of the 2 receptors has not been fully evaluated, CCR2 appears to be present on monocytes, lymphocytes, mast cells, and basophils, whereas CCR3 is expressed at very high levels on eosinophils and basophils and at lower levels on subsets of Th2-type cells (28, 29). Together these 2 receptors may extensively support the recruitment and/or activation of the different cell populations found within the asthmatic airway. In addition to the above receptors, recent data have begun to unravel some of the expression patterns and responses associated with a number of other receptors that may also be related to allergic responses. It appears that T lymphocytes that produce IL-4 (Th2-type) have significantly higher expression of CCR3, CCR4, and/or CCR8 (28–30). These results suggest that these receptors may preferentially be involved in the recruitment of lymphocytes that would specifically create a proallergic milieu and lead to increased airway damage and altered physiology. Although this hypothesis has not been tested, data should soon be available to address these issues. Interestingly, some of the chemokine receptors, especially CCR3, have now been identified on epithelial cells. The impact and functional consequence of a G protein–coupled event in airway epithelial cells has not yet been realized, but is likely to be of significant biologic importance.
From a pharmacologic point of view, G protein–coupled serpentine receptors (7 transmembrane) offer an excellent opportunity for intervention. However, the signaling pathways of these receptors have not been thoroughly evaluated. What has been elucidated is the type of G protein, usually GI, that is involved in chemotactic responses. Examining Ca2+ flux can routinely monitor these signaling pathways. However, data have indicated that Ca2+ flux is not always coupled with movement of cells and that if one monitors only the former parameter, significant information may be neglected (31). Specifically, different subunits of the G-protein signaling pathway direct Ca2+ flux and chemotaxis (Figure 1), with the α subunit inducing Ca2+ flux and the β/γ subunits together responsible for chemotaxis. Therefore, these functions may be separated based upon the type of chemokine-receptor interaction that the cell receives. Thus, it is likely that different cell types under the influence of diverse inflamed environments may respond in a distinct manner to the same chemokine receptor stimulus. Unfortunately, at the present time, specific inhibitors of individual signaling pathways are in insufficient supply for use in experimental procedures.
Figure 1.
The ligation of G protein–coupled chemokine receptors induces multiple pathways linked to different G-protein subunits. The classic analysis of chemokine receptor activation is the measurement of Ca2+ flux, but several other pathways exist that may influence cellular activation and mediator production. The α subunit appears to be coupled to at least 4 different pathways that can influence arachidonic acid metabolism, as well as to MAP kinase activation pathways. The other 2 subunits, β and γ, combine to mediate the chemotactic process and initiate the Ser/Thr and Tyr kinase and phosphatase pathways. Altogether, G-protein activation pathways may help to regulate or activate the leukocytes after they become ligated. It is unclear how the activation of specific pathways is managed under different chemokine stimuli.
Genetically, there have been numerous polymorphisms described in chemokine receptor genes. The CCR5 δ32 mutation is directly related to altering HIV infectivity in human populations in vivo, while a CCR2 64I (a G→A substitution) also has an effect on progression of HIV (8). These mutations may also have an effect on the incidence of other diseases, such as rheumatoid arthritis and diabetes mellitus (32, 33). Interestingly, a recent study demonstrated a number of polymorphisms in the CCR3 gene, with the most frequent occurring in 26% of individuals studied (34). Whether these or other polymorphisms in chemokine receptor genes affect the prevalence or severity of asthma will be an interesting subject for future study.
Regulation of chemokines and their receptors.
During an immune response, the ability to recruit and activate leukocytes is dependent upon the local production of a particular chemokine and the cellular expression of the appropriate receptor. As reviewed above, a number of CC chemokines have the ability to participate in an allergic asthmatic response. Although it appears that common pathways, such as NFκB- and AP-1–induced promoter activation, mediate chemokine production, chemokines are differentially regulated by specific cytokines. Eotaxin, MCP-1, and MCP-4 are all upregulated by IL-4 and/or IL-13, presumably via a STAT6-dependent pathway (35, 36). These chemokines, therefore, would be preferentially produced during a Th2-type allergic response. In contrast, RANTES and MIP-1α are both induced by Th1-type and regulated by Th2-type cytokines, and therefore may only play a minor role in a Th2-driven allergic response (37). However, recent data have indicated that allergic responses can develop with Th1 and Th2 components, both of which can contribute to the damage within the airway. In addition, when one examines exacerbations of asthmatic responses, it appears that viruses capable of inducing Th1-type responses are among the most common causes of asthmatic exacerbations. These responses may be accompanied by significant levels of chemokines, such as RANTES and MIP-1α, that are capable of inducing the migration of leukocytes, including eosinophils. In accordance with this concept, RANTES and MIP-1α are produced in exaggerated quantities by airway epithelial cells infected with asthma-associated viruses such as rhinovirus and respiratory syncytial virus (38, 39). Thus, the idea that an event or feature of asthma is mediated by a single chemokine or receptor in all cases may not be a functional hypothesis.
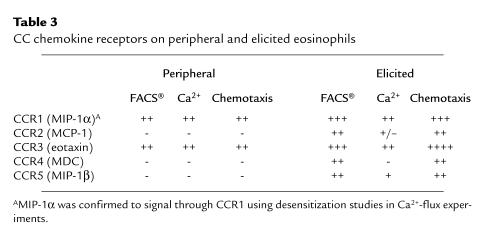
The regulation of chemokine receptors will also be an important issue to unravel. At present, little is known about the regulation of receptors on cells. However, what is known correlates well with the expression of specific responses. As mentioned above, it appears that certain Th-cell subsets, as well as eosinophils, preferentially express certain CC chemokine receptors. However, much of this data comes from peripheral or tissue-cultured cell populations. Recent data in our laboratory have demonstrated that once leukocytes begin to migrate from the peripheral circulation into a site of an immune/inflammatory response, receptor expression changes. In most cases, the cells begin to express a wide range of chemokine receptors, perhaps allowing them to better interact with their inflamed environment and become susceptible to further regulation by a number of chemokines. This effect appears relevant for lymphocytes and neutrophils, as well as for eosinophils (our unpublished data). As outlined in Table 3, a number of differences are found in chemokine receptor expression by isolated murine peripheral eosinophils compared with elicited eosinophils. When examined by flow cytometric analysis, the eosinophils isolated from the peripheral circulation expressed only CCR1 and CCR3. The eosinophils elicited and isolated from the peritoneum of sensitized mice had increased expression of CCR1 and CCR3 compared with the peripheral cells, with detectable surface expression of CCR2, CCR4, and CCR5. These latter observations indicate and explain the ability of eosinophils to utilize multiple chemokines as they move from vessel to airway. Chemotactic and/or Ca2+-flux assays using specific chemokines have verified the expression of the additional receptors on the elicited cells. Thus, chemokine receptors should not only be examined on peripheral cell populations, but also on cells at the site of tissue response. The expression of the additional receptors on leukocytes as they migrate into the tissue may drastically alter their function and survival within the inflamed airway.
Table 3.
CC chemokine receptors on peripheral and elicited eosinophils
Chemokines play an important role in asthma at multiple levels. The coordinated production of specific chemokines and the expression of distinct subsets of receptors likely contribute to the evolution, intensity, and severity of the asthmatic response. Chemokines were originally described as mediators of leukocyte recruitment. However, recent evidence suggests that they can also influence the outcome of the immune response by altering the profile of cytokines that are produced and by activating/degranulating multiple effector cell populations. In view of the large number of chemokines and the promiscuous binding pattern for multiple receptors, it is unlikely that a single chemokine- or chemokine receptor–induced mechanism will be identified for therapy. Instead, a multimechanistic approach may be the most promising. Future investigations into the effector functions of chemokines and mechanisms of activation of chemokine receptors should elucidate both the roles of individual moieties during various phases of the asthmatic response and the pathways they employ in mediating these effects. Only then can the true functions of individual chemokines be identified and appropriate molecules targeted.
References
- 1.Backman KS, Greenberger PA, Patterson R. Airways obstruction in patients with long-term asthma consistent with ‘irreversible asthma.’. Chest. 1997;112:1234–1240. doi: 10.1378/chest.112.5.1234. [DOI] [PubMed] [Google Scholar]
- 2.Corrigan CJ, Kay AB. T-cell/eosinophil interactions in the induction of asthma. Eur Respir J Suppl. 1996;22:72s–78s. [PubMed] [Google Scholar]
- 3.Kay AB. T cells as orchestrators of the asthmatic response. Ciba Found Symp. 1997;206:56–67; discussion 67–70, 106–110. [PubMed] [Google Scholar]
- 4.Rossi GL, Olivieri D. Does the mast cell still have a key role in asthma? Chest. 1997;112:523–529. doi: 10.1378/chest.112.2.523. [DOI] [PubMed] [Google Scholar]
- 5.Foresi A, et al. Eosinophils, mast cells, and basophils in induced sputum from patients with seasonal allergic rhinitis and perennial asthma: relationship to methacholine responsiveness. J Allergy Clin Immunol. 1997;100:58–64. doi: 10.1016/s0091-6749(97)70195-7. [DOI] [PubMed] [Google Scholar]
- 6.Lamblin C, et al. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med. 1998;157:394–402. doi: 10.1164/ajrccm.157.2.97-02099. [DOI] [PubMed] [Google Scholar]
- 7.Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Locati M, Murphy PM. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425–440. doi: 10.1146/annurev.med.50.1.425. [DOI] [PubMed] [Google Scholar]
- 9.Stellato C, et al. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J Clin Invest. 1997;99:926–936. doi: 10.1172/JCI119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkard SG, Westwick J, Millar AB. Production of interleukin-8, RANTES and MCP-1 in intrinsic and extrinsic asthmatics. Eur Respir J. 1997;10:2097–2104. doi: 10.1183/09031936.97.10092097. [DOI] [PubMed] [Google Scholar]
- 11.Holgate ST, et al. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997;156:1377–1383. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 12.Juji T, et al. Polymorphism in RANTES chemokine promoter affects HIV-1 disease progression. Proc Natl Acad Sci USA. 1999;96:4581–4585. doi: 10.1073/pnas.96.8.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jose PJ, et al. Eotaxin: cloning of an eosinophil chemoattractant cytokine and increased mRNA expression in allergen-challenged guinea-pig lungs. Biochem Biophys Res Commun. 1994;205:788–794. doi: 10.1006/bbrc.1994.2734. [DOI] [PubMed] [Google Scholar]
- 14.Heath H, et al. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugherty BL, et al. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsner J, Hochstetter R, Kimmig D, Kapp A. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur J Immunol. 1996;26:1919–1925. doi: 10.1002/eji.1830260837. [DOI] [PubMed] [Google Scholar]
- 17.Kitayama J, Mackay CR, Ponath PD, Springer TA. The C-C chemokine receptor CCR3 participates in stimulation of eosinophil arrest on inflammatory endothelium in shear flow. J Clin Invest. 1998;101:2017–2024. doi: 10.1172/JCI2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 161:7047–7053. [PubMed] [Google Scholar]
- 19.Gonzalo JA, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukacs NW, et al. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–4404. [PubMed] [Google Scholar]
- 21.Campbell EM, et al. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2–/– mice: the role of mast cells. J Immunol. 1999;163:2160–2167. [PubMed] [Google Scholar]
- 22.Stafford S, et al. Monocyte chemotactic protein-3 (MCP-3)/fibroblast-induced cytokine (FIC) in eosinophilic inflammation of the airways and the inhibitory effects of an anti-MCP-3/FIC antibody. J Immunol. 1997;158:4953–4960. [PubMed] [Google Scholar]
- 23.Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams JT. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–1172. doi: 10.1006/bbrc.1993.2599. [DOI] [PubMed] [Google Scholar]
- 24.Lukacs NW, et al. C-C chemokine-induced eosinophil chemotaxis during allergic airway inflammation. J Leukoc Biol. 1996;60:573–578. doi: 10.1002/jlb.60.5.573. [DOI] [PubMed] [Google Scholar]
- 25.Karpus WJ, et al. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 26.Kimata H, et al. RANTES and macrophage inflammatory protein 1 alpha selectively enhance immunoglobulin E (IgE) and IgG4 production by human B cells. J Exp Med. 1996;183:2397–2402. doi: 10.1084/jem.183.5.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Ambrosio D, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 31.Ward SG, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 32.Garred HO, et al. CC chemokine receptor 5 polymorphism in rheumatoid arthritis. J Rheumatol. 1998;25:1462–1465. [PubMed] [Google Scholar]
- 33.Szalai C, et al. Chemokine receptor CCR2 and CCR5 polymorphisms in children with insulin-dependent diabetes mellitus. Pediatr Res. 1999;46:82–84. doi: 10.1203/00006450-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann N, Bern JA, Rothenberg ME. Polymorphisms in the human CC chemokine receptor-3 gene. Biochim Biophys Acta. 1998;14442:170–176. doi: 10.1016/s0167-4781(98)00162-6. [DOI] [PubMed] [Google Scholar]
- 35.Goebeler M, et al. Interleukin-13 selectively induces monocyte chemoattractant protein-1 synthesis and secretion by human endothelial cells. Involvement of IL-4R alpha and Stat6 phosphorylation. Immunology. 1997;91:450–457. doi: 10.1046/j.1365-2567.1997.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mochizuki M, Bartels J, Mallet AI, Christophers E, Schroder JM. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol. 1998;160:60–68. [PubMed] [Google Scholar]
- 37.Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokine MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–3604. [PubMed] [Google Scholar]
- 38.Olszewsha-Pazdrac B, et al. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with RSV. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scroth MK, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]