Abstract
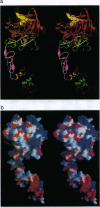
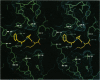
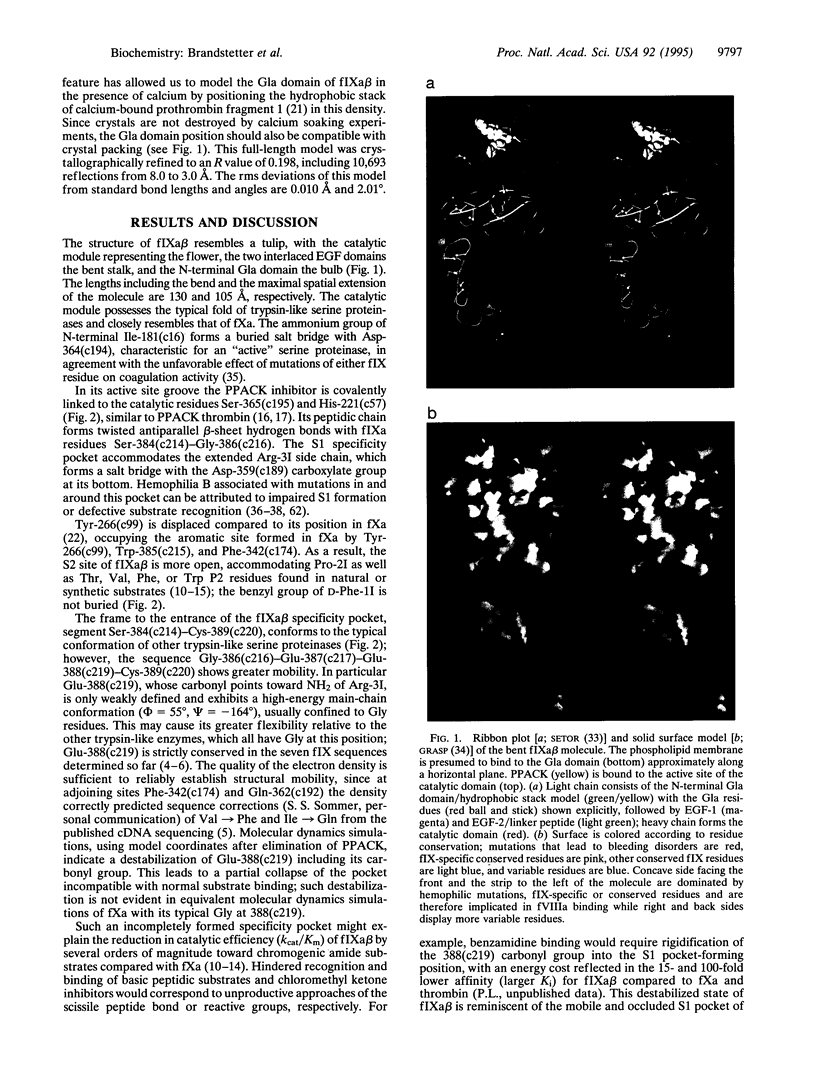
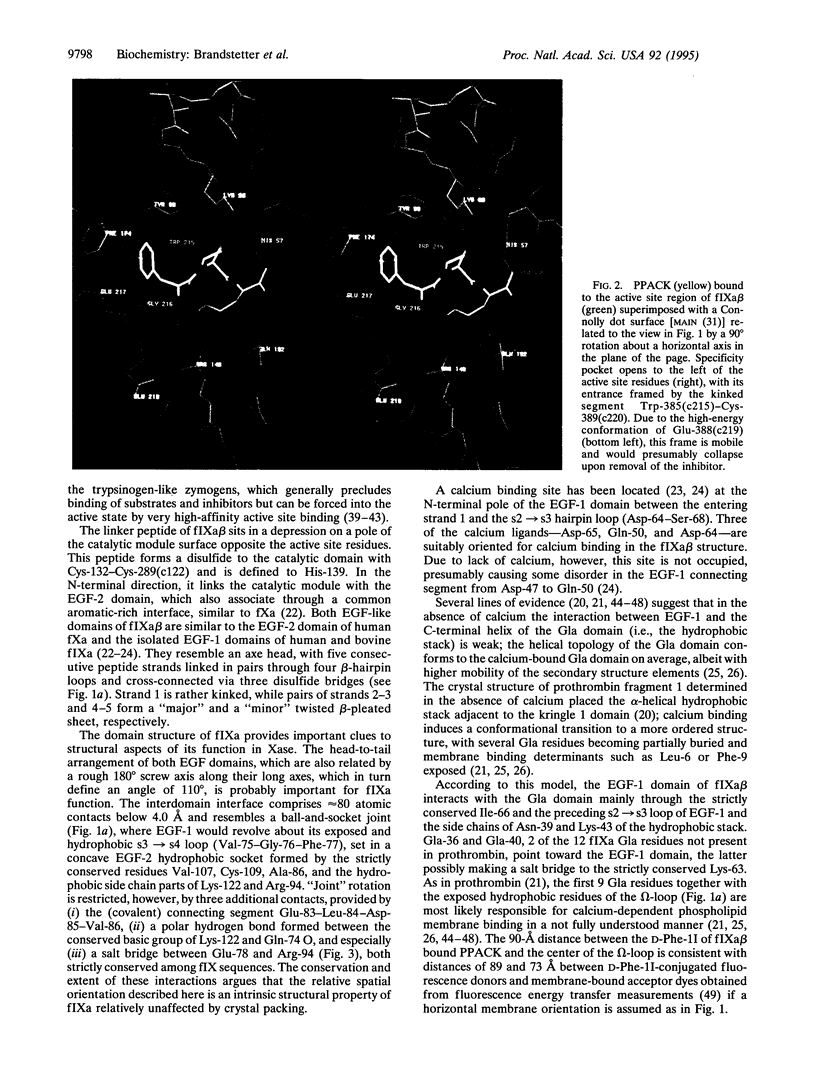
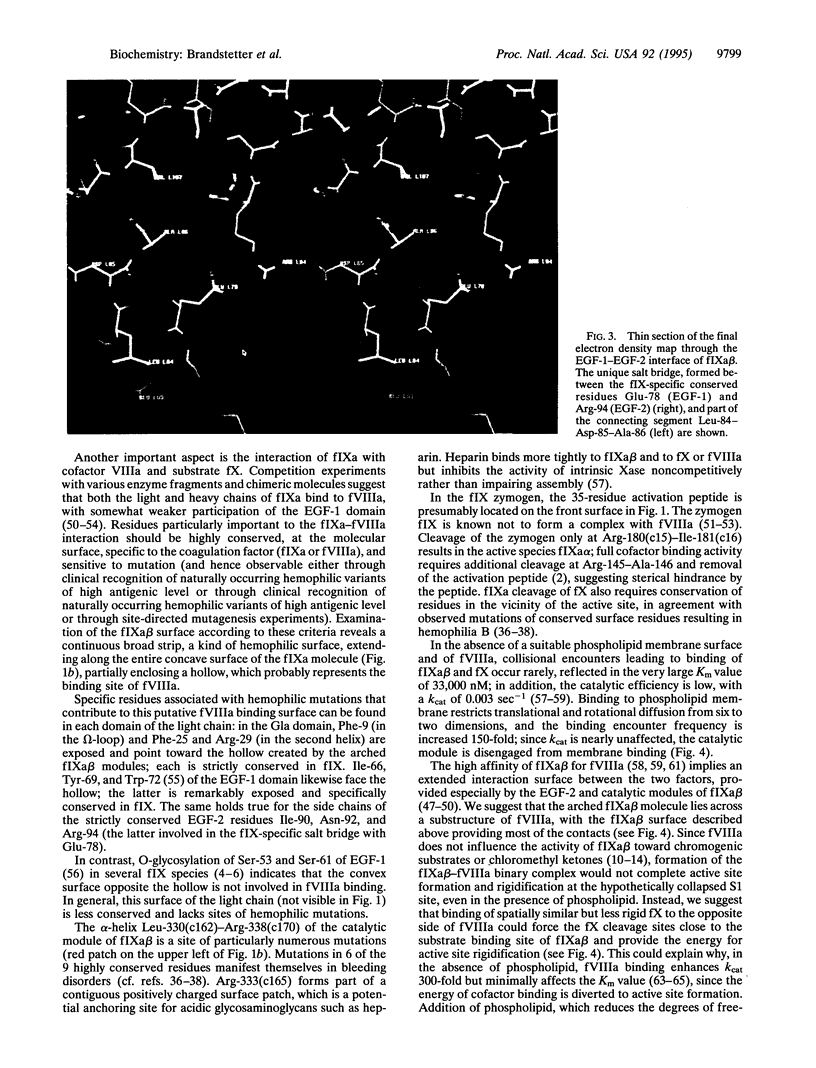
Hereditary deficiency of factor IXa (fIXa), a key enzyme in blood coagulation, causes hemophilia B, a severe X chromosome-linked bleeding disorder afflicting 1 in 30,000 males; clinical studies have identified nearly 500 deleterious variants. The x-ray structure of porcine fIXa described here shows the atomic origins of the disease, while the spatial distribution of mutation sites suggests a structural model for factor X activation by phospholipid-bound fIXa and cofactor VIIIa. The 3.0-A-resolution diffraction data clearly show the structures of the serine proteinase module and the two preceding epidermal growth factor (EGF)-like modules; the N-terminal Gla module is partially disordered. The catalytic module, with covalent inhibitor D-Phe-1I-Pro-2I-Arg-3I chloromethyl ketone, most closely resembles fXa but differs significantly at several positions. Particularly noteworthy is the strained conformation of Glu-388, a residue strictly conserved in known fIXa sequences but conserved as Gly among other trypsin-like serine proteinases. Flexibility apparent in electron density together with modeling studies suggests that this may cause incomplete active site formation, even after zymogen, and hence the low catalytic activity of fIXa. The principal axes of the oblong EGF-like domains define an angle of 110 degrees, stabilized by a strictly conserved and fIX-specific interdomain salt bridge. The disorder of the Gla module, whose hydrophobic helix is apparent in electron density, can be attributed to the absence of calcium in the crystals; we have modeled the Gla module in its calcium form by using prothrombin fragment 1. The arched module arrangement agrees with fluorescence energy transfer experiments. Most hemophilic mutation sites of surface fIX residues occur on the concave surface of the bent molecule and suggest a plausible model for the membrane-bound ternary fIXa-FVIIIa-fX complex structure: fIXa and an equivalently arranged fX arch across an underlying fVIIIa subdomain from opposite sides; the stabilizing fVIIIa interactions force the catalytic modules together, completing fIXa active site formation and catalytic enhancement.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S. S., Rawala-Sheikh R., Cheung W. F., Stafford D. W., Walsh P. N. The role of the first growth factor domain of human factor IXa in binding to platelets and in factor X activation. J Biol Chem. 1992 Apr 25;267(12):8571–8576. [PubMed] [Google Scholar]
- Arni R. K., Padmanabhan K., Padmanabhan K. P., Wu T. P., Tulinsky A. Structures of the noncovalent complexes of human and bovine prothrombin fragment 2 with human PPACK-thrombin. Biochemistry. 1993 May 11;32(18):4727–4737. doi: 10.1021/bi00069a006. [DOI] [PubMed] [Google Scholar]
- Astermark J., Björk I., Ohlin A. K., Stenflo J. Structural requirements for Ca2+ binding to the gamma-carboxyglutamic acid and epidermal growth factor-like regions of factor IX. Studies using intact domains isolated from controlled proteolytic digests of bovine factor IX. J Biol Chem. 1991 Feb 5;266(4):2430–2437. [PubMed] [Google Scholar]
- Astermark J., Hogg P. J., Björk I., Stenflo J. Effects of gamma-carboxyglutamic acid and epidermal growth factor-like modules of factor IX on factor X activation. Studies using proteolytic fragments of bovine factor IX. J Biol Chem. 1992 Feb 15;267(5):3249–3256. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Maki S. L. A monoclonal antibody to factor IX that inhibits the factor VIII:Ca potentiation of factor X activation. J Biol Chem. 1985 Sep 25;260(21):11574–11580. [PubMed] [Google Scholar]
- Baron M., Norman D. G., Harvey T. S., Handford P. A., Mayhew M., Tse A. G., Brownlee G. G., Campbell I. D. The three-dimensional structure of the first EGF-like module of human factor IX: comparison with EGF and TGF-alpha. Protein Sci. 1992 Jan;1(1):81–90. doi: 10.1002/pro.5560010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow R. T., Parker E. T., Krishnaswamy S., Lollar P. Inhibition by heparin of the human blood coagulation intrinsic pathway factor X activator. J Biol Chem. 1994 Oct 28;269(43):26796–26800. [PubMed] [Google Scholar]
- Bode W., Mayr I., Baumann U., Huber R., Stone S. R., Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Schwager P., Huber R. The transition of bovine trypsinogen to a trypsin-like state upon strong ligand binding. The refined crystal structures of the bovine trypsinogen-pancreatic trypsin inhibitor complex and of its ternary complex with Ile-Val at 1.9 A resolution. J Mol Biol. 1978 Jan 5;118(1):99–112. doi: 10.1016/0022-2836(78)90246-2. [DOI] [PubMed] [Google Scholar]
- Bode W. The transition of bovine trypsinogen to a trypsin-like state upon strong ligand binding. II. The binding of the pancreatic trypsin inhibitor and of isoleucine-valine and of sequentially related peptides to trypsinogen and to p-guanidinobenzoate-trypsinogen. J Mol Biol. 1979 Feb 5;127(4):357–374. doi: 10.1016/0022-2836(79)90227-4. [DOI] [PubMed] [Google Scholar]
- Bode W., Turk D., Karshikov A. The refined 1.9-A X-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human alpha-thrombin: structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. 1992 Apr;1(4):426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Ii S., Yoon H. S., Phillips J. A., 3rd, Sommer S. S. Missense mutations and evolutionary conservation of amino acids: evidence that many of the amino acids in factor IX function as "spacer" elements. Am J Hum Genet. 1991 Oct;49(4):820–838. [PMC free article] [PubMed] [Google Scholar]
- Butenas S., Orfeo T., Lawson J. H., Mann K. G. Aminonaphthalenesulfonamides, a new class of modifiable fluorescent detecting groups and their use in substrates for serine protease enzymes. Biochemistry. 1992 Jun 16;31(23):5399–5411. doi: 10.1021/bi00138a023. [DOI] [PubMed] [Google Scholar]
- Byrne R., Link R. P., Castellino F. J. A kinetic evaluation of activated bovine blood coagulation factor IX toward synthetic substrates. J Biol Chem. 1980 Jun 10;255(11):5336–5341. [PubMed] [Google Scholar]
- Castillo M. J., Kurachi K., Nishino N., Ohkubo I., Powers J. C. Reactivity of bovine blood coagulation factor IXa beta, factor Xa beta, and factor XIa toward fluorogenic peptides containing the activation site sequences of bovine factor IX and factor X. Biochemistry. 1983 Mar 1;22(5):1021–1029. doi: 10.1021/bi00274a004. [DOI] [PubMed] [Google Scholar]
- Cheung W. F., Hamaguchi N., Smith K. J., Stafford D. W. The binding of human factor IX to endothelial cells is mediated by residues 3-11. J Biol Chem. 1992 Oct 15;267(29):20529–20531. [PubMed] [Google Scholar]
- Duffy E. J., Lollar P. Intrinsic pathway activation of factor X and its activation peptide-deficient derivative, factor Xdes-143-191. J Biol Chem. 1992 Apr 15;267(11):7821–7827. [PubMed] [Google Scholar]
- Duffy E. J., Parker E. T., Mutucumarana V. P., Johnson A. E., Lollar P. Binding of factor VIIIa and factor VIII to factor IXa on phospholipid vesicles. J Biol Chem. 1992 Aug 25;267(24):17006–17011. [PubMed] [Google Scholar]
- Freedman S. J., Furie B. C., Furie B., Baleja J. D. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995 Apr 7;270(14):7980–7987. doi: 10.1074/jbc.270.14.7980. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Legaz M. E., Davie E. W. The mechanism of activation of bovine factor X (Stuart factor) by intrinsic and extrinsic pathways. Biochemistry. 1974 Dec 17;13(26):5290–5299. doi: 10.1021/bi00723a006. [DOI] [PubMed] [Google Scholar]
- Giannelli F., Green P. M., Sommer S. S., Lillicrap D. P., Ludwig M., Schwaab R., Reitsma P. H., Goossens M., Yoshioka A., Brownlee G. G. Haemophilia B: database of point mutations and short additions and deletions, fifth edition, 1994. Nucleic Acids Res. 1994 Sep;22(17):3534–3546. doi: 10.1093/nar/22.17.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi N., Roberts H., Stafford D. W. Mutations in the catalytic domain of factor IX that are related to the subclass hemophilia Bm. Biochemistry. 1993 Jun 29;32(25):6324–6329. doi: 10.1021/bi00076a004. [DOI] [PubMed] [Google Scholar]
- Hase S., Kawabata S., Nishimura H., Takeya H., Sueyoshi T., Miyata T., Iwanaga S., Takao T., Shimonishi Y., Ikenaka T. A new trisaccharide sugar chain linked to a serine residue in bovine blood coagulation factors VII and IX. J Biochem. 1988 Dec;104(6):867–868. doi: 10.1093/oxfordjournals.jbchem.a122571. [DOI] [PubMed] [Google Scholar]
- Hertzberg M. S., Ben-Tal O., Furie B., Furie B. C. Construction, expression, and characterization of a chimera of factor IX and factor X. The role of the second epidermal growth factor domain and serine protease domain in factor Va binding. J Biol Chem. 1992 Jul 25;267(21):14759–14766. [PubMed] [Google Scholar]
- Hughes P. E., Morgan G., Rooney E. K., Brownlee G. G., Handford P. Tyrosine 69 of the first epidermal growth factor-like domain of human factor IX is essential for clotting activity. J Biol Chem. 1993 Aug 25;268(24):17727–17733. [PubMed] [Google Scholar]
- Jacobs M., Freedman S. J., Furie B. C., Furie B. Membrane binding properties of the factor IX gamma-carboxyglutamic acid-rich domain prepared by chemical synthesis. J Biol Chem. 1994 Oct 14;269(41):25494–25501. [PubMed] [Google Scholar]
- Kam C. M., Kerrigan J. E., Plaskon R. R., Duffy E. J., Lollar P., Suddath F. L., Powers J. C. Mechanism-based isocoumarin inhibitors for blood coagulation serine proteases. Effect of the 7-substituent in 7-amino-4-chloro-3-(isothioureidoalkoxy)isocoumarins on inhibitory and anticoagulant potency. J Med Chem. 1994 Apr 29;37(9):1298–1306. doi: 10.1021/jm00035a009. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Smith K. J., McMullen B. A. Proteolytic inactivation of blood coagulation factor IX by thrombin. Blood. 1985 Dec;66(6):1302–1308. [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Kurachi S., Furukawa M., Yao S. N. Biology of factor IX. Blood Coagul Fibrinolysis. 1993 Dec;4(6):953–973. [PubMed] [Google Scholar]
- Lin S. W., Smith K. J., Welsch D., Stafford D. W. Expression and characterization of human factor IX and factor IX-factor X chimeras in mouse C127 cells. J Biol Chem. 1990 Jan 5;265(1):144–150. [PubMed] [Google Scholar]
- Lollar P., Fass D. N. Inhibition of activated porcine factor IX by dansyl-glutamyl-glycyl-arginyl-chloromethylketone. Arch Biochem Biophys. 1984 Sep;233(2):438–446. doi: 10.1016/0003-9861(84)90465-x. [DOI] [PubMed] [Google Scholar]
- Lollar P., Knutson G. J., Fass D. N. Stabilization of thrombin-activated porcine factor VIII:C by factor IXa phospholipid. Blood. 1984 Jun;63(6):1303–1308. [PubMed] [Google Scholar]
- Lollar P., Parker C. G., Kajenski P. J., Litwiller R. D., Fass D. N. Degradation of coagulation proteins by an enzyme from Malayan pit viper (Akistrodon rhodostoma) venom. Biochemistry. 1987 Dec 1;26(24):7627–7636. doi: 10.1021/bi00398a015. [DOI] [PubMed] [Google Scholar]
- McRae B. J., Kurachi K., Heimark R. L., Fujikawa K., Davie E. W., Powers J. C. Mapping the active sites of bovine thrombin, factor IXa, factor Xa, factor XIa, factor XIIa, plasma kallikrein, and trypsin with amino acid and peptide thioesters: development of new sensitive substrates. Biochemistry. 1981 Dec 8;20(25):7196–7206. doi: 10.1021/bi00528a022. [DOI] [PubMed] [Google Scholar]
- Medved L. V., Vysotchin A., Ingham K. C. Ca(2+)-dependent interactions between Gla and EGF domains in human coagulation factor IX. Biochemistry. 1994 Jan 18;33(2):478–485. doi: 10.1021/bi00168a012. [DOI] [PubMed] [Google Scholar]
- Mutucumarana V. P., Duffy E. J., Lollar P., Johnson A. E. The active site of factor IXa is located far above the membrane surface and its conformation is altered upon association with factor VIIIa. A fluorescence study. J Biol Chem. 1992 Aug 25;267(24):17012–17021. [PubMed] [Google Scholar]
- Nishimura H., Takao T., Hase S., Shimonishi Y., Iwanaga S. Human factor IX has a tetrasaccharide O-glycosidically linked to serine 61 through the fucose residue. J Biol Chem. 1992 Sep 5;267(25):17520–17525. [PubMed] [Google Scholar]
- Nishimura H., Takeya H., Miyata T., Suehiro K., Okamura T., Niho Y., Iwanaga S. Factor IX Fukuoka. Substitution of ASN92 by His in the second epidermal growth factor-like domain results in defective interaction with factors VIIa/X. J Biol Chem. 1993 Nov 15;268(32):24041–24046. [PubMed] [Google Scholar]
- Padmanabhan K., Padmanabhan K. P., Tulinsky A., Park C. H., Bode W., Huber R., Blankenship D. T., Cardin A. D., Kisiel W. Structure of human des(1-45) factor Xa at 2.2 A resolution. J Mol Biol. 1993 Aug 5;232(3):947–966. doi: 10.1006/jmbi.1993.1441. [DOI] [PubMed] [Google Scholar]
- Roberts H. R. Molecular biology of hemophilia B. Thromb Haemost. 1993 Jul 1;70(1):1–9. [PubMed] [Google Scholar]
- Rydel T. J., Ravichandran K. G., Tulinsky A., Bode W., Huber R., Roitsch C., Fenton J. W., 2nd The structure of a complex of recombinant hirudin and human alpha-thrombin. Science. 1990 Jul 20;249(4966):277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Koeberl D. D., Sommer S. S. Direct sequencing of the activation peptide and the catalytic domain of the factor IX gene in six species. Genomics. 1990 Jan;6(1):133–143. doi: 10.1016/0888-7543(90)90458-7. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Turner R. T., Bolander M. E. Restriction-site PCR: a direct method of unknown sequence retrieval adjacent to a known locus by using universal primers. PCR Methods Appl. 1993 May;2(4):318–322. doi: 10.1101/gr.2.4.318. [DOI] [PubMed] [Google Scholar]
- Selander-Sunnerhagen M., Ullner M., Persson E., Teleman O., Stenflo J., Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992 Sep 25;267(27):19642–19649. doi: 10.2210/pdb1ccf/pdb. [DOI] [PubMed] [Google Scholar]
- Seshadri T. P., Tulinsky A., Skrzypczak-Jankun E., Park C. H. Structure of bovine prothrombin fragment 1 refined at 2.25 A resolution. J Mol Biol. 1991 Jul 20;220(2):481–494. doi: 10.1016/0022-2836(91)90025-2. [DOI] [PubMed] [Google Scholar]
- Soriano-Garcia M., Padmanabhan K., de Vos A. M., Tulinsky A. The Ca2+ ion and membrane binding structure of the Gla domain of Ca-prothrombin fragment 1. Biochemistry. 1992 Mar 10;31(9):2554–2566. doi: 10.1021/bi00124a016. [DOI] [PubMed] [Google Scholar]
- Stoylova S., Mann K. G., Brisson A. Structure of membrane-bound human factor Va. FEBS Lett. 1994 Sep 12;351(3):330–334. doi: 10.1016/0014-5793(94)00881-7. [DOI] [PubMed] [Google Scholar]
- Sunnerhagen M., Forsén S., Hoffrén A. M., Drakenberg T., Teleman O., Stenflo J. Structure of the Ca(2+)-free Gla domain sheds light on membrane binding of blood coagulation proteins. Nat Struct Biol. 1995 Jun;2(6):504–509. doi: 10.1038/nsb0695-504. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi J., Padmanabhan K. P., Mann K. G., Tulinsky A. The isomorphous structures of prethrombin2, hirugen-, and PPACK-thrombin: changes accompanying activation and exosite binding to thrombin. Protein Sci. 1994 Dec;3(12):2254–2271. doi: 10.1002/pro.5560031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacey A. I., Krawczak M., Kakkar V. V., Cooper D. N. Determinants of the factor IX mutational spectrum in haemophilia B: an analysis of missense mutations using a multi-domain molecular model of the activated protein. Hum Genet. 1994 Dec;94(6):594–608. doi: 10.1007/BF00206951. [DOI] [PubMed] [Google Scholar]
- Wang D., Bode W., Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A resolution. J Mol Biol. 1985 Oct 5;185(3):595–624. doi: 10.1016/0022-2836(85)90074-9. [DOI] [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., Tans G., Rosing J., Hemker H. C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981 Apr 10;256(7):3433–3442. [PubMed] [Google Scholar]
- van Dieijen G., van Rijn J. L., Govers-Riemslag J. W., Hemker H. C., Rosing J. Assembly of the intrinsic factor X activating complex--interactions between factor IXa, factor VIIIa and phospholipid. Thromb Haemost. 1985 Jun 24;53(3):396–400. [PubMed] [Google Scholar]