Abstract
A number of tremorogenic β-carboline alkaloids have been found in common plant-derived foodstuffs, beverages, and inhaled substances. Because of their natural presence in the food chain, there is a growing concern regarding the potential risks of certain essen tial tremors associated with the long-term low-level dietary exposure to these alkaloids. The purpose of this study was to develop an effective analytical method to determine blood levels of two major β-car boline derivatives, harmane and harmine. Human blood was extracted with ethyl acetate and methyl-t-butyl ether (2:98) under an alkaline condition. After evaporation of organic solvent, the samples were re- constructed in methanol. The samples were fraction ated on a 250 × 4.6-mm C18 reversed-phase column with an isocratic mobile system consisting of 17.5 mM potassium phosphate buffer (ph 6.5) and methanol (30: 70), followed by an on-line fluorescence detection. The method had the detection limit to determine 206 and 81 pg/ml of harmane and harmine, respectively, in 10 ml of human blood. The intraday precision (C.V.) at 25 ng/ml was less than 6.7 and 3.4% for harmane and harmine, respectively. The interday precision was 7.3% for harmane and 5.4% for harmine. The method has proven sensitive, reproducible, and thus useful for both laboratory and clinical studies of β-carboline toxicities.
A number of tremorogenic β-carboline alkaloids such as harmane, harmine, harmaline, and ibogaine have been found in common plant-derived foodstuffs (wheat, rice, corn, barley, soybeans, rye, grapes, mushrooms, vinegar). plant -derived beverages (wine. beer. whisky. brandy, sake). and plant-derived inhaled substances (tobacco) (1, 6). These substances are also endogenous to animal tissue (6, 9) and have been isolated in beef and sardines (1). Laboratory animals exposed to these chemicals result in an acute action tremor (5). Because of their natural presence in the food chain, it is conceivable that the route of exposure in humans would be from dietary sources, smoking. and consumption of alcoholic beverages. In fact, the occurrence of β-carbo-lines in human blood under normal physiological conditions has been reported in the literature (2, 3, 8). Given their increasing toxicological importance, development of a simple and rapid analytical approach for clinical detection and quantitation of β-carboline derivatives in humans is highly desirable.
Several methods have been used for the quantitation of β-carboline derivatives in body fluids. Zetler et al. took advantage of the unique fluorescence of β-carbo-lines to estimate nine derivatives in rat brains (10). The method, however, lacked specificity as the authors failed to separate the mixed β-carbolines in tissue samples prior to fluorescent detection. Rommelspacher et al. employed thin-layer gel-plate separation and mul tiple extraction followed by high-performance liquid chromatography (HPLC) to detect 6-0H-tetrahy-dronorharmane (6-0H-THN) in rat and human sam ples (9). The substantial precolumn preparation in that study limited its application to clinical monitoring of this and other β-carbolines. Moncrieff also reported an HPLC method for tissue harmane, harmine, and harmaline (4). However, the quantitation of three compounds required three different mobile phases and three separate HPLC runs. A better HPLC method was developed by Adachi et al. (1) for determination norharmane and harmane in foodstuffs. Nevertheless, the adaptability of this method to biological remains uncertain. Moreover, to the best of our knowledge, there has been no report regarding the quantitation of harmane and harmine in human blood normal physiological conditions.
The objective of this study was to develop a simple and rapid analytical method to determine the concentrations of two major β-carboline derivatives, harmane and harmine, in human and rat blood. We have established a one-step extraction procedure followed HPLC separation and fluorescent quantitation of blood harmane and harmine. By using this method, we have demonstrated that these two tremorogenic β-carboline alkaloids are indeed presented in human and rat blood.
MATERIALS AND METHODS
Materials
Chemicals were obtained from the following sources: potassium phosphates, ethyl acetate, methyl-t-butyl ether, and methanol from Fisher Scientific (Pittsburgh, PA); and harmane (1-methyl-9H-pyrido[3,4-β]indole), harmine (7-methoxy-1-methyl-9H-pyrido[3,4-β]indole), and harmol (1-methyl-9H-pyrido[3.4-β]indol-7-ol) from Sigma (St. Louis, MO). All reagents were of analytical grade, HPLC grade, or the best available pharmaceutical grade.
Instrumentation
A Perkin–Elmer Model LC-250 binary liquid chromatographic system equipped with an LC-600 autosampler and an LS-40 fluorescence detector were used for analysis. Separation was accomplished using an ion-interaction, reversed-phase Econosphere C18 column (ODS2, 5 μm, 250 × 4.6 mm) attached to a Spherisorb guard column (ODS2, 5 μm, 10 × 4.6 mm). Both analytical and guard columns were purchased from Alltech (Deerfield, IL).
An isocratic mobile phase consisted of 17.5 mM potassium phosphate buffer (equal molar concentration of both monobasic and dibasic potassium salts with a pH of 6.5 adjusted by phosphoric acid} and methanol (30:70). The mobile phase was filtered through a 0.45-μm filter and degassed with helium. The column was frequently washed with 100% methanol. A 50-μl aliquot of samples was injected and the separation performed at room temperature at a flow rate of 1.0 ml/min at pressures between 2200 and 2500 psi. The detector was set at an excitation wavelength of 300 nm and an emission wavelength of 435 nm. A Macintosh computer equipped with Mac Integrator II (Rainin) was used to collect and analyze the data. Retention times were about 7–8 min for harmane and 10–11 min for harmine.
Sample preparation
Human and rat blood was collected in a heparinized tube and immediately frozen at –20°C. The samples were processed for extraction within 3 days. At the time of analysis, samples were thawed at room temperature. One volume (9–12 ml) of blood was mixed with half a volume (5–6 ml) of 1.0 M NaOH (2: 1) in a 50-ml conical Sarstedt tube. Following vortex for 30 s, the samples were placed on a horizontal rotator and shaken at room temperature for 30 min to destroy blood cells and solublize protein components. The extraction solution consisting of ethyl acetate and methyl-t-butyl ether (2:98) was added in a volume equivalent to the total volume of blood and NaOH (about 15 ml). The tube was vigorously shaken by hand for 1–2 min, followed by shaking on a horizontal rotator at room temperature for 45 min. After centrifugation at 3000g for 10 min, the upper organic phase (about 15 ml) was separated and transferred to another tube. The extraction procedure was repeated two additional times. The organic phase was combined and evaporated under nitrogen to dryness. The samples were reconstructed in 0.25 ml of methanol, transferred to autosampler vials with sealed caps, and stored in a refrigerator prior to HPLC analysis within 2 weeks.
Standard curves were constructed using harmane and/or harmine standards dissolved in the mobile phase to produce final concentrations of 0, 10, 25, 50, and 100 ng/ml followed by HPLC.
Detection limit and recovery
The detection limit is defined as the concentration of the drug which produces a signal/noise ratio of 3 (7) and thus is computed as
| [1] |
SD in the equation represents standard deviation of the measured mean. Harmane and harmine standards (final concentration of 25 ng/ml) were repeatedly injected for HPLC 12 times. Standard curves were established at the same time.
A recovery study was conducted by adding 10 ng of standard harmane and harmine in 3 ml of blood from human or rat. Another 3 ml of blood from the same human or rat without adding standards served as the blood blank, and the standards added in methanol without blood served as the control. All three groups of samples underwent the extraction procedure as described above. The absolute recovery was calculated from a standard curve by dividing the amounts of standards obtained after extraction (subtracting the values of blood blanks) by the amounts of standards originally added (Eq. [2]). The relative recovery was estimated by dividing the amounts of standards recovered from blood by those recovered from methanol controls (Eq . [3]). In some experiments, harmol (10 ng/ml) was added as an internal standard.
| [2] |
| [3] |
Precision and stability studies
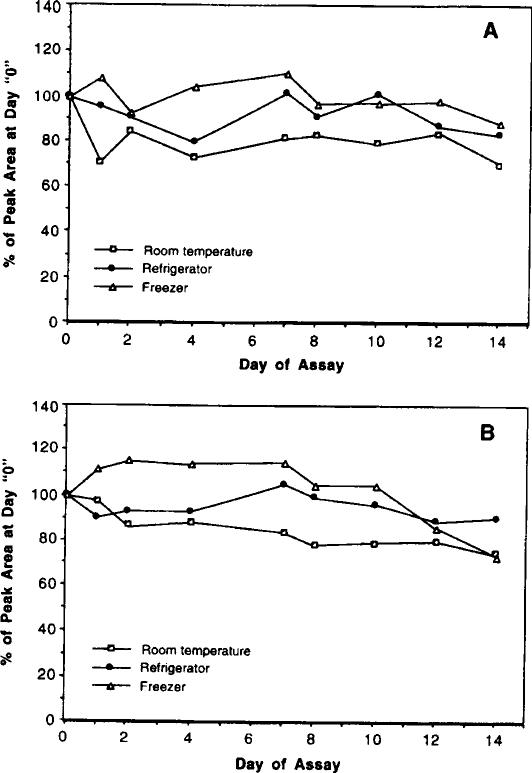
Both precision and stability studies were conducted at a final harmane and harmine concentration of 25 ng/ml. For intraday precision, the analytical procedure was repeated 10 to 12 times. The interday precision study was performed using the same procedure for 2 weeks. To study sample stability, harmane and harmine standards in methanol were allowed to stand in a refrigerator (4 °C), freezer (–20°C), or at room temperature and assayed by HPLC at day 0, 1, 2, 4, 7, 8, 10, 12, and 14.
RESULTS AND DISCUSSION
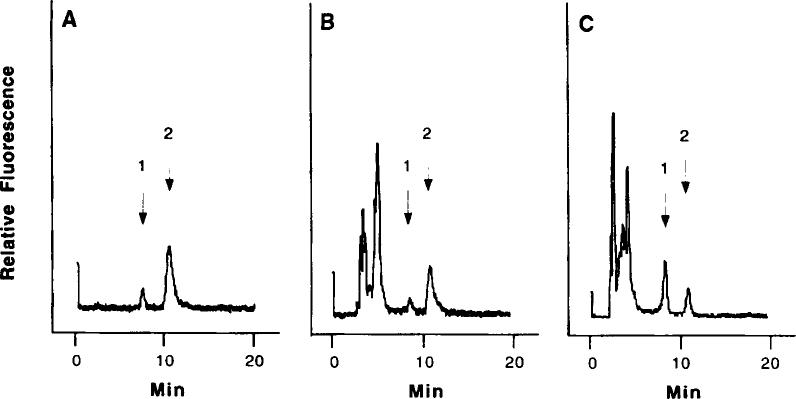
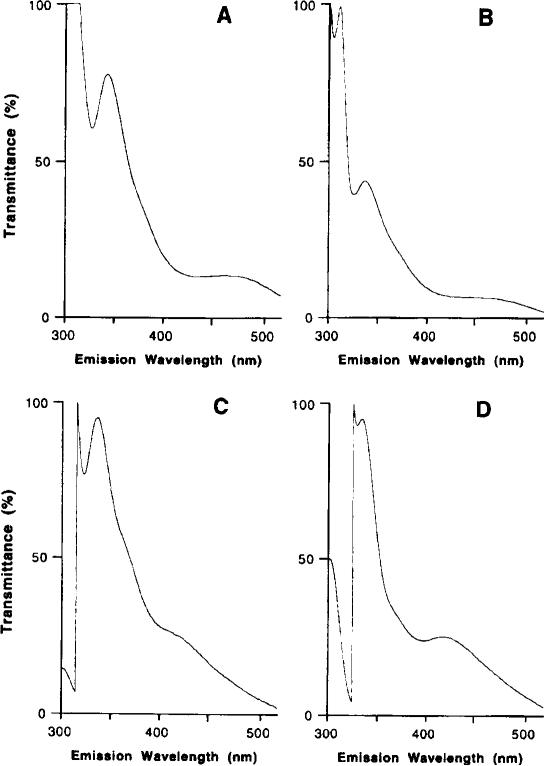
This method was established for determination of harmane and harmine in human blood under normal physiological conditions. It coupled one-step extraction with subsequent direct HPLC analysis. The method also took advantage of the unique fluorescent characteristics of harmane and harmine, which increases the assay sensitivity and eliminates interference from other substances present in the blood. For example, harmaline, another β-carboline analog, has a relative fluorescence about 71 times less than harmine at this wavelength setting. Both harmane and harmine were effectively separated under the current HPLC conditions (Fig. 1A). The emission wavelength scan of the fractions collected from HPLC further confirms that the emission spectra of blood standards resemble those of standards made in methanol (Fig. 2).
FIG. 1.
Typical HPLC traces of harmane and harmine. (A) Harmane and harmine standards in methanol (2.5 ng/50 μl); (B) harmane and harmine (6 ng) added in 10 ml of human blood; and (C) human blood sample from a patient (ID # 1803-0) without addition of harmane and harmine. Arrows indicate (1) harmane and (2) harmine.
FIG. 2.
Emission spectra of harmane and harmine in collected HPLC fractions. Harmane and harmine standards were made in either methanol or blood. The blood standards underwent extraction. followed by HPLC. The methanol standards were directly injected onto HPLC without extraction. The peak fractions at 7–8 and 10–11 min of each injection were collected and scanned for emission spec trum at an excitation wavelength of 300 nm. (A) Fraction of harmane in methanol; (B) fraction of harmane in blood; (C) fraction of harmine in methanol; and (D) fraction of harmine in blood.
This method can detect 240 and 100 pg of harmane and harmine, respectively, injected onto HPLC in 50 μl of the final assay solution. Concerning the absolute recovery of both compounds in blood (59% for harmane and 64% for harmine, Table 1), the method can detect 2 and 0.8 ng of harmane and harmine, respectively, in 0.25 ml of reconstructed HPLC solution after extraction. Depending on the initial blood volume available for extraction, the method can detect 21 0 pg/ml of harmane and 80 pg/ml of harmine, respectively, in 10 ml of human blood. Typical HPLC traces of harmane and harmine standard in human blood extracts and one blood sample from a patient are illustrated in Figs. 1B and 1C.
TABLE 1.
Recovery, Precision, and Linearity of HPLC Determination of Harmane and Harmine
| Harmane (%) | Harmine (%) | |
|---|---|---|
| Absolute recoverya | ||
| Human blood | 59.13 ± 5.30 | 63.93 ± 3.19 |
| Human blood (low concentration)b | 59.70 ± 1.71 | 56.90 ± 2.76 |
| Rat blood | 56.27 ± 6.65 | 58.40 ± 9.30 |
| Relative recoverya | ||
| Human blood | 104.6 ± 9.53 | 104.2 ± 6.83 |
| Rat blood | 99.43 ± 12.4 | 94.47 ± 14.1 |
| Intraday precision (25 ng/ml)c | ||
| At room temperature | 6.69 | 3.36 |
| Interday precision (25 ng/ml)c | ||
| At room temperature | 10.8 | 9.67 |
| In refrigerator (4°C) | 7.32 | 5.44 |
| In freezer (–20°C) | 6.28 | 9.49 |
| Correlation coefficient (r)d | 0.9990 ± 0.0010 | 0.9993 ± 0.0006 |
Note. Data represent means ± SD (n = 4–6) unless otherwise specified.
Concentrations tested at 3.3 ng/ml of blood for both harmane and harmine unless otherwise specified.
Concentrations tested at 0.6 ng/ml for harmane and 0.3 ng/ml for harmine.
Data represent percentages coefficient of variation (n = 10–12).
Concentrations of standard curves ranged from 0 to 100 ng/ml.
Moncrieff described an HPLC method to quantitate harmane, harmine, and harmaline in CSF and plasma without sample extraction (4). No data from humans were presented. We failed to detect harmane and harmine in human plasma by using that method, apparently because of extremely low amounts of these β-carbolines in less than 1 ml of plasma. In addition, Moncrieffs method required three different mobile phases and wavelength settings and separate HPLC runs to quantitate these compounds.
The intraday precision, measured as a coefficient of variation (CV) at 25 ng/ml, was less than 7% (n = 12) for harmane and 3% for harmine, respectively. The day-to-day precision was determined under different sample storage conditions. Both harmane and harmine in assay solution appeared to be somewhat unstable at room temperature. There were 30 and 26% losses in the peak areas of harmane and harmine, respectively, after a 2-week storage at room temperature (Fig. 3). The interday precision at room temperature was 11% for harmane and 10% for harmine (Table 1). In contrast, the samples stored in a refrigerator (4oC) for 2 weeks showed less fluctuation in the peak areas than did those stored at room temperature or in a freezer (Fig. 3). The interday precisions for samples in refrigerator were 7 and 5% for harmane and harmine, respectively. Since the possibility for β-carboline derivatives to be metabolized or autooxidized in blood cannot be excluded at present, we strongly recommend that the blood samples be processed for extraction within 3 days of collection.
FIG. 3.
Stability of harmane (A) and harmine (B) (25 ng/ml) in HPLC assay solution. Standard harmane and harmine were added to the assay solution at day 0. The samples were processed for HPLC analyses at the time indicated. Data represent means of three to four separate assays.
The recoveries of standard harmane and harmine from human and rat blood were made at 3.3 ng/ml. The absolute recovery of both compounds from human and rat blood ranged between 56 and 64% (Table 1), while the relative recoveries, compared to the compounds recovered from methanol, were between 95 and 105%. When harmol was used as the internal standard, it was well separated from harmane and harmine with a retention time of 5–6 min; however, the absolute recovery of harmol in blood was only about 2–3%, much less than those of harmane and harmine. The poor recovery of the internal standard may introduce an error in sample quantitation. Since the method without the internal standard was highly repeatable and reproducible, we recommend the use of absolute recovery of harmane and harmine to calculate the blood concentrations of these two β-carboline derivatives. Other suitable internal standards remain to be explored.
ACKNOWLEDGMENTS
This research was supported in part by NIEHS Grants P30-ES09089, R01-ES08146 (W.Z.), and R01-NS01863 (E.D.L.) and by the Paul Beeson Physician Faculty Scholars in Aging Research Award (E.D.L.).
REFERENCES
- 1.Adachi J, Mizoi Y, Naito T, Yamamoto K, Fujiwara S, Ninomiya I. Determination of β-carbolines in foodstuffs by high performance liquid chromatography and high performance liquid chromatograph–mass spectrometry. J. Chromatogr. 1991;538:331–339. doi: 10.1016/s0021-9673(01)88854-3. [DOI] [PubMed] [Google Scholar]
- 2.Allen RF, Beck O, Borg S, Skroder R. Analysis of 1-methyl-1,2,3,4-tetrahydro-β-carboline in human urine and cerebrospinal fluid by gas chromatography–mass spectrometry. Eur. J. Mass Spectrom. 1980;1:171–177. [Google Scholar]
- 3.Bidder TA, Shoemaker DW, Boettger HG, Evans M, Cummins JT. Harmane in human platelets. Life Sci. 1979;25:157–164. doi: 10.1016/0024-3205(79)90387-4. [DOI] [PubMed] [Google Scholar]
- 4.Moncrieff J. Determination of pharmacological levels of harmane, harmine and harmaline in mammalian brain tissue, cerebrospinal fluid and plasma by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. 1989;496:269–278. doi: 10.1016/s0378-4347(00)82576-1. [DOI] [PubMed] [Google Scholar]
- 5.O'Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine induced degeneration of Purkinje cells: A model of indirect, trans-synaptic excitotoxicity. J. Neurosci. 1997;17:8828–8841. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poindexter EH, Carpenter RD. The isolation of harmane and norharmane from tobacco and cigarette smoke. Phytochemistry. 1962;1:215–221. [Google Scholar]
- 7.Ren S, Scheuer ML, Zheng W. Determination of lamotrigine in biological materials by a simple and rapid liquid chromatographic method. Ther. Drug Monitor. 1998;20:209–214. doi: 10.1097/00007691-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Rommelspacher H, Strauss S, Lindemann J. Excretion of tetrahydroharmane and harmane into the urine of man and rat after load with ethanol. FEES Lett. 1980;109:209–212. doi: 10.1016/0014-5793(80)81088-x. [DOI] [PubMed] [Google Scholar]
- 9.Rommelspacher H, Barbey M, Strauss S, Greiner B, Fahndrich E. In: Beta-Carbolines and Tetrahydroisoquinolines. Bloom F, Barchas J, Sandler M, Usdin E, editors. A. R. Liss; New York: 1982. pp. 41–55. [Google Scholar]
- 10.Zetler G, Singbartl G, Schlosser L. Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7:237–248. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]