Abstract
Multiple forms of candidiasis are clinically important in humans. Established murine models of disseminated, oropharyngeal, vaginal, and cutaneous candidiasis caused by Candida albicans are described in this unit. Detailed materials and methods for C. albicans growth and detection are also described.
Keywords: Candida albicans, mouse model, fungal infection
INTRODUCTION
The fungus Candida albicans and other related Candida species are commensal organisms in healthy humans but opportunistic pathogens in immunocompromised patients. Mucocutaneous presentations include oropharyngeal candidiasis (OPC) involving the mouth, pharynx, and esophagus; cutaneous or dermal candidiasis involving the skin; and vaginal candidiasis involving the female genital mucosa. Disseminated disease is a serious problem in hospital settings, occurring when Candida breaches an epithelial or skin barrier, for example via an intravenous catheter or abdominal surgery.
Understanding of both the host response and the mechanisms of fungal virulence has been aided by the development of animal models that recapitulate human disease with remarkable fidelity. These models allow researchers to probe the main host immune components by use of genetically modified mice (knockouts and transgenics) or mice treated with depleting antibodies. In addition, requirements for microbial virulence can be probed using different strains of Candida, either from clinical isolates or genetically modified laboratory strains. Together, these provide a powerful tool to dissect the interplay between pathogen and host.
The main mouse models used to recapitulate human candidiasis are discussed in this unit. Other excellent articles on this topic can also be found in the literature (Clancy et al., 2009; Yano and Fidel, 2011; Solis and Filler, 2012). C. albicans is used in each system described here, as relatively little is known about other Candida species in human or experimental models of infection. C. albicans does not normally colonize the mucosa of mice, but under various conditions of immunosuppression, disease can be induced, which is discussed for each model in the following sections.
Basic Protocol 1 describes a method for inducing disseminated candidiasis in adult mice. Support Protocol 1 describes how to grow and maintain cultures of C albicans. The Alternate Protocol describes a method for inducing gastric disseminated candidiasis in adult immunocompromised BALB/c mice. Basic Protocol 2 provides a method for inducing oropharyngeal candidiasis in mice. Immunosuppression with cortisone acetate (Support Protocol 2) renders wild-type mice susceptible to OPC, with consistent oral fungal burden, as well as histological and visual evidence of invasive hyphal formation on the oral mucosa. Basic Protocol 3 describes a model of vaginal candidiasis in estrogenized CBA/J mice. Basic Protocol 4 describes a model for cutaneous candidiasis in adult C57BL/6 mice. Finally, Support Protocol 3 describes the periodic acid–Schiff (PAS) staining method for fungi in infected tissues used to visualize Candida.
BASIC PROTOCOL 1: DISSEMINATED CANDIDIASIS IN ADULT MICE
Blood-borne candidiasis is the 4th most common hospital-acquired infection, caused by perturbation of physical barriers such as intravenous catheters. The mortality rate for disseminated candidiasis in humans averages 40%, and may reach as high as 80%. Disseminated candidiasis caused by intravenous injection of C. albicans in mice closely mimics human disseminated C. albicans infection (Odds, 1998; Spellberg et al., 2005). Following intravenous injection of C. albicans into the bloodstream, the fungus disseminates to multiple organs. Kidneys are the principal infection site, with progressive infection occuring over a range of inoculum doses (MacCallum and Odds, 2005). Additional tissue can be collected, including brain, spleen, liver, lungs, heart, and peripheral blood for fungal burden determination. Intravenous injection of C. albicans is lethal in adult mice, with the cause of death attributed to fungal sepsis, depending on mouse strain. This protocol describes disseminated candidiasis in adult mice.
Materials
C. albicans blastospores, SC5314 or CAF2-1 strain (Support Protocol 1)
Phosphate-buffered saline (APPENDIX 2A), sterile
70% ethanol in a spray bottle
6- to 8-week-old C57BL/6 mice
Tissue homogenizer (GentleMACS Dissociator, Miltenyi Biotec)
YPD agar (BD Difco) 100 mm × 15 mm plates (optionally containing 50 μg/ml ampicillin)
1-ml tuberculin syringes
27-G, 1/2-in. needles
Dissecting pins
Sterile dissecting tools
Scale for weighing organs
GentleMACS C-tubes (Miltenyi Biotec)
Additional reagents and equipment for preparing Candida suspension (Support Protocol 1), injection of mice (UNIT 1.6), euthanasia of mice (UNIT 1.8), and PAS staining (Support Protocol 3)
Inoculate mice
-
1
Prepare C. albicans blastospore cell suspension of chosen strain at desired concentration (Support Protocol 1) in PBS.
Inoculum concentrations will depend on the experiment objectives and should be researched prior to proceeding with experiments. A typical working range is 1 × 105 to 1 × 106 viable blastospores per mouse. -
2
Inject the inoculum intravenously via the lateral or dorsal tail vein (UNIT 1.6) using a 1-ml tuberculin syringe and a 27-G, 1/2-in. needle.
Standard injection volumes range from 100 to 200 μl. Ensure inoculum is mixed well before injection.
Monitor mice
-
3
Observe mice daily for disease symptoms.
These include weight loss, ruffled coat due to reduced grooming, increased/decreased movement, abnormal posture (e.g., hunched back), and trembling.For survival studies, sacrifice mice when they have reached a permissible percentage loss of body weight (normally 20% to 25%) or when the mice look moribund. Record date of death as the following day. For tissue burden and histology studies, sacrifice mice at a pre-determined end point.
Animal sacrifice, organ harvest, and fungal burden determination
-
4
Sacrifice mice by CO2 asphyxiation followed by cervical dislocation (UNIT 1.8).
-
5
Spray hands and clean tools with 70% ethanol after the handling of each mouse.
-
6
Wet the fur of the chest and abdomen with 70% ethanol.
-
7
Pin the euthanized mouse to a clean surface with the abdomen facing up.
-
8
Elevate the skin with sterilized forceps and cut along the ventral midline from the groin to the sternum.
-
9
Cut through the peritoneal muscle wall and membrane. Use blunt side of scissors to exclude the abdominal organs from the incision site.
-
10
Locate the kidneys on the dorsal wall of the abdominal cavity, lateral to the spine and great vessels. Remove both kidneys with forceps in a swift movement.
-
11
Weigh kidneys and store on ice in sterile PBS until processed.
-
12
Homogenize kidneys in 1 ml sterile PBS (per kidney) in a GentleMACS C-tube and store on ice until required.
-
13
Prepare 1:10 serial dilutions of the homogenates in sterile PBS. Plate 25 to 50 μl of each dilution in triplicate on YPD plates. Incubate at 30°C for 24 to 48 hr.
Optional: YPD plates containing antibiotics can be used to exclude the growth of bacteria. However, only C. albicans should be detected on antibiotic-free plates, as the internal organs are normally sterile environments free of bacteria.Sample dilutions will depend on the expected degree of fungal invasion and should be determined empirically. -
14
Count the number of colonies and calculate as colony forming units (CFU)/g tissue.
Organs may not be uniformly colonized by C. albicans, and so it is recommended that fungal burden and histology be performed on separate, whole organs. -
15
For histological analysis, stain tissue sections with PAS (see Support Protocol 3).
ALTERNATE PROTOCOL: GASTRIC DISSEMINATED CANDIDIASIS IN ADULT IMMUNOCOMPROMISED MICE
Without administration of immunosuppressing agents and antibiotics, healthy adult mice are resistant to gastrointestinal (GI) colonization and subsequent dissemination by C. albicans. The degree of persistence and the extent of fungal GI colonization may vary depending on the mouse strain and the C. albicans isolate. However, gnotobiotic athymic beige or cytokine-deficient mice can be productively infected without administration of immunocompromising agents. In this protocol, BALB/c mice are fed with vancomycin to reduce endogenous bacteria, and neutrophils are depleted by antibody administration (Bistoni et al., 1993).
Materials
Adult male and female BALB/c (H-2d) mice, 6 to 8 weeks old
0.2 mg/ml vancomycin in drinking water
C. albicans blastospores, 3153 serotype A (3153A; ATCC)
Phosphate-buffered saline (PBS; APPENDIX 2A)
RB6-8C5 MAb (anti-mouse Ly6G from BioXCell)
Cyclophosphamide (optional)
9-cm Saboraud’s dextrose plates (see recipe) containing 50 μg/ml chloramphenicol
24-G feeding needle (Popper & Sons) attached to a 1.0-ml syringe or 1-ml tuberculin syringe with a blunt-end 21-G needle tipped with polyethylene tubing (25-mm i.d.; 61-mm o.d.; Clay Adams)
Sterile dissecting tools including metal punch
Tissue homogenizer (Polytron-Kinematica)
Additional reagents and equipment for restraint of mice (UNIT 1.3), injection of mice (UNIT 1.6), and euthanasia of mice (UNIT 1.8)
Inoculate mice
-
1
Feed adult BALB/c mice 0.2 mg/ml vancomycin in their drinking water beginning 3 days before yeast inoculation and continue for the duration of the experiment.
Tetracycline (1 mg/ml) or clindamycin (0.24 mg/ml) are alternative antibiotics to decrease endogenous flora. -
2
Prepare a cell suspension of 2.0 × 108 C. albicans blastospores (strain 3153A) in 0.05 ml sterile PBS.
-
3
Inject mice intragastrically with blastospores as follows:
Restrain mice manually as described in (UNIT 1.3).
To facilitate intragastric delivery of the inoculum, extend the mouse’s neck to align the oropharynx with the esophagus.
Fill the 1-ml syringe of a feeding needle with the inoculum.
Introduce the feeding needle into the left diastema and gently push caudally toward the right ramus of the mandible.
-
When the mouse begins to swallow, gently insert the feeding needle or tube into the esophagus and introduce the inoculum.
The length of the feeding tube can be estimated by measuring the distance from the nose to the last rib.Alternatively, mice can be deprived of water for 8 hr and then orally inoculated by receiving water containing 106 cells/ml as their sole water source for 24 hr. For inoculation by feeding chow containing C. albicans, see Samonis et al. (1990).
-
4
Immediately following inoculation, inject mice intraperitoneally (UNIT 1.6) with 100 μg RB6-8C5 MAb in 0.2 ml PBS, to deplete neutrophils. Repeat at 1 and 2 weeks post-inoculation.
The RB6-8C5 MAb must be purchased as affinity-purified antibody. Alternatively, administer cyclophosphamide (100 mg/kg) intraperitoneally at 7-day intervals, 1 and 2 weeks prior to sacrifice.
Animal sacrifice, organ harvest, and fungal burden determination
-
5
Sacrifice mice at 4 weeks after fungal challenge by cervical dislocation (UNIT 1.8) and dissect under aseptic conditions. Remove the GI tract, rinse with sterile PBS, and separate the tongue, esophagus, stomach, small intestines, cecum, Peyer’s patches, and mesenteric lymph nodes. Also dissect the liver, spleen, lungs, and kidneys. Weigh each tissue before homogenization.
Stomach, esophagus, and intestines are the primary sites of colonization. Liver, spleen, lungs, and kidneys are removed for evaluation of fungal dissemination, if required by experimental design. Individual GI tissues and visceral organs can be homogenized separately, or two pooled homogenates can be prepared (one containing GI tissues, one containing visceral tissues). -
6
Using a Polytron homogenizer, homogenize tissues for 10 sec at high speed (~15,000 rpm) in 5.0 ml ice-cold sterile PBS in the following order: spleen, kidneys, liver, lungs, GI tract. For pooled homogenates, use 5.0 ml PBS; for individual organs, use 1 to 2 ml.
-
7
Plate 100 μl of tissue sample dilutions on 9-cm Sabouraud’s dextrose agar plates containing 50 μg/ml chloramphenicol. Incubate plates upside down at 37°C for 48 hr.
-
8
Count the number of colonies and calculate as colony forming units (CFU)/g tissue.
To avoid consideration of random growth of a few colonies, growth of >10 CFU per 0.1 ml tissue homogenate is considered indicative of fungal GI colonization or disseminated infection.
BASIC PROTOCOL 2: OROPHARYNGEAL CANDIDIASIS (OPC) IN MICE
Humans are typically colonized with Candida species in the oral cavity without pathology. However, immunodeficiency (especially of CD4+ cells as in HIV/AIDS) is associated with recurrent OPC (“thrush”; Glocker and Grimbacher, 2010). In contrast to humans, mice do not bear C. albicans as a commensal organism (Suegara et al., 1979; Iliev et al., 2012). However, similar to humans, wild-type adult mice are resistant to oropharyngeal colonization and disease with C. albicans (and other Candida species), exhibiting complete clearance of fungi within 3 to 4 days of oral inoculation with no evidence of oral mucosal plaque formation. Immunosuppression with cortisone acetate renders wild-type mice susceptible to OPC, with consistent oral fungal burden, histological and visual evidence of invasive hyphal formation on the oral mucosa (Fig. 19.6.1A), and an approximately 50% rate of dissemination (Kamai et al., 2001). Useful controls in this model are sham-infected mice or wild-type infected mice as negative controls. This protocol describes OPC in adult mice.
Figure 19.6.1.
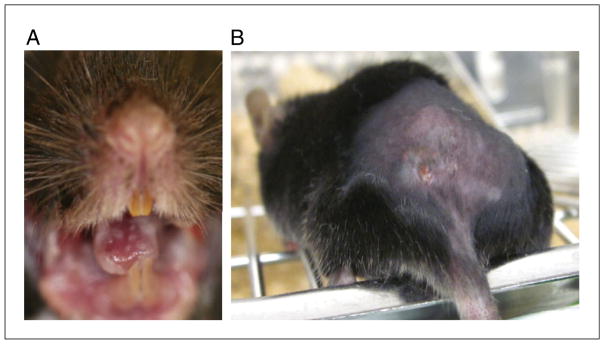
Mucocutaneous candidiasis in mice. (A) Image of oral plaques on a cortisone-acetate treated wild-type mouse. (B) Image of lesion that forms early in dermal candidiasis approximately 24 to 48 hr after injection.
Materials
Cortisone acetate suspension (Support Protocol 2)
0.05% (v/v) Tween 20/PBS: add 10 μl Tween 20 to 20 ml sterile PBS (see APPENDIX 2A for PBS)
C. albicans blastospore suspension, CAF2-1 strain (2 × 107 cells/ml; Support Protocol 1)
Phosphate-buffered saline (PBS; APPENDIX 2A), sterile
5- to 7-week-old C57BL/6 mice
Ketamine
Xylazine
0.9% (w/v) NaCl solution, sterile
YPD plates of C. albicans (Support Protocol 1)
Sterile lubricant eye ointment
10% neutral buffered formalin
RNALater (Life Technologies) or liquid N2
Scale for weighing mice and tissues
26-G needle
1-ml tuberculin syringe
Cotton plugs
Cotton-tip applicators, autoclaved (3 in., pack of 1000, Fisher)
Cage illuminated with heat lamp
Sterile dissecting tools including straight scissors and medium-point forceps
GentleMACS C-tubes (Miltenyi Biotec)
Straight razor blades
Additional reagents and equipment for preparing cortisone acetate suspension (Support Protocol 2), injection of mice (UNIT 1.6), euthanasia of mice (UNIT 1.8), and processing the kidneys for yeast infection (Basic Protocol 1)
Immunosuppress positive control mice [every 48 hr starting on “day minus one” (day –1) with day 0 being time of infection]
-
1
Prepare cortisone acetate suspension of 22.5 mg/ml in 0.05% Tween 20/PBS (see Support Protocol 2) in advance and store at −80°C.
-
2
Weigh the mice. Immediately prior to injection, load a 26-G needle attached to a 1-ml tuberculin syringe with the resuspended 22.5 mg/ml cortisone acetate suspension at a volume of 10 μl/g of mouse.
-
3
Scruff the mouse caudal to the nape of the neck and inject the mouse subcutaneously (UNIT 1.6).
Inoculate mice
-
4
Prepare cotton balls from cotton plugs, excluding strings securing plugs. Lightly roll cotton to form a ball, weighing 0.0025 g (range 0.0023 to 0.0027 g). Package in aluminum foil and autoclave prior to use.
-
5
Prepare C. albicans suspension in PBS immediately prior to inoculation procedure with a final cell density of 2 × 107 cells/ml (see Support Protocol 1). Prepare sterile microcentrifuge tubes with sterile PBS for sham controls and pre-infection swab of the oral cavity.
-
6
Administer ketamine/xylazine anesthetic intraperitoneally (UNIT 1.6) to each mouse several minutes before the inoculation.
Anesthesia with ketamine (100 mg/kg of mouse) and xylazine (10 mg/kg of mouse) administered intraperitoneally provides approximately 1 hr of anesthesia, followed by an additional 30 min of decreased oral function. The anesthetic agents are diluted in 0.9% sterile solution to yield final volumes of 100 to 200 μl per mouse and administered with 26-G needles on 1-ml tuberculin syringes. -
7
To assess for pre-existing colonization, moisten a sterile cotton-tipped swab in sterile PBS and swab the oral cavity. Streak the cotton-tipped swab on a YPD plate, then discard the swab.
Plates are incubated at 30°C for 2 days and colonies enumerated (see Support Protocol 1). -
8
Using forceps, saturate a cotton ball in the C. albicans blastospore suspension (shaken well to resuspend) or PBS and insert sublingually in the mouse. Keep cotton ball in place for 75 min.
-
9
Apply sterile lubricant eye ointment to both eyes with a sterile cotton tip applicator.
-
10
Administer 1 ml of 0.9% sterile NaCl subcutaneously in two locations on the back to rehydrate the mouse.
-
11
Place the mouse in a cage illuminated with a heat lamp at a distance of approximately 4 feet.
-
12
Monitor animals for level of anesthesia throughout the procedure. If mice regain motor function more than 30 min prior to the end of the procedure, administer booster anesthetic intraperitoneally (UNIT 1.6) with 50 mg ketamine/kg of mouse, diluted in sterile 0.9% NaCl.
-
13
At 75 min, remove the cotton ball from the mouth with forceps. Administer an additional 1 ml of sterile 0.9% NaCl subcutaneously in two locations on the back.
-
14
Return mice to the cage and monitor until motor function has fully recovered.
-
15
Monitor mice daily for weight loss, activity level, appearance, and grooming habits.
Moribund mice are sacrificed immediately, including mice with impaired ambulation, greater than 25% weight loss, lethargy, difficulty breathing, or neurological disturbances.
Animal euthanasia, organ harvest, and fungal burden determination
-
16
Sacrifice mice (UNIT 1.6) and dissect, homogenize, and plate out kidney as in Basic Protocol 1, steps 1 to 14.
-
17
Open the jaw and dissect tongue with scissors.
The tongue tissue can be divided longitudinally at the midline with a razor blade into two specimens, weighed, and placed in the appropriate fluid. For histology, tissue is placed in 1 ml 10% neutral buffered formalin on ice. For RT-PCR, tissue is placed in 1 ml RNALater on ice or flash frozen in liquid nitrogen. For fungal burden, tissue is homogenized in 0.5 ml sterile PBS in a gentleMACS C-tube on ice, and plated similarly to the kidney tissue in Basic Protocol 1, steps 13 to 14.Additional tissue may also be collected at this time, including peripheral blood, spleen, brain, liver, salivary glands and cervical lymph nodes for other studies.
BASIC PROTOCOL 3: VAGINAL CANDIDIASIS IN MICE
Recurrent vulvovaginal candidiasis (VVC) is a common fungal infection in women, and can be a problem even in the absence of evident immunodeficiency (Yano and Fidel, 2011). Wild-type mice are resistant to VVC, but susceptibility can be induced under conditions of pseudoestrus. Persistent vaginal infection is found in estrogenized mice after vaginal challenge with C. albicans. This protocol describes a model of vaginal candidiasis in estrogenized CBA/J mice.
Materials
Female CBA/J (J-2α) mice, 8 to 10 weeks of age
Estradiol valerate (Sigma, cat. no. E1631) dissolved in sesame oil (Sigma, cat. no. S3547) immediately before use
C. albicans blastospores, 3153 serotype A (3153A; ATCC) (Support Protocol 1)
Phosphate-buffered saline (PBS; APPENDIX 2A), sterile
70% ethanol
Refrigerated centrifuge
Sterile filter (0.22-μm)
Dissecting equipment, including curved forceps
Additional reagents and equipment for injection of mice (UNIT 1.6), preparation of C. albicans suspension (Support Protocol 1), anesthesia of mice (Basic Protocol 2), determination of fungal burden (Basic Protocol 1, steps 13 to 14), paraffin sectioning (UNIT 21.4), and PAS staining (Support Protocol 3)
Inject female CBA/J mice subcutaneously in the lower abdomen (UNIT 1.6) with 0.1 to 0.5 mg estradiol valerate in 20 to 100 μl sesame oil 3 to 6 days prior to vaginal inoculation, and repeat weekly until completion of the study (see Anticipated Results).
Prepare a cell suspension of 2.5 × 106 (or a desired inoculum in the range of 5 × 104- to 5 × 108) C. albicans blastospores from stationary-phase culture in 0.02 ml sterile PBS (see Support Protocol 1).
Inoculate 0.02 ml yeast cell suspension or sterile PBS (for negative-control mice) into the vagina using a micropipettor and sterile tips. Insert the tip to the end of the vaginal lumen, close to the cervix. Perform inoculation under anesthesia (Basic Protocol 2) or rapidly to minimize mouse distress.
Perform vaginal lavage on anesthetized mice (for serial measurements on days 4, 7, and 10 post-inoculation) or euthanized mice (for terminal measurement). To do this, wash the vagina by aspirating 100 μl PBS back and forth several times. Avoid trauma to the wall of the lumen if performing serial measurements.
Determine fungal burden from vaginal lavage fluid as in Basic Protocol 1, steps 13 to 14.
Centrifuge the remaining vaginal lavage fluid 5 min at 300 × g, 5°C.
Filter sterilize supernatant vaginal lavage fluid and store at −70°C for ELISA or other future studies.
-
For histological examination, excise the vagina from a euthanized mouse:
Place the mouse on its back and spray the groin with 70% ethanol.
Expose the vaginal opening by angling the urinary orifice with forceps.
Insert curved forceps into the vaginal lumen and grasp the cervix.
Invert the vagina by pulling gently on the cervix.
-
Trim the vagina at the base of the opening and trim the cervix. Invert the tissue before sectioning (UNIT 21.4) for PAS staining (Support Protocol 3).
Smear preparations of vaginal lavage fluid (by Papanicolaou technique) can be prepared on a glass slide and stained to evaluate for PMNs. Wet mount preparations of vaginal lavage fluid can be evaluated for the presence of hyphae.
Collect lumbar lymph nodes to assess the systemic immune response to the inoculation.
BASIC PROTOCOL 4: DERMAL/CUTANEOUS CANDIDIASIS IN ADULT MICE
C. albicans is a commensal organism of the skin in healthy humans. Certain immunodeficiency syndromes predispose individuals to dermal candidiasis, including Hyper-IgE/Job’s Syndrome, Autoimmune Polyendocrinopathy-1 (APS1, deficiency in the AIRE gene), and various other rare mutations that impact the IL-17/Th17 pathway (Huppler et al., 2012). Dermal infection of healthy, adult mice with C. albicans can be established without use of immunosuppressive agents such as cortisone acetate. C. albicans is inoculated into the dorsal back skin of various strains of mice. Healing rates, fungal burden, leukocyte infiltration, and epidermal hyperplasia are the major readouts for disease susceptibility. This protocol describes cutaneous candidiasis in adult C57BL/6 mice.
Materials
5-7 week old C57BL/6 mice
C. albicans pseudohyphae, CAF2-1 strain (Support Protocol 1)
YPD medium (e.g., BD Difco) containing 10% (v/v) fetal bovine serum (FBS)
10% neutral buffered formalin
RNALater (Life Technologies) or liquid N2
Phosphate-buffered saline (PBS; APPENDIX 2A), sterile
Inverted microscope
Electric razor
Hemacytometer
1-ml tuberculin syringe and 25-G needle
Dissecting pins
Sterile dissecting tools including straight scissors and forceps4-mm punch biopsy tool
Pellet pestles (Sigma, cat. no. Z359947)
Additional reagents and equipment for preparation of Candida suspension (Support Protocol 1), injection of mice (UNIT 1.6), and sacrifice of mice and plating of organ homogenates (Basic Protocol 1),
Prepare mice for infection
-
1
Prepare mice by shaving the dorsal region at least 24 hr before injection. In preparation for inoculation, singly cage mice and designate mice for either the longitudinal clinical study or histology and fungal burden assays.
Inoculate mice
-
2
Prepare a C. albicans overnight culture as in Support Protocol 1. Adjust culture to inoculation densities of 5 × 104 cells/ml and 5 × 106 cells/ml in YPD with 10% FBS, incubate with shaking at 37°C for 2 hr, and confirm switch to pseudohyphae by microscopic examination using a hemacytometer, in order to confirm that at least 95% of blastospores have switched to the virulent pseudohyphal form.
-
3
Inject pseudohyphae in a 50-μl volume of PBS intradermally (UNIT 1.6) into the deep dermis and superficial fat of the shaved dorsal region of each mouse using 25-G needles. Inject a separate cohort of mice with PBS for sham control.
Proper injection will result in the formation of a wheal.For clinical scoring and fungal burden determination, 5 × 106 cells/ml should be inoculated, while an inoculum of 5 × 104 cells/ml is used to ensure recovery of intact skin tissue for histology.Attempts should be made to place the injection at the same location for each mouse to aid in determination of healing and harvesting tissue at the relevant tissue site.
Clinical course of C. albicans-infected mice
-
4
Assess mice visually and with photographs at least three times weekly at injection site for presence of nodules, ulceration, erythema, and crusting.
-
5
Score disease positively if any one of these four clinical features is present—mice are considered healed when the four disease parameters are absent.
Animal euthanasia, tissue harvest, and fungal burden determination
-
6
For the cohort of mice selected for histological evaluation and fungal burden determination, sacrifice mice on Day 4 as described in Basic Protocol 1.
-
7
Pin the euthanized mouse to a clean surface with the shaved dorsal region facing up.
-
8
Elevate the skin with sterilized forceps and snip the skin, place the scissors in the incision, and separate skin from the deeper layers by blunt dissection.
-
9
Cut through skin and remove the entire area of shaved skin.
-
10
Place tissue, epidermis side down, on firm, sterile surface and excise a 4-mm punch section of skin where injection site was located.
-
11
For histology, place tissue in 1 ml of 10% neutral buffered formalin. For RT-PCR, place tissue in 1 ml RNALater on ice or flash frozen in liquid nitrogen.
-
12
Weigh tissue section being used for fungal burden assay and place in 200 μl of PBS and homogenize with a pellet pestle on ice.
-
13
Plate homogenates as in Basic Protocol 1.
SUPPORT PROTOCOL 1: CANDIDA ALBICANS GROWTH AND MAINTENANCE
C. albicans can grow as a single cell (yeast) or as multicellular filamentous colonies (mycelium) composed of branching, septate hyphae ~2 to 10 μm in diameter (Fig. 19.6.1). In addition, the organism can assume a pseudohyphal morphology, wherein the cells resemble elongated blastospore cells and are linked together. Cultivation in cornmeal agar stimulates the formation of characteristic thick-walled chlamydospores. The optimal growth temperature is 25° to 37°C. Growth can be aerobic or anaerobic. Colonies are usually smooth white, but may become cream colored or tan with age. They are glabrous, creamy, or membranous and may have a fringe of submerged hyphae. Colony morphology, sugar utilization pattern, germ-tube formation, and serologic reactions are useful diagnostic procedures for C. albicans identification. For details on fungus growth, refer to Odds (1988).
Materials
Candida albicans [laboratory strain CAF2-1 (Conti et al., 2009) or SC5314 (ATCC no. MYA-2876)] or other strain as called for in protocol
YPD broth (BD Difco)
YPD agar (BD Difco) in 100 × 15–mm plates
15% (v/v) glycerol
Phosphate-buffered saline (PBS; APPENDIX 2A)
50-ml conical tubes, sterile (e.g., BD Falcon)
Centrifuge
Additional reagents and equipment for counting cells with a hemacytometer (APPENDIX 3A)
C. albicans maintenance
-
1
Grow C. albicans at 30°C in YPD broth for 15 to 24 hr with shaking or on YPD agar in 100 × 15–mm plates for 24 to 48 hr.
Store plates with C. albicans up to 2 weeks at 4°C. -
2
Prepare stock cultures at 1–5 × 107 cells/ml in 15% (v/v) glycerol in water and maintain for very long periods at −80°C or in liquid nitrogen.
Inoculum preparation
-
3
Inoculate 10 ml of YPD broth with 1 colony of C. albicans from a YPD culture plate (stored at 4°C).
-
4
Incubate the tube at 30°C with shaking for 14 to 18 hr (mucosal candidiasis) or 18 to 24 hr (disseminated candidiasis). Be consistent with culture time of C. albicans, as virulence can change depending on growth phase.
-
5
Transfer 1 ml (or appropriate volume) of overnight C. albicans culture to a sterile microcentrifuge tube.
-
6
Centrifuge cells 1 min at 800 × g, room temperature.
-
7
Discard supernatant and wash cells with sterile PBS 1 min at 800 × g, room temperature. Remove supernatant
-
8
Wash again as in step 7.
-
9
Discard supernatant and resuspend in 1 ml (or appropriate volume) sterile PBS for intravenous or mucocutaneous inoculation.
-
10
Count cells using a hemacytometer (APPENDIX 3A).
Cell concentration must be accurately calculated with hemacytometer for disseminated infection, since variation in inoculum doses can impact infectivity of C. albicans (MacCallum and Odds, 2005; Lionakis et al., 2011).Alternatively, cell density can be determined using diluted culture on a spectrophotometer. Optical density of C. albicans culture varies on different spectrophotometers, requiring titration on an individual machine compared to an initial visual determination of cell numbers. -
11
Dilute cells in sterile PBS to the desired inoculum concentration. Keep C. albicans suspension on ice until use. Aim to use inoculum immediately.
Once inoculum has been prepared, cell suspension can be counted to confirm inoculum concentration. -
12
Plate out 1:10 serial dilutions of C. albicans inoculum on YPD agar plates and incubate for 24 to 48 hr at 30°C. Count CFU to determine viable cell concentration.
SUPPORT PROTOCOL 2: PREPARATION OF CORTISONE ACETATE SUSPENSION
This protocol describes how to prepare the cortisone acetate suspension used in Basic Protocol 2.
Materials
Cortisone 21-acetate (Sigma)
0.05% Tween 20/PBS: add 10 μl Tween 20 to 20 ml sterile PBS (see APPENDIX 2A for PBS)
50-ml conical tube (BD Falcon)
Probe-type sonicator
In a 50-ml conical tube, freshly prepare 20 ml of 0.05% Tween 20/PBS.
Add 450 mg of cortisone 21-acetate powder to 20 ml 0.05% Tween 20/PBS and vortex briefly.
Sonicate the suspension on ice for 30 to 45 sec using a probe-type sonicator.
Immediately and quickly dispense 500-μl aliquots into pre-labeled, sterile 1.5 ml microcentrifuge tubes and store at −80°C.
SUPPORT PROTOCOL 3: PERIODIC ACID–SCHIFF STAINING OF TISSUE SECTIONS
Diagnostic procedures of fungal infections include the staining of fungi in infected tissues. Periodic acid–Schiff (PAS) stain is recommended for observing fungi in tissues and smears. Fungi stain specifically by virtue of the presence of polysaccharides and chitin in the fungal cell wall.
Materials
Fixed, paraffin-embedded, 4-μm tissue sections (e.g., with modified Bouin’s fixative; UNIT 15.9)
Xylol
95% and 100% (v/v) ethanol
-
Periodic acid–Schiff (PAS) stain (see recipe) consisting of:
1% periodic acid
Basic fuchsin solution
Zinc (or sodium) hydrosulfite solution
Light-green stain
Mounting medium
Place fixed, paraffin-embedded, 4-μm tissue sections in xylol for 15 min to deparaffinize.
Rinse quickly in 100% ethanol and then in distilled water.
Immerse in 1% (v/v) periodic acid for 10 min.
Rinse in tap water for 5 to 10 min.
Immerse in basic fuchsin solution for 2 min.
Rinse in tap water for 30 sec.
Immerse in zinc (or sodium) hydrosulfite solution for 30 min.
Rinse in tap water for 3 to 5 min.
Immerse in light-green stain for 2 min.
Rinse briefly in tap water.
Dehydrate using the following series of rinses: ~10 sec in 95% ethanol, 1 min in 100% ethanol, and twice for ~1 min in xylol.
-
Mount in mounting medium and examine by light microscopy.
The fungi stain bright red or purplish red after periodic acid hydrolysis, which releases aldehydes that can combine with Schiff reagent. The carbohydrates in the cell walls take the red stain as a result of the reaction.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see APPENDIX 5.
Periodic acid–Schiff (PAS) stain
Basic fuchsin solution
0.1 g basic fuchsin
5.0 g 95% (v/v) ethanol
95.0 ml H2O
Light-green stain
1.0 g light green
0.25 ml glacial acetic acid
100.0 ml 80% (v/v) ethanol
Zinc (or sodium) hydrosulfite solution
1.0 g zinc (or sodium) hydrosulfite
0.5 g tartaric acid
100.0 ml H2O
1% (v/v) aqueous solution of periodic acid
-
Do not allow to dry
Glacial acetic acid and periodic acid are stored at 4°C, and all other PAS components at room temperature. Storage times are per manufacturer’s recommendations.
Sabouraud’s dextrose plates
40 g glucose
10 g neopeptone or polypeptone (consisting of 5 g pancreatic digest of casein and 5 g peptic digest of animal tissue)
15 to 20 g agar
1 liter demineralized, distilled H2O
Final pH, 5.6 ± 0.2
Prepare suspension and mix well. While stirring constantly, heat to boiling point and boil for 1 min. Distribute and autoclave at 121°C for 15 min. Incubate overnight at 37°C for sterility control.
For plates: Reheat to melt and distribute on Petri dishes under sterile conditions. Add any desired antibiotics to melted agar that has cooled to ≤50°C. Store plates upside down (to avoid dehydration) for up to several days at 4°C.
COMMENTARY
Background Information
Disseminated candidiasis
The most severe form of disease caused by C. albicans is disseminated infection, which is often fatal. The intravenous challenge model of disseminated infection is commonly used to determine C. albicans strain virulence; it is also used to test antifungal drug efficacies, as well as the host immune response towards C. albicans (Noble et al., 2010; Szabo and MacCallum, 2011). Because a high inoculum of Candida is used, there is no direct human disease correlate to the disseminated model. However, the two protocols described above mimic the human disease that is normally caused by penetration of physical barriers by C. albicans, resulting from medical implants or direct penetration of the GI tract.
Mucocutaneous candidiasis
Mucosal models of both oral and dermal candidiasis have been developed that, although they are initiated with a high inoculum of fungus, recapitulate clinical parameters of human disease quite faithfully. These models have been valuable tools to demonstrate the various host immune components involved in protection to C. albicans (Conti et al., 2009; Kagami et al., 2010) as well as identify virulence genes associated with disease (Sun et al., 2010). Although not discussed here, a recent model of candidiasis induced by dentures has been described (Johnson et al., 2012).
Vulvovaginal candidiasis
Wild-type mice are not susceptible to VVC. The induction of an estrogenized state imparts susceptibility without perturbing the normal immune function of the mice. Although humans are susceptible without exogenous estrogen treatment, hormonal changes are known to influence resistance to mucosal colonization and predispose to recurrent vaginitis (Fidel and Sobel, 1996). Cutaneous candidiasis is common in immunocompromised patients as well, but can also infect warm, moist tissue even in healthy individuals.
Immunity to candidiasis
Significant advances in understanding host immunity to Candida infections have been made in the last 5 years. Most striking is recent data that demonstrate a strongly protective role for the Th17/IL-17 pathway in protection against candidiasis, particularly the mucocutaneous manifestation (Conti and Gaffen, 2010; Hernández-Santos and Gaffen, 2012). Mice deficient in formation of IL-17-producing cells or in IL-17 signaling are highly susceptible to disseminated, oral, and dermal candidiasis models (Huang et al., 2004; Conti et al., 2009; Ho et al., 2010; Kagami et al., 2010). These findings corroborate newer discoveries in humans in which patients with targeted genetic defects in these pathways present with chronic mucocutanous candidiasis (Puel et al., 2010, 2011; Huppler et al., 2012). Whereas IL-17/Th17 cells are protective in fungal infections, they are pathogenic in autoimmune settings, and efforts are underway to test the efficacy of biologic drugs targeting IL-17 and its receptor for use in autoimmunity (Patel et al., 2013). The protective role of IL-17 in fungal infection must therefore be considered as a risk factor in these autoimmune settings.
Critical Parameters and Troubleshooting
Disseminated candidiasis
When comparing responses against baseline levels, useful controls in the disseminated model are sham (PBS)–infected mice. Factors affecting susceptibility to infection include mouse strain (MacCallum and Odds, 2005; Carvalho et al., 2012), inoculum concentration (MacCallum and Odds, 2005; Lionakis et al., 2011), and C. albicans strain, which should be taken into consideration when planning experiments. Numerous studies have demonstrated considerable differences in virulence between clinical isolates during disseminated infection, which is believed to be at least partly due to differences in expression of virulence factors such as adhesion proteins and proteinases between strains (Chaffin et al., 1998; MacCallum et al., 2009). Furthermore, C. albicans strains of differing virulence have different effects on the immune response. The kidneys are the principal site of infection in the disseminated infection model and are commonly used as a readout of the severity of disease. However, C. albicans infects multiple organs to different degrees, which should be taken into consideration for both the acute disseminated and GI disseminated models.
Oral candidiasis
The standard OPC model is 5 days (Solis and Filler, 2012). However, at this time the bulk of the response is from the innate immune compartment (Bär et al., 2012; Hernández-Santos et al., 2013), and therefore this model is not useful for studying adaptive immunity. Harvesting organs at Day 2 is best suited for studies of gene expression or cellular influx, but even wild-type mice have a significant fungal load at this time. Harvest at Day 4 to 5 is most appropriate for comparative studies of fungal burden between mouse strains, since wild-type mice (at least those not subjected to immunodeficiency regimens) clear the fungus by this time point. This model can be adjusted to study the adaptive immune response—i.e., since wild-type mice recover fully, they can be retained for several weeks to allow time for an adaptive response to be generated and analyzed (Bär et al., 2012; Hernández-Santos et al., 2013).
Dermal candidiasis
The standard dermal model recommends monitoring mice three times a week for healing (Kagami et al., 2010). Depending on the knockout strain of mouse being tested, daily monitoring may be necessary. Fungal load is quite variable in this model, so fairly large cohorts may be needed to observe statistically significant differences.
Vulvovaginal candidiasis
Significant variation in fungal burden is frequently noted between mice in the vulvovaginal model. Therefore, larger cohorts of 7 to 10 mice may be needed for statistical purposes (Yano and Fidel, 2011). In addition, different strains of C. albicans result in different fungal burdens. Altering the inoculum may be necessary for strains that are less virulent than 3153A in this model (Taylor et al., 2000). Sham-inoculated, estrogen-treated mice are an important control cohort, due to the changes in vaginal flora observed with exogenous estrogen treatment in mice. Measurement of neutrophil numbers in lavage fluid is used as a surrogate for in-flammatory symptoms, since clinical symptoms such as itching or swelling cannot be assessed.
Anticipated Results
Disseminated candidiasis
Mice are injected intravenously with C. albicans via the lateral tail vein into the bloodstream. Infection spreads rapidly throughout the body following intravenous injection of C. albicans, with the fungus invading multiple organs such as the kidneys, brain, liver, lungs, and heart within hours of entry into the bloodstream, in line with progressive disseminated infection in humans. Fungal growth is rapidly controlled in most organs, but progressive infection occurs in the kidneys. Cause of death is attributed to fungal sepsis, with mice also developing renal failure shortly before death.
The degree of persistence of GI candidiasis varies with different clinical isolates of C. albicans as well as with mouse strain. The cardial-atrium fold of the gastric mucosa is the major site of colonization, with infection not easily detected in other GI areas. Similar histopathological lesions are observed in adult immuno-compromised BALB/c and DBA/2 mice with GI candidiasis.
Oral candidiasis
Wild-type mice inoculated orally with C. albicans exhibit mild weight loss and oral fungal burden without dissemination for the first 48 hr after inoculation. These transient symptoms are followed by weight gain and complete clearance of the yeast by days 3 to 4 after inoculation. Cortisone-treated wild-type mice are profoundly ill, with weight loss of 25% by day 4 to 5 after inoculation, decrease in grooming and activity, and oral fungal burdens of 1 × 105 to 5 × 106 CFU/g tongue tissue. On gross examination, the oral mucosa has thick, adherent white plaques on the tongue, palate, and buccal mucosa (Fig. 19.6.1A). Approximately 50% of cortisone-treated mice will show evidence of dissemination with detectable fungal burden in the kidneys, although this may be missed if only one kidney is sampled. Mice that are susceptible due to isolated specific genetic deletions often exhibit mild-moderate fungal burdens of around 1 × 104 CFU/g tongue tissue.
Dermal candidiasis
At the site of injection, a wound forms within 24 to 48 hr of inoculation with Candida albicans (see Fig. 19.6.1B). Typical lesions will first present as a nodule with ulceration and redness, with crusting occurring as the lesion heals. Wild-type mice inoculated with C. albicans dermally clear the organism completely by Day 20, with complete wound healing by this point.
Vulvovaginal candidiasis
Smear preparations of vaginal lavage fluid stained by Papanicolaou technique show that the majority of infiltrating leukocytes are neutrophils. Very few neutrophils are detected in untreated mice. Serial vaginal lavage does not alter colony counts, and vaginal colonization/infection with Candida persists for weeks in estrogen-treated inoculated mice. Untreated mice remain negative for Candida, and non-estrogen-treated inoculated mice fail to establish persistent vaginal colonization. Typical fungal burdens are 5 × 104 to 5 × 105 CFU/100 μl lavage fluid with states of consistent colonization. Lumbar lymph nodes may become enlarged in inoculated mice with up to 10-fold increase in leukocyte cellular recoveries compared to uninoculated mice.
Time Considerations
Candida culture
Setting up an overnight C. albicans culture takes 10 min. Allow 1 hr for generating the C. albicans inoculum.
Mouse injections (general)
The time taken to inject mice will depend on the number of mice and the efficiency of injections, but a conservative estimate is 1 hour for 10 mice.
GI and disseminated infections
In the presence of neutrophil-depleting MAbs, 3 days of vancomycin treatment are required prior to inoculation. For 20 mice, about 3 hr are required to prepare the suspension and perform intragastric inoculations and i.p. antibody injections. Antibody injections (30 min) are repeated at 1 and 2 weeks. Animals are sacrificed at 4 weeks. For acute disseminated infections, 2 hr are required to prepare the suspension and perform i.v. inoculations, and animals are sacrificed 1 to 2 days later.
OPC
Positive control mice are treated with cortisone starting 1 day prior to inoculation. It takes 2 hr to prepare the C. albicans suspension and inoculate 20 mice, followed by 75 min of monitoring of anesthetized mice prior to removal of the cotton ball from the oral cavity. Mice must be monitored until they recover from sedation, up to an additional 45 min. It takes approximately 4 hr to harvest tongues and kidneys and prepare homogenized organ cultures from 20 mice. Cultures are enumerated after 2 days of incubation.
Vaginal candidiasis
Mice are treated with estrogen for 3 days prior to inoculation. It takes 3 hr to prepare the suspension and perform 20 vaginal inoculations. It takes 3 hr to prepare lavage cultures, which are enumerated after 2 days of incubation.
Dermal candidiasis
Mice need to be shaved 24 hr before infection. Inoculum preparation is the same as in the other models, except extra time must be allotted to allow incubation of pseudohyphae. Intradermal injections do not require anesthesia, but speed depends on the skill of the experimenter to inject mice that are not sedated. In the dermal model, mice must be monitored and scored for healing for an extended period (minimum 3 to 4 weeks).
Acknowledgments
HRC was supported by NIH grant DE023293. S.L.G. was supported by NIH grants AR054389 and DE022550. A.R.H. was supported by the Children’s Hospital of Pittsburgh of UPMC and a Pediatric Infectious Disease Society Award (funded by the Stanley A. Plotkin Sanofi Pasteur Fellowship Award).
Footnotes
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Literature Cited
- Bär E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, LeibundGut-Landmann S. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol. 2012;188:5636–5643. doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- Bistoni F, Cenci E, Mencacci A, Schiaffella E, Mosci P, Puccetti P, Romani L. Mucosal and systemic T helper cell function after intragastric colonization of adult mice with Candida albicans. J Infect Dis. 1993;168:1449–1457. doi: 10.1093/infdis/168.6.1449. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Giovannini G, De Luca A, D’Angelo C, Casagrande A, Iannitti RG, Ricci G, Cunha C, Romani L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol Immunol. 2012;9:276–286. doi: 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin WL, Lopez-Ribot JL, Casanova M, Gozalbo D, Martinez JP. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy C, Cheng S, Nguyen M. Animal models of candidiasis. In: Cihlar R, Calderone R, editors. Candida albicans: Methods and Protocols. Vol. 499. Humana Press; Totowa, New Jersey: 2009. pp. 65–76. [Google Scholar]
- Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010;12:518–527. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti H, Shen F, Nayyar N, Stocum E, JNS, Lindemann M, Ho A, Hai J, Yu J, Jung J, Filler S, Masso-Welch P, Edgerton M, Gaffen S. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel PL, Jr, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335–348. doi: 10.1128/cmr.9.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker E, Grimbacher B. Chronic mucocutaneous candidiasis and congenital susceptibility to Candida. Curr Opin Allergy Clin Immunol. 2010;10:542–550. doi: 10.1097/ACI.0b013e32833fd74f. [DOI] [PubMed] [Google Scholar]
- Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Santos N, Huppler AR, Peterson AC, Khader SA, KCM, Gaffen SL. Th17 cells confer long term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Shen F, Conti H, Patel N, Childs E, Peterson A, Hernandez-Santos N, Kolls J, Kane L, Ouyang W, Gaffen S. IL-17RC is required for immune signaling via an extended SEFIR domain in the cytoplasmic tail. J Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Huppler AR, Bishu S, Gaffen SL. Mucocutaneous candidiasis: The IL-17 pathway and implications for targeted immunotherapy. Arthritis Res Ther. 2012;14:217. doi: 10.1186/ar3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DP, Brown GD, Underhill DM. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CC, Yu A, Lee H, Fidel PL, Jr, Noverr MC. Development of a contemporary animal model of Candida albicans–associated denture stomatitis using a novel intraoral denture system. Infect Immun. 2012;80:1736–1743. doi: 10.1128/IAI.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler S. New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun. 2011;3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum DM, Odds FC. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses. 2005;48:151–161. doi: 10.1111/j.1439-0507.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJ, Gow NA, Odds FC. Property differences among the four major Candida albicans strain clades. Eukaryot Cell. 2009;8:373–387. doi: 10.1128/EC.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC. Pathogenesis of candidosis. In: Odds FC, editor. Candida and Candidosis. Bailliere Tindall; Oxford: 1998. pp. 252–278. [Google Scholar]
- Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72:116–123. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: A role for IL-17 cytokines? Curr Opin Immunol. 2010;22:467–474. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowji S, Bustamante J, Wright J, Liu L, Lim H, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman N, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonis G, Anaissie EJ, Rosenbaum B, Bodey GP. A model of sustained gastrointestinal colonization by Candida albicans in healthy adult mice. Infect Immun. 1990;58:1514–1517. doi: 10.1128/iai.58.6.1514-1517.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nat Protoc. 2012;7:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. Mice with disseminated can-didiasis die of progressive sepsis. J Infect Dis. 2005;192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- Suegara N, Siegel JE, Savage DC. Ecological determinants in microbial colonization of the murine gastrointestinal tract: Adherence of Torulopsis pintolopesii to epithelial surfaces. Infect Immun. 1979;25:139–145. doi: 10.1128/iai.25.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo EK, MacCallum DM. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol Lett. 2011;320:1–8. doi: 10.1111/j.1574-6968.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- Taylor BN, Fichtenbaum C, Saavedra M, Slavinsky IJ, Swoboda R, Wozniak K, Arribas A, Powderly W, Fidel PL., Jr In vivo virulence of Candida albicans isolates causing mucosal infections in people infected with the human immunodeficiency virus. J Infect Dis. 2000;182:955–959. doi: 10.1086/315768. [DOI] [PubMed] [Google Scholar]
- Yano J, Fidel PL., Jr Protocols for vaginal inoculation and sample collection in the experimental mouse model of Candida vaginitis. J Vis Exp. 2011 doi: 10.3791/3382. pii 3382. [DOI] [PMC free article] [PubMed] [Google Scholar]


