Abstract
MDMA (±3,4-methylenedioxymethamphetamine, ‘ecstasy’) is reportedly used recreationally because it increases feelings of sociability and interpersonal closeness. Prior work suggests that the pro-social effects of MDMA may be mediated by release of oxytocin. A direct examination of plasma levels of oxytocin after acute doses of oxytocin and MDMA, in the same individuals, would provide further evidence for the idea that MDMA produces its prosocial effects by increasing oxytocin. Fourteen healthy MDMA users participated in a 4-session, double-blind study in which they received oral MDMA (0.75 and 1.5 mg/kg), intranasal oxytocin (20 IU or 40 IU), and placebo. Plasma oxytocin concentrations, as well as cardiovascular and subjective effects were assessed before and at several time points after drug administration. MDMA (1.5 mg/kg only) increased plasma oxytocin levels to a mean peak of 83.7 pg/ml at approximately 90–120 minutes, compared to 18.6 pg/ml after placebo. Intranasal oxytocin (40 IU, but not 20 IU) increased plasma oxytocin levels to 48.0 pg/ml, 30–60 min after nasal spray administration. MDMA dose-dependently increased heart rate, blood pressure, feelings of euphoria (e.g., ‘High’ and ‘Like Drug’), and feelings of sociability, whereas oxytocin had no cardiovascular or subjective effects. The subjective and cardiovascular responses to MDMA were not related to plasma oxytocin levels, although the N was small for this analysis. Future studies examining the effects of oxytocin antagonists on responses to MDMA will help to determine the mechanism by which MDMA produces pro-social effects.
Keywords: MDMA, oxytocin, mood, plasma, pharmacokinetics, humans
INTRODUCTION
The recreational drug ±3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) is typically used in social settings and produces feelings of sociability and interpersonal closeness (Bravo, 2001; Kelly et al., 2006; Rodgers et al., 2006; Sumnall et al., 2006). In controlled studies, acute doses of MDMA increase self-reports of euphoria, friendliness and closeness to others (Harris et al., 2002; Tancer and Johanson, 2003; Bedi et al., 2010; Hysek and Liechti, 2012; Kirkpatrick et al., 2012). MDMA also improves recognition of positive mental states, such as friendliness in others (Hysek et al., 2012a), while impairing recognition of negative states such as expressions of anger or fear (Bedi et al., 2010; Hysek et al., 2012a). Thus, MDMA may facilitate social behavior both by producing positive and pro-social subjective states, and by modulating sensitivity to positive and negative emotions in others. While it is known that MDMA is a potent releaser of the monoamine neurotransmitters: dopamine (DA), serotonin (5HT), and norepinephrine (NE) (Rothman et al., 2001; Han and Gu, 2006; Verrico et al., 2007), there is also evidence that it produces its social effects by releasing oxytocin (OT).
OT is a peptide important in mammalian social bonding (Bos et al., 2012). In humans, MDMA increases OT levels in blood plasma (Dumont et al., 2009; Hysek et al., 2012a; Hysek et al., 2013), which are correlated with increased subjective feelings of sociability (Dumont et al., 2009). In rats, MDMA increases the release of OT via 5HT1A receptors in the brain (Thompson et al., 2007), and both exogenous OT and MDMA increase “adjacent lying”, thought to be indicative of prosocial behavior (Ramos et al., 2013). In humans, other serotonergic drugs, such as d-fenfluramine also increase OT (Lee et al., 2003), and intranasal OT (IN-OT; 18–40 IU) produces psychological effects that are consistent with pro-social, anxiolytic and affiliative effects (Kosfeld et al., 2005; Lim and Young, 2006; Domes et al., 2007a, b; Zak et al., 2007; Di Simplicio et al., 2009; Bos et al., 2012; Shahrestani et al., 2013). Some of these effects, such as enhanced recognition of positive emotions (Marsh et al., 2010) and impaired recognition of negative emotions (Di Simplicio et al., 2009), closely resemble the effects observed with MDMA.
We found some support for the idea that the prosocial effects of MDMA may be mediated by OT in a recent study. Healthy MDMA users (N=65) completed measures of subjective sociability and social and emotional processing after MDMA (0.75 and 1.5 mg/kg) and IN-OT (20 and 40 IU) (Kirkpatrick et al., 2014). Interestingly, although the drugs differed on many measures, both increased feelings of sociability. IN-OT subjective responses were small but positively correlated to MDMA responses on two subjective measures of sociability, suggesting that individuals who were sensitive to MDMA-related subjective effects were similarly sensitive to IN-OT subjective effects. However, it is still unknown if the similarities between the two drugs were partially due to similar effects on endogenous oxytocin release. Several studies report that IN-OT increases plasma OT levels although the pharmacokinetics of intranasally administered OT are not fully understood (Domes et al., 2010; Gossen et al., 2012; Striepens et al., 2013). Additionally, it is difficult to compare the plasma levels of OT across studies with MDMA and exogenously administered IN-OT because of differences in procedures and assay methods. To our knowledge, there have been no studies in which MDMA and IN-OT were administered in the same subjects, to determine and physiological outcomes such as plasma OT levels and its time course of effects.
Thus, in this study we tested single doses of oral MDMA (0.75 and 1.5 mg/kg) and IN-OT (20 IU and 40 IU) in healthy young adults, using a mixed between- and within-subjects design. We assessed the drugs’ effects on plasma OT levels, cardiovascular measures and several self-reported measures of subjective drug effects and feelings of sociability. We hypothesized that 1) both MDMA and IN-OT would dose-dependently increase plasma OT levels; 2) MDMA would dose-dependently enhance self-report measures of sociability and “positive” mood; and 3) MDMA-related increases in feelings of sociability would be positively correlated to increases in plasma OT.
METHODS
Participants
Healthy adults (N=14) who reported having used MDMA 4–40 times in their lifetime were recruited via newspaper, community bulletin board, and online advertisements. Potential participants completed an initial telephone and an in-person psychiatric evaluation and medical examination, including an electrocardiogram and physical examination. Inclusion criteria were: age between 18 and 35, at least a high school education, fluency in English, and BMI between 18 and 30. Candidates were excluded if they smoked more than 10 tobacco cigarettes per day, if they had any significant cardiovascular, neurological, or major psychiatric illness including all Axis I disorders or sinus infection or other condition blocking access to the olfactory epithelium.
Participants provided written informed consent prior to participation. They were told they might receive a stimulant (such as amphetamine or ecstasy), a sedative (such as Valium), a cannabinoid, a hormone (such as OT), or placebo. Participants were instructed to consume their normal amount of caffeine before sessions, but were asked to refrain from tobacco use for 9 hrs, and other drug use for 48 hrs, prior to each session. Women not using hormonal contraceptives were tested only during the follicular phase (days 2–14; White et al., 2002). Participants were debriefed following the study. This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board at the University of Chicago in accordance with the Code of Federal Regulations (Title 45, Part 46) adopted by the National Institutes of Health and the Office for Protection from Research Risks of the US Federal Government.
Design
The study used a within-and-between-subjects, double-dummy design in which participants received two doses of MDMA (0.75 and 1.5 mg/kg), one dose of IN-OT (20 or 40 IU), and placebo. After an initial orientation session, participants completed four outpatient sessions separated by at least five days as a washout period (Abraham et al, 2009). Dosing order was randomized. On each session participants ingested a capsule (placebo or MDMA) and received a nasal spray (placebo or IN-OT). Eight participants received 20 IU IN-OT and 8 participants received 40 IU. Blood samples were collected before and at several time points for 4 hours after drug administration. Participants’ mood states and cardiovascular measures were monitored regularly. Plasma OT levels for a total of two participants in the 20 IU group were univariate outliers and likely occurred because of blood sampling issues resulting in hemolysis. These two participants were not included in the analyses.
Procedure
Sessions were conducted between 0900h and 1330h in order to minimize any diurnal variation in biological measures. Upon arrival participants provided urine and breath samples to confirm abstinence from alcohol (as measured by an Alco-Sensor III Breathalyzer, Intoximeters Inc., St Louis, MO), amphetamine, cocaine and opiates (as measured by urine toxicology: Ontrak TesTstik, Roche Diagnostic Systems Inc., Somerville, NJ), and marijuana (as measured by a saliva test: Oratect, Branan Medical Corp., Irvine, CA), and women were tested for pregnancy. Sessions were rescheduled if the participant tested positive for drugs. An intravenous catheter was inserted into the participant’s non-dominant arm for blood sampling. At 0920h, pre-capsule measures of heart rate and blood pressure were obtained, a blood sample was obtained, and participants completed self-report mood and drug effects questionnaires (see below). At 0930h, participants ingested capsules containing either MDMA or placebo, and at 1000h they received an intranasal spray containing either IN-OT or placebo (see below). Physiological and subjective measures were obtained at 1030, 1100, 1130, and 1330h (i.e., 60, 90, 120, and 240 min post capsule administration). During times when no measures were scheduled the participants were allowed to relax and watch movies or read. At 1330 pm, the catheter was removed and the participants were discharged provided that their heart rate and blood pressure had returned to baseline levels.
Physiological measures
Cardiovascular measures
Heart rate and blood pressure were measured at regular intervals throughout the sessions using portable monitors (Life Source, A&D, Tokyo, Japan).
Plasma oxytocin levels
At each time point, the study nurse drew a 10 ml blood sample drawn into a pre-cooled purple top tube containing disodium EDTA. The samples were placed on ice and then were centrifuged in a refrigerated 4°C centrifuge (3000 rpm, for 15 min) at a consistent post-draw interval for all sessions. They were then stored immediately in a −80°C freezer. Prior to assaying, samples were first purified by solid phase extraction (Seltzer et al 2010). One ml of plasma was run through solid phase extraction (Sep-Pak Light C18 cartridges) and eluted with 1ml 80% acetonitrile. Three hundred μl of ethanol was added to ensure that the proteins were all denatured and then the sample was dried and resuspended in Assay Buffer. Samples were then analyzed by enzyme immunoassay (EIA) using the Assay Designs EIA kit (Assay Designs, Inc. Ann Arbor, MI, USA). This kit has been validated in a range of species and across different biological media including urine (Wismer-Fries et al., 2005; Carter et al., 2007; Gray et al., 2007; Seltzer and Ziegler, 2007; Seltzer et al., 2010; Snowdon et al., 2010; Feldman et al., 2011). The specificity of the antibody used in this assay has been repeatedly validated via high-performance liquid chromatography (HPLC) and results across different species and biological media indicate that the assay antibody binds only to intact OT and does not show cross-reactivity with other peptide hormones (Wismer-Fries et al., 2005; Carter et al., 2007; Seltzer and Ziegler, 2007). This assay should be considered a reliable but conservative measure of OT, since the assay antibody responds primarily to the intact OT molecule and not to OT metabolites (Seltzer and Ziegler, 2007).
Subjective Effects
Participants completed subjective effect questionnaires before and at several time points after capsule and nasal spray administration. They completed a series of visual analog scales (VAS: 0 to 100 mm; ‘not at all’ to ‘extremely’) that consisted of adjectives describing several MDMA-related mood effects (i.e., ‘I feel…’ ‘Anxious,’ ‘Dizzy,’ ‘Elated,’ ‘Restless,’ ‘Sedated,’ ‘Stimulated’) and “prosocial” effects (i.e., ‘I feel…’ ‘Confident,’ ‘Friendly,’ ‘Insightful,’ ‘Loving’, ‘Lonely,’ ‘Playful,’ ‘Sociable’). They also completed the drug-effect questionnaire (DEQ), a visual analogue questionnaire designed to assess the extent to which participants experienced the effects of the drugs: ‘Feel Drug’, ‘Feel High’, ‘Like Drug’, ‘Dislike Drug’, and ‘Want More’ (Fischman and Foltin, 1991; Justice and De Wit, 2000). Each item was presented with a 100-mm line labeled ‘not at all’ at one end and ‘extremely’ at the other end.
Drugs
Drugs were administered in randomized order, under double-blind conditions. Capsules and nasal sprays were prepared by The University of Chicago Hospitals investigational pharmacy. MDMA powder (0.75 and 1.5 mg/kg) was encapsulated in 00 opaque capsules with lactose filler. Placebo capsules contained only lactose. These MDMA doses were selected based on our previous studies indicating that the drug reliably increases positive mood and alters emotional processing at these doses (Bedi et al., 2009, 2010). Intranasal OT (20 and 40 IU) doses were prepared within 24 hours of use. A single dose of Pitocin (OT Injection USB; Monarch Pharmaceuticals; concentration: 10 or 20 IU Pitocin/1 ml) was transferred into two, 1 ml intranasal atomizers (MAD300 by LMA Inc., San Diego, CA). Placebo nasal sprays consisted of Ocean Spray Nasal Solution (Valeant Pharmaceuticals, Bridgewater, NJ). The doses of 20 and 40 IU IN-OT were chosen based on previous studies utilizing intranasally administered OT and the structurally similar neuropeptide, vasopressin (Bos et al., 2012; MacDonald et al., 2011). Nasal sprays were administered by trained personnel in four doses to each nostril over the course of 15 minutes. During the administration, participants sat comfortably in reclined position, with their heads tilted back to maximize absorption.
Data Analysis
Acute drug-related effects
To characterize the acute effects of MDMA, subjective, cardiovascular, and plasma data were analyzed with repeated measures analyses of variance (ANOVAs) with two within-subject factors. The within-subjects factors were Drug Dose (placebo, 0.75 and 1.5 mg/kg MDMA) and Time of assessment. Planned t-tests were conducted to compare mean responses between the doses: 1) placebo versus all active MDMA doses and 2) 0.75 mg/kg versus 1.5 mg/kg MDMA.
Similarly, to characterize the acute effects if IN-OT, subjective, cardiovascular, and plasma data were analyzed with ANOVAs with two within-subject factors and one between-subjects factor. The within-subjects factors were Drug Dose (placebo and active OT) and Time of assessment. The between-subjects factor was OT dose level Group (20, 40 IU). Planned t-tests were conducted to compare mean responses between the doses: 1) placebo and active OT in each OT dose level group and 2) 20 IU group versus 40 IU group.
Correlations between MDMA-related “prosocial” subjective effects and plasma oxytocin
We conducted Pearson’s correlational analyses to investigate the relationship between subjective responses to MDMA and MDMA-induced increases in plasma OT. To summarize subjective and physiological effects across the entire session, we calculated area-under-the-curve (AUC) for plasma OT levels and each prosocial subjective item, relative to the participant’s pre-drug baseline, using the trapezoidal method (Tallarida and Murray, 1981). Subjective ratings from MDMA sessions were compared to plasma OT levels.
For all analyses and comparisons, p values were considered statistically significant at less than 0.05 with Bonferroni adjustments for multiple comparisons.
RESULTS
Sample Characteristics
In total, 14 volunteers (2 Female, 12 Male) completed the study. They were 25.4 ± 3.7 (mean ± SD) years old, had a BMI of 23.5 ± 2.9, and had completed 14.7 ± 1.5 years of formal education. They had used MDMA a mean of 13.5 ± 12.0 times (range 4–40 lifetime); on average their last use of MDMA was 22.1 ± 35.1 months prior to study participation (range 0.25–120 months). Ten participants currently drank caffeinated beverages (1–3 cups/day), seven smoked tobacco (1–20 cigarettes/month), thirteen drank alcohol (2–18 drinks/week), and ten currently smoked marijuana (1–30 days/month). Participants who received 20 or 40 IU IN-OT did not differ on any demographic measure.
Acute Drug-related Effects
Plasma Oxytocin Levels
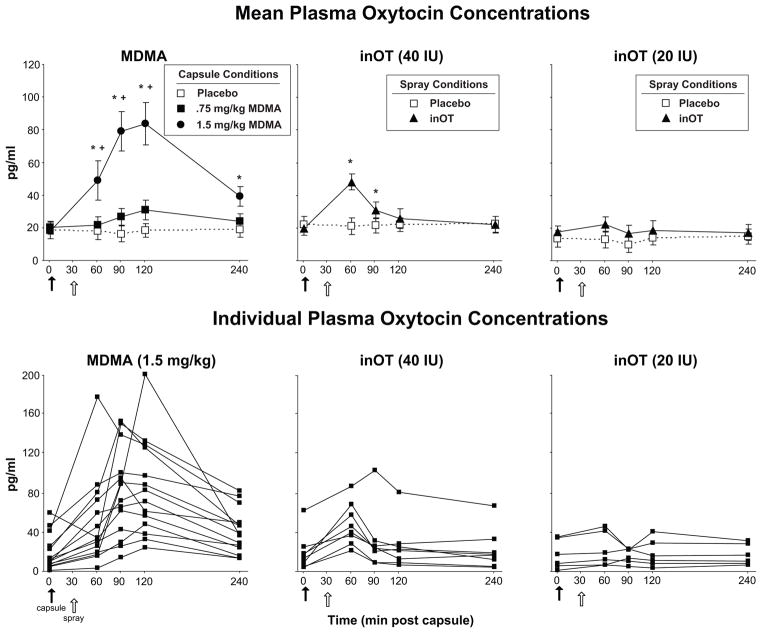
MDMA (1.5 mg/kg) but not MDMA (0.75 mg/kg) significantly increased plasma OT levels over the course of the session (Figure 1, top left panel; Dose x Time interaction: F[8, 104] = 13.9, p < 0.001, χ2 = 0.17). Mean peak plasma concentrations were reached at 90–120 min after capsule ingestion, and all 14 participants showed some increase in OT levels following the larger MDMA dose (Figure 1; bottom left panel). Plasma OT levels following MDMA (0.75 mg/kg) were slightly increased compared to placebo but this difference did not reach significance (t[13] = 2.96, p = 0.07, d = 0.79).
Figure 1.
Top panels: Mean plasma OT concentrations scores following administration of MDMA, IN-OT, or placebo as a function of dose and time. Bottom panels: Individual plasma OT concentrations scores following administration of placebo, MDMA (1.5 mg/kg: left panel), or IN-OT (20 IU and 40 IU: center and right panels) as a function of time. The closed arrow denotes time of capsule administration. The open arrow denotes time of nasal spray administration. An * indicates 1.5 mg/kg MDMA significantly different from placebo (p<0.05). A + indicates 1.5 mg/kg MDMA significantly different from 0.75 mg/kg MDMA (p<0.05). Error bars represent one SEM.
Relative to placebo, IN-OT (40 IU, but not 20 IU) increased mean plasma OT levels following the nasal spray (t[7] = 3.77, p < 0.05, d = 1.33). The increased plasma level was significant 30 min after nasal spray administration (Figure 1, top middle and right panels; t[7] = 5.66, p < 0.01, d = 2.00). Individual responses to each active nasal spray are shown in Figure 1 (bottom middle and right panels).
Post hoc t-tests revealed that peak plasma levels were greater following the larger MDMA dose compared to either IN-OT dose (20 IU: t[5] = 4.8; 40 IU: t[7] = 3.3, p < 0.05 for both comparisons, d = 3.22 and 1.16, respectively).
Cardiovascular and Subjective Effects
MDMA dose-dependently increased heart rate and blood pressure compared to placebo (Main effect of Dose: F[2, 26] = 17.5–35.2, p < 0.001, χ2 = 0.26–0.36; Table 3), and the larger dose produced a greater cardiovascular response (i.e., heart rate and systolic pressure) compared to the lower dose (t[13] = 3.2–5.7, p < 0.05 for both comparisons, d=0.85–1.51). Peak cardiovascular effects occurred between 90 and 120 min.
MDMA produced robust increases on several self-reported ratings of euphoria and feelings of sociability. For example, both MDMA doses increased ratings of ‘Feel Drug,’ ‘Like Drug,’ and ‘Want More’ (Table 1; Figure 2 left panel, Main effect of Dose: F[2, 26] = 20.0–47.7, p < 0.001, χ2 = 0.24–0.30), and this effect was greater with the larger dose compared to the lower dose (t[13] = 3.3–5.8, p < 0.01 for all comparisons, d=0.88–1.54). Additionally, both MDMA doses significantly increased ratings of ‘Insightful’ and the larger dose increased ratings of ‘Sociable’ compared to placebo (Figure 2 middle and right panels, Main effect of Dose: F[2, 26] = 7.9–18.6, p < 0.01, χ2 = 0.18–0.26). MDMA also increased ratings of positive mood states such as ‘Elated’ and negative states such as ‘Restless’ (Table 1). The drug’s effects on all subjective ratings peaked 60–120 min after capsule ingestion.
Table 1.
MDMA-related mean (SEM) heart rate, blood pressure, and self-report ratings over the entire session, calculated as change from pre-capsule.
| Drug Condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | 0.75 mg/kg MDMA |
1.5 mg/kg MDMA |
Main Effect of Dose | ||||||
| Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | F (2,26) | p | χ2 | |
| Cardiovascular measures | |||||||||
| Heart Rate | 1.7 | (0.7) | 8.7 | (1.7) * | 16.0 | (2.9) *§ | 17.5 | <0.001 | 0.26 |
| Systolic Pressure | 1.2 | (2.5) | 14.5 | (1.9) * | 27.1 | (2.2) *§ | 35.2 | <0.001 | 0.36 |
| Diastolic Pressure | 0.4 | (1.2) | 6.7 | (1.2) * | 11.5 | (1.2) * | 23.8 | <0.001 | 0.27 |
| Drug Effects Questionnaire | |||||||||
| Feel Drug | 6.4 | (2.3) | 24.4 | (4.7) * | 44.4 | (3.9) *§ | 47.7 | <0.001 | 0.27 |
| Feel High | 5.4 | (2.3) | 20.6 | (4.8) * | 43.7 | (3.2) *§ | 59.7 | <0.001 | 0.28 |
| Like Drug | 5.0 | (3.0) | 22.4 | (4.7) * | 47.8 | (4.0) *§ | 39.6 | <0.001 | 0.30 |
| Dislike Drug | 7.9 | (2.9) | 12.8 | (2.9) | 15.3 | (5.5) | 4.7 | 0.018 | 0.06 |
| Want More | 6.4 | (3.1) | 22.5 | (6.1) * | 41.5 | (6.2) *§ | 20.0 | <0.001 | 0.24 |
| Visual Analog Scales | |||||||||
| Anxious | 2.2 | (4.0) | 11.5 | (4.3) | 14.2 | (4.0) | 2.3 | 0.124 | 0.04 |
| Confident | −3.9 | (1.9) | 1.3 | (2.4) | 10.1 | (4.9) | 5.2 | 0.013 | 0.17 |
| Dizzy | 5.3 | (3.2) | 6.4 | (5.4) | 15.4 | (6.4) | 5.9 | 0.008 | 0.06 |
| Elated | 2.6 | (1.6) | 16.0 | (4.4) * | 33.3 | (5.4) *§ | 23.3 | <0.001 | 0.25 |
| Friendly | −3.2 | (2.5) | 5.0 | (3.0) | 17.8 | (4.3) * | 13.6 | <0.001 | 0.24 |
| Insightful | 0.7 | (1.7) | 10.5 | (4.8) | 23.7 | (5.4) * | 18.6 | <0.001 | 0.26 |
| Lonely | −0.1 | (1.8) | 4.8 | (3.7) | 11.1 | (5.5) | 1.1 | 0.363 | 0.03 |
| Loving | 0.3 | (1.6) | 4.7 | (2.8) | 28.6 | (5.8) *§ | 18.2 | <0.001 | 0.26 |
| Playful | −4.5 | (2.5) | 5.4 | (2.9) | 15.7 | (5.6) | 4.5 | 0.021 | 0.12 |
| Restless | 7.2 | (3.0) | 18.1 | (4.2) | 27.3 | (5.5) * | 10.2 | 0.001 | 0.13 |
| Sedated | 1.8 | (3.7) | 0.6 | (2.6) | 5.0 | (2.8) | 0.8 | 0.464 | 0.01 |
| Sociable | −3.6 | (2.2) | 4.5 | (3.8) | 11.8 | (5.2) * | 7.9 | 0.002 | 0.18 |
| Stimulated | 4.2 | (2.0) | 19.2 | (4.5) | 36.3 | (5.3) * | 40.4 | <0.001 | 0.28 |
significantly different from placebo
significantly different from 0.75 mg/kg
Figure 2.
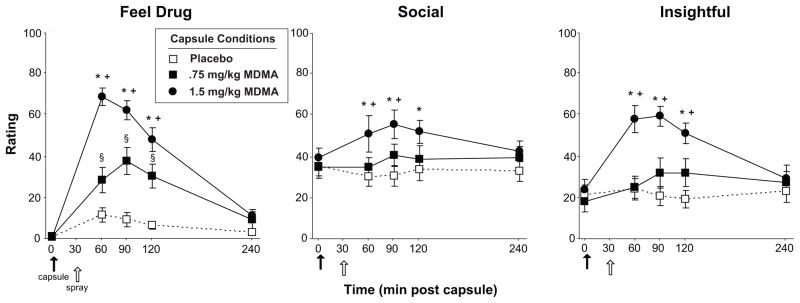
Selected mean scores on subjective ratings following administration of MDMA or placebo as a function of dose and time. The closed arrow denotes time of capsule administration. The open arrow denotes time of nasal spray administration. An * indicates 1.5 mg/kg MDMA significantly different from placebo (p<0.05). A + indicates 1.5 mg/kg MDMA significantly different from 0.75 mg/kg MDMA (p<0.05). A § indicates 0.75 mg/kg MDMA significantly different from placebo (p<0.05). Error bars represent one SEM.
Mean ratings over the course of the entire session for all cardiovascular and self-report measures following IN-OT administration are provided in Table 2. Overall neither dose of IN-OT (20 or 40 IU) produced systematic changes in heart rate, blood pressure, or subjective effects compared to placebo.
Table 2.
Oxytocin-related mean (SEM) heart rate, blood pressure, and self-report ratings over the entire session, calculated as change from pre-capsule. There were no significant differences between oxytocin and placebo.
| Oxytocin Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 IU (N=6) | 40 IU (N=8) | ||||||||||
| Placebo | Oxytocin | Placebo | Oxytocin | Dose x Group | |||||||
| Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | F (1,12) | p | χ2 | |
| Cardiovascular measures | |||||||||||
| Heart Rate | 0.8 | (1.1) | 0.2 | (1.4) | 2.6 | (0.9) | 1.1 | (1.2) | 0.1 | 0.717 | 0.00 |
| Systolic Pressure | 2.2 | (3.9) | 5.2 | (3.2) | 0.2 | (3.3) | −1.7 | (2.8) | 1.1 | 0.309 | 0.01 |
| Diastolic Pressure | −0.3 | (1.8) | 3.3 | (1.5) | 1.1 | (1.6) | −0.7 | (1.3) | 5.3 | 0.041 | 0.05 |
| Drug Effects Questionnaire | |||||||||||
| Feel Drug | 6.1 | (3.5) | 3.0 | (1.6) | 6.8 | (3.0) | 2.7 | (1.4) | 0.0 | 0.861 | 0.00 |
| Feel High | 4.6 | (3.5) | 0.3 | (1.2) | 6.2 | (3.0) | 3.5 | (1.0) | 0.0 | 0.841 | 0.00 |
| Like Drug | 5.9 | (4.5) | 3.2 | (5.2) | 4.1 | (3.9) | 9.6 | (4.5) | 0.1 | 0.797 | 0.00 |
| Dislike Drug | 8.3 | (4.3) | 4.2 | (2.9) | 7.5 | (3.8) | 2.5 | (2.5) | 0.2 | 0.649 | 0.00 |
| Want More | 7.9 | (4.6) | 2.8 | (5.6) | 4.9 | (4.0) | 10.1 | (4.8) | 1.3 | 0.284 | 0.02 |
| Visual Analog Scales | |||||||||||
| Anxious | 2.1 | (6.1) | 0.3 | (2.3) | 2.4 | (5.3) | −0.7 | (2.0) | 3.0 | 0.107 | 0.01 |
| Confident | −5.8 | (2.9) | −1.6 | (3.2) | −2.1 | (2.5) | −5.2 | (2.8) | 0.2 | 0.663 | 0.00 |
| Dizzy | 4.0 | (4.9) | 0.1 | (3.9) | 6.5 | (4.2) | 2.7 | (3.4) | 0.2 | 0.658 | 0.00 |
| Elated | 1.8 | (2.4) | −1.5 | (2.4) | 3.4 | (2.1) | 1.5 | (2.1) | 1.2 | 0.293 | 0.01 |
| Friendly | −4.0 | (3.8) | −1.7 | (3.7) | −2.4 | (3.3) | −2.3 | (3.2) | 1.1 | 0.32 | 0.00 |
| Insightful | 2.0 | (2.6) | 2.1 | (2.8) | −0.7 | (2.3) | 2.8 | (2.4) | 0.7 | 0.414 | 0.00 |
| Lonely | −0.2 | (2.7) | −1.8 | (1.4) | 0.0 | (2.3) | 1.3 | (1.2) | 0.5 | 0.503 | 0.00 |
| Loving | −1.4 | (2.5) | −2.8 | (2.7) | 2.0 | (2.1) | 1.2 | (2.3) | 0.0 | 0.895 | 0.00 |
| Playful | −8.1 | (3.7) | −3.5 | (1.9) | −0.9 | (3.2) | 1.9 | (1.6) | 0.2 | 0.683 | 0.00 |
| Restless | 8.3 | (4.6) | 0.1 | (2.7) | 6.1 | (4.0) | 1.5 | (2.3) | 0.6 | 0.452 | 0.00 |
| Sedated | 6.1 | (5.6) | −1.7 | (5.2) | −2.6 | (4.8) | 5.1 | (4.5) | 0.7 | 0.421 | 0.01 |
| Sociable | −6.0 | (3.3) | 0.7 | (3.2) | −1.3 | (2.8) | −2.6 | (2.8) | 0.8 | 0.396 | 0.00 |
| Stimulated | 2.9 | (3.0) | 3.7 | (3.1) | 5.6 | (2.6) | 3.2 | (2.7) | 3.6 | 0.081 | 0.01 |
Correlations between MDMA-related prosocial subjective effects and plasma oxytocin levels
None of the subjective or cardiovascular responses to MDMA (0.75 or 1.5 mg/kg) were significantly correlated with plasma OT levels (N=14; r = −0.46–0.38; p = 0.100–0.984).
DISCUSSION
The current findings show that single doses of either oral MDMA or IN-OT dose-dependently increased plasma OT levels. Following the larger MDMA dose (i.e., 1.5 mg/kg), plasma OT levels were significantly increased 60 minutes after capsule administration and remained elevated throughout the session. Interestingly, there was substantial individual variability in both the magnitude and time course of MDMA-induced plasma OT response. However, MDMA-induced plasma OT response was unrelated to drug-related mood response. Intranasal OT (40 IU) produced only a brief elevation in plasma OT, detectable only at 30 and 60 minutes after administration. Thus, both MDMA and IN-OT increased plasma OT levels, as reported previously (Dumont et al., 2009; Domes et al., 2010; Gossen et al., 2012; Hysek et al., 2012a), but the time course and magnitude of the effect were markedly different. Compared to MDMA (1.5 mg/kg), IN-OT (40 IU) produced earlier, smaller in magnitude, transient increases in plasma levels. Overall, these results replicate and extend previous studies by demonstrating the effects of a range of MDMA and IN-OT doses on plasma OT response.
The larger MDMA dose increased plasma OT concentrations, although there was variability in response patterns across participants. Plasma OT was significantly elevated within 60 minutes following capsule administration (i.e., at the first time point) and peaked at 120 minutes. The magnitude and time course are consistent with previous studies (Dumont et al., 2009; Hysek et al., 2012a), replicating that MDMA administration results in a marked OT release that can be measured in peripheral plasma. The lower dose of MDMA (0.75 mg/kg) did not significantly increase plasma OT levels, suggesting that there may be a qualitative difference in the subjective and behavioral effects of the drug depending upon the dose level. These data indicate that future studies investigating the pro-social effects of MDMA should use relatively larger doses. Interestingly, there was substantial individual variability in drug response: participants differed in both the peak (between 60 and 120 minutes) and magnitude (between a 20 and 150 pg/ml increase from baseline) of drug response. It is not known whether this variability in plasma response was related to individual differences in pharmacokinetics of the drug or to differences in sensitivity to MDMA-related prosocial behavioral effects. Unfortunately, one limitation of this study is that we did not include measures of social behavior or response to social stimuli. However, a post hoc analysis revealed that MDMA-induced increases in heart rate significantly covaried with some prosocial subjective effects (e.g., ratings of ‘Sociable’ and ‘Friendly’), but not with more general drug-related effects (e.g., ratings of ‘Feel Drug’ and ‘Feel High’). This suggests that variability in physiological response to MDMA may partially explain variability in its prosocial effects and will need to be further examined in future studies.
MDMA also dose-dependently increased ratings of euphoria (i.e., drug liking) as well as ratings of sociability such as feelings of friendliness, playfulness, and insightful, consistent with previous reports (Tancer and Johanson, 2003; Bedi et al., 2009, 2010; Hysek and Liechti, 2012; Hysek et al., 2012b; Kirkpatrick et al., 2012, 2014). In a recent study we found some support that the subjective prosocial effects of MDMA may be related to OT function (Kirkpatrick et al., 2014). Thus, we predicted that MDMA-related sociability ratings would be related to plasma OT concentrations. The current results do not support this hypothesis. Nevertheless, this is consistent with previous studies indicating that plasma OT levels were not correlated with several measures of pro-social feelings and behavior (Hysek et al., 2012a, 2013), but contrary to one previous study showing significant within-subject correlations between MDMA-induced plasma OT and two subjective measures of sociability (Dumont et al., 2009). However, the differences in statistical approaches between the current study and Dumont et al. make it difficult to compare the two studies. The relationship between plasma OT and MDMA-related prosocial effects remains to be determined. Of course, it is unclear whether the presence – or absence – of a significant correlational relationship between hormone levels and subjective response would support a true physiological link between MDMA-related subjective response and oxytocin levels in the plasma. Regardless, these data suggest that MDMA produces many of its prosocial subjective effects through other neurochemical mechanisms. For example, a recent study indicates that both MDMA and exogenous oxytocin produces pro-social behavior via involvement of vasopressin receptors (Ramos et al., 2013). The ability of oxytocin to target homologous brain vasopressin receptors may also explain how exogenous oxytocin reverses social deficits found in oxytocin-receptor knock-out mice (Sala et al., 2011).
Intranasal OT also increased plasma OT levels, albeit to a lesser extent. Plasma OT concentrations following the 40 IU IN-OT dose were lower than MDMA-related levels (OT = 37 pg/ml vs MDMA = 84 pg/ml), peaked relatively early (i.e., 30–60 minutes vs 120 min after administration), and were short-lived (30 min vs 3 hours). However, responses were less variable than the OT levels after MDMA (7 of the 8 subjects exhibited peak response 30 min after administration, compared to a range of peak response times for MDMA). Overall, the pharmacokinetic time course of IN-OT is consistent with recent reports showing that 24–26 IU IN-OT increased plasma OT levels immediately following drug administration (Gossen et al., 2012; Striepens et al., 2013). We did not observe an increase in plasma OT following the 20 IU dose. However, the sample size in this study was small (N=6 for 20 IU and N=8 for 40 IU) and more subjects may have provided power to detect a subtle effect. Future investigations might investigate a wider range of intranasal doses using a within-subjects design and larger sample size.
The current results should be interpreted in the context of several potential limitations. One limitation of our study, and others investigating the central effects of OT, is that we measured OT levels in plasma, and we do not know how these levels correspond to OT levels in the brain. Some evidence suggests that OT has a more sustained action in the CNS compared to the periphery (Mens et al., 1983), suggesting that behavior may be influenced by central OT even after plasma levels have returned to baseline. It has been shown that OT and the closely related nanopeptide, arginine vasopressin, can be measured in cerebral spinal fluid (CSF) after intranasal administration (Born et al., 2002; Striepens et al., 2013), and that plasma oxytocin levels may not be related to CSF levels following acute administration. Future studies should further investigate the correspondence of central and peripheral OT levels and how these might relate to prosocial behaviors. Another limitation of the current study is that we utilized different routes of administration for OT (intranasal) and MDMA (oral). This difference makes it potentially difficult to directly compare the effects of the two drugs due to differences in rates of absorption and distribution. However, in order to minimize expectancies, participants received both a capsule and a nasal spray during each session. Finally, the formulation of IN-OT used in the current study (i.e., Pitocin) is less concentrated than the formulation used in typical oxytocin studies (i.e., Syntocin). This difference may influence absorption rates of the drug and thus, future studies directly comparing MDMA and IN-OT might utilize a range of oxytocin formulations.
In conclusion, MDMA dose-dependently increased plasma OT concentrations and feelings of euphoria and sociability. The larger dose of intranasal OT also increased plasma OT levels but these increases were relatively low and short-lived compared to those produced by MDMA. Additionally, MDMA-induced increases in mean plasma OT concentrations were unrelated with mean levels of subjective sociability in our study. MDMA-related subjective effects may be mediated by mechanisms that are not reflected in plasma OT levels, such as central OT, vasopressin, or monoamine neurotransmitter signaling. Thus, future studies will need to parse out contributions of monoamine and central neuropeptide brain pathways to the prosocial effects of MDMA. These data provide further information about the pharmacokinetics of plasma OT following administration of two drugs believed to produce prosocial behavioral effects in humans.
Acknowledgments
Supported by DA026570 (Harriet de Wit PI)
We thank Jon Solamillo and the clinical staff of the General Clinical Research Center at The University of Chicago for technical assistance. Toni Ziegler and Dan Wittwer of the Wisconsin National Primate Research Center are gratefully acknowledged for assay services.
Funding: This research was supported by NIDA R01 DA02812 and R21 DA026579, and the Institute for Translational Medicine (University of Chicago Medical Center). No funding agency was involved in the design of the study, analysis of the data, or preparation of the manuscript.
Footnotes
Contributors: Authors HdW, RL, SJ, and MK designed the study and wrote the protocol. MK and SF managed and competed the literature searches and analyses. MK wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflicts of Interest: The authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham TT, Barnes AJ, Lowe RH, Kolbrich Spargo EA, Milman G, Pirnay SO, et al. Urinary MDMA, MDA, HMMA, and HMA excretion following controlled MDMA administration to humans. J Anal Toxicol. 2009;33:439–446. doi: 10.1093/jat/33.8.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of +/−3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68:1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe RM, Honk JV. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: A review of single administration studies. Front Neuroendocrinol. 2012;33:17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bravo GL. What does MDMA feel like? In: Holland J, editor. Ecstasy: The complete guide. A comprehensive look at the risks and benefits of MDMA. Park Street Press; Rochester, US: 2001. [Google Scholar]
- Carter CS, Pournajafi-Nazarloo P, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Behavioral associations and potential as a salivary biomarker. Ann NY Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J Psychopharmacol. 2009;23:241–248. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007a;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007b;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Sweep FC, van der Steen R, Hermsen R, Donders AR, Touw DJ, van Gerven JM, Buitelaar JK, Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci. 2009;4:359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Grunder G, Spreckelmeyer KN. Oxytocin plasma concentrations after single intranasal oxytocin administration - a study in healthy men. Neuropeptides. 2012;46:211–215. doi: 10.1016/j.npep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Gray PB, Parkin JC, Samms-Vaughan ME. Hormonal correlates of human paternal interactions: A hospital-based investigation in urban Jamaica. Horm Behav. 2007;52:499–507. doi: 10.1016/j.yhbeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology (Berl) 2012a;222:293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME. Effects of MDMA alone and after pretreatment with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology (Berl) 2012;224:363–376. doi: 10.1007/s00213-012-2761-6. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola VG, Vischer N, Donzelli M, Krahenbuhl S, Grouzmann E, Huwyler J, Hoener MC, Liechti ME. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One. 2012b;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Preller KH, Quednow BB, Liechti ME. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci. 2013 Oct 28; doi: 10.1093/scan/nst161. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AJ, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Parsons JT, Wells BE. Prevalence and predictors of club drug use among club-going young adults in New York city. J Urban Health. 2006;83:884–895. doi: 10.1007/s11524-006-9057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2012;219:109–122. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and Intranasal Oxytocin on Social and Emotional Processing. Neuropsychopharmacology 2014. 2014 Jan 22; doi: 10.1038/npp.2014.12. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lee R, Garcia F, van de Kar LD, Hauger RD, Coccaro EF. Plasma oxytocin in response to pharmaco-challenge to D-fenfluramine and placebo in healthy men. Psychiatry Res. 2003;118:129–136. doi: 10.1016/s0165-1781(03)00070-2. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 2010;209:225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, Van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, et al. Acute Prosocial Effects of Oxytocin and Vasopressin When Given Alone or in Combination with 3,4-Methylenedioxymethamphetamine in Rats: Involvement of the V1A Receptor. Neuropsychopharmacology. 2013;38:2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J, Buchanan T, Pearson C, Parrott AC, Ling J, Hefferman TM, Scholey AB. Differential experiences of the psychobiological sequelae of ecstasy use: quantitative and qualitative data from an internet study. J Psychopharmacol. 2006;20:437–446. doi: 10.1177/0269881105058777. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE. Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): A radiolabeled clearance study and endogenous excretion under varying social conditions. Horm Behav. 2007;51:436–442. doi: 10.1016/j.yhbeh.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, Guastella AJ. The Impact of a Single Administration of Intranasal Oxytocin on the Recognition of Basic Emotions in Humans: A Meta-Analysis. Neuropsychopharmacology. 2013;38:1929–1936. doi: 10.1038/npp.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm Behav. 2010;58:614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20:670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of pharmacologic calculations. Springer; Heidelberg, Germany: 1981. [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl) 2007;189:489–503. doi: 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wismer-Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]