Abstract
Context
Ceramide causes endothelial apoptosis and emphysema-like changes in animal models.
Objectives
Test if plasma sphingomyelin, a major precursor of ceramide, would predict longitudinal increase in the percentage of emphysema-like lung on computed tomography (CT).
Materials and methods
3,840 participants had their plasma sphingomyelin measured at baseline examination and their pulmonary emphysema measured on cardiac CT scans at baseline and on follow-up visits. Mixed effects models were used to adjust for potential confounders.
Results
one standard deviation increase in sphingomyelin predicted a 0.12 % per year (95% CI: 0.02 to 0.22; p = 0.019) greater increase of percent emphysema.
Discussion and conclusion
Higher plasma levels of sphingomyelin predicted greater annual increase in quantitatively measured percent emphysema.
Keywords: Sphingomyelin, Ceramide, Emphysema, Computed Tomography
INTRODUCTION
Pulmonary emphysema is defined structurally by the presence of abnormal, permanent enlargement of airspaces distal to the terminal bronchioles with destruction of airway walls and without fibrosis.(Pauwels et al., 2001) Emphysema overlaps incompletely with chronic obstructive pulmonary disease (COPD),(Soriano et al., 2003) which is defined by airflow limitation that is not fully reversible.(Celli et al., 2004) Emphysema is not uncommon in the general population (Auerbach et al., 1972) and, assessed on computed tomography (CT), is associated with increased mortality and symptoms.(Haruna A et al., 2010, Zulueta et al., 2012)
In addition to protease-antiprotease imbalance, the pathogenesis of emphysema involves oxidative stress, inflammation, and cellular apoptosis.(Tuder et al., 2006, Petrache et al., 2011) All of these processes involve up-regulation of ceramide,(Petrache et al., 2005) a second-messenger lipid. Up-regulation of ceramide induces endothelial and epithelial apoptosis via caspases activation and death cell receptor clustering leading to pulmonary emphysema.(Petrache et al., 2011) Ceramide may also contribute to oxidative stress (Hannun and Obeid, 2002) and proteolytic effects in the lung.(Reunanen et al., 1998)
Sphingomyelin, a sphingolipid, is a basic constituent of cell membranes, an integral component of plasma phospholipids, and a major source of ceramide.(Levade et al., 1999) Plasma sphingomyelin is internalized into cells via apolipoprotein B and E receptor-mediated transport and hydrolyzed by lysosomal sphingomyelinase (L-aSMase) into intracellular ceramide (Levade et al., 1999) or can be degraded extracellularly by secretory acid sphingomyelinase (S-aSMase) into paracellular ceramide.(Petrache et al., 2011) Hence plasma sphingomyelin contributes to the intracellular and paracellular pool of ceramide in the lung, both of which are implicated in apoptotic signaling.(Petrache et al., 2011, Petrache and Petrusca DN, 2013, Medler et al., 2008)
Ceramide is increased in human lung specimens from patients with emphysema,(Petrache et al., 2005) but whether plasma levels of sphingomyelin predict progression of emphysema in human is unknown. We tested the hypothesis that plasma levels of sphingomyelin are associated with greater increases in the percentage of emphysema-like lung (percent emphysema) on CT scan and, secondarily, decline in lung function, in a large prospective cohort study.
METHODS
Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of subclinical cardiovascular disease that recruited 6,814 participants in 2000-02 at six clinical sites.(Bild et al., 2002) Written informed consent was obtained from all participants. The protocols were approved by the institutional review boards of all collaborating institutions and by the National Heart, Lung, and Blood Institute.
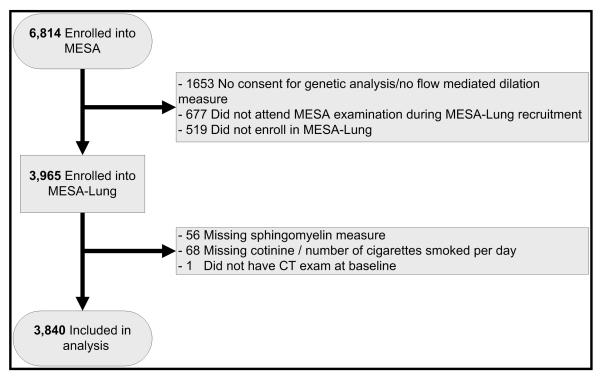
The MESA Lung Study enrolled 3,965 MESA participants who completed baseline measures of flow-mediated dilation, consented to genetic analyses and underwent a MESA examination between 2004 and 2006 (Figure 1). Participants missing information on sphingomyelin or smoking (n=125) were excluded from the current analysis.
Figure 1.
Flow chart of the Multi-Ethnic Study of Atherosclerosis (MESA) and MESA Lung study recruitment.
Plasma Sphingomyelin
Plasma sphingomyelin levels were measured in a blinded fashion using a rapid, sensitive, and high-throughput four step enzymatic assay, as previously described by one of the coauthors.(Hojjati and Jian, 2006) This approach has been previously validated against the classic method(Bligh and Dyer, 1959, Bartlett, 1959) and the two methods were found to be well correlated (r=0.91, P<0.01).(Jiang XC et al., 2000) The interassay coefficient of variation was 1.7 ± 0.05%.(Hojjati and Jian, 2006)
Percent of Emphysema-like Lung
Quantitative measures of emphysema were performed on the lung fields of cardiac CT scans, which imaged approximately 70% of the lung volume from the carina to the lung bases. CT scans were performed at full inspiration on multi-detector CT (MDCT) and electron-beam tomography (EBT) scanners following a standardized protocol.(Carr et al., 2005) Two scans were performed at each visit; the scan with the higher air volume was used for analyses except in cases of discordant scan quality, in which case the higher quality scan was used.(Hoffman et al., 2009)
Image attenuation was assessed using modified Pulmonary Analysis Software Suite(Zhang et al., 2006) at a single reading center by trained readers without knowledge of other participant information. To correct for variation in scanner calibration, scatter, and beam hardening, we adjusted all CT values for the attenuation of air outside the body, which should measure −1,000 Hounsfield Units (HU). Percent emphysema was defined as the percentage of the total voxels in the lung which fell below −910 HU. This threshold was chosen based upon pathology comparisons(Coxson et al., 1995) and the generally mild degree of emphysema in this sample. Sensitivity analyses were performed using a −950 HU threshold. Percent emphysema measures from the carina to lung base are highly correlated (r=0.99) with full-lung measures on the same full-lung scans in smokers(Reddy and Barr RG, 2005) and have been previously validated against full-lung scans in this cohort.(Hoffman et al., 2009)
All 3,840 participants underwent scanning at the baseline visit, 32% were randomly selected to undergo repeat scanning at a median follow-up of 19 months; another 59% underwent repeat scanning at a median follow-up of 37 months, and a randomly selected 26% underwent scanning a third time at a median follow-up of 56 months. Scanner changes were minimized: 60% of patients were scanned on the same scanner over the seven-year study period, 39% had one scanner change, and one percent had two (see Online Supplement Table 1 for list of scanners and Online Supplement Table 2 for consistency of scanning). Calibration of scanners was confirmed throughout the study via calcium hydroxyapatite phantoms (Carr et al., 2005) and assessment of attenuation of air outside the body, which showed very stable results over time (Online Supplement Figure 1). Exceptions were Toshiba Aquillion scanners, which were used briefly at one site, and a General Electric (GE) Pro16 scanner.
Spirometry
Spirometry was conducted in 2004-06 and 2010-12 in accordance with the American Thoracic Society/European Respiratory Society guidelines(Miller et al., 2005) on a dry-rolling-sealed spirometer (Occupational Marketing, Inc., Houston, TX). All spirometry exams were reviewed by one investigator.(Hankinson et al., 2010) Airflow limitation was defined as the forced expiratory volume in 1 second (FEV1) to the forced vital capacity (FVC) ratio less than the lower limit of normal.(Pellegrino et al., 2005)
Smoking and Other Covariates
Age, gender, race/ethnicity, education, family history of emphysema, asthma, and number of cigarettes smoked per day were self-reported. Use of lipid lowering agents was ascertained by medication questionnaire.(Psaty et al., Volume 45, Issue 6, June 1992, Pages 683–692) Current smoking status at baseline was verified with urinary cotinine level (Immulite 2000 Nicotine Metabolite Assay; Diagnostic Products Corp., Los Angeles, CA).(Rodriguez et al., 2010) Total serum cholesterol, high density lipoprotein (HDL), LDL, triglycerides, systolic and diastolic blood pressure, height and weight were measured using standard techniques.
Statistical Analysis
The cohort was stratified by quintiles of sphingomyelin for descriptive purposes. Mixed effects models were used to assess the relationship of baseline sphingomyelin level as a continuous term to change in percent emphysema over time. The initial model for percent emphysema included sphingomyelin, time since baseline exam, their interaction and age, gender, race/ethnicity, height, weight, body mass index (BMI), and CT scanner model and milliamperes (mAs). The full model additionally included educational attainment, number of cigarettes smoked per day among current smokers, pack-years (for ever-smokers), urinary cotinine level, family history of emphysema, diagnosis of asthma before the age of 45, total serum cholesterol, HDL, LDL, triglycerides, systolic and diastolic blood pressure, lipid lowering agents, and study site. Height, weight, BMI, scanner model, mAs, and cigarettes per day were treated as time-varying covariates. Model distributional assumptions were confirmed.
Effect modification was assessed in the full multivariate model. Secondary analyses were stratified by smoking history, race/ethnicity, and gender; excluded participants with restriction on spirometry defined as FVC below the lower limit of normal(Hankinson et al., 1999) with FEV1 to FVC ratio above 0.70; limited to participants without scanner change; stratified by CT scanner type; after the exclusion of scans acquired on the Toshiba Aquillion and GE Pro16 scanners, and additionally controlling for restrictive lung disease measured by high attenuation areas (HAAs).(Lederer et al., 2009) Analyses for lung function used a similar statistical approach, and cross-sectional analyses utilized linear regression.
Statistical significance was defined as two-tailed P-value<0.05. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC) and R statistical software, version 2.6 (R Foundation for Statistical Computing, Vienna).
RESULTS
The 3,840 participants had a mean age at baseline of 61±10 years, 50% were male, and the race/ethnic distribution was 36% non-Hispanic White, 26% African-American, 23% Hispanic, and 16% Asian. Thirteen percent were current cigarette smokers, 39% had smoked in the past, and 48% had never smoked. The mean plasma sphingomyelin was 48±14 mg/dL and the median percent emphysema-910 Hounsfield Units was 18% (IQR=9, 29) at baseline. Twelve percent of participants had airflow limitation.
There were 8,333 measures of percent emphysema over a median follow-up of 3.1 years (range 0.9, 6.8) with most participants assessed at two time points. The mean rate of percent emphysema increase was 0.30% per year (95% CI: 0.21, 0.39) during the follow-up.
Table 1 shows the characteristics of the participants at baseline, stratified by quintiles of plasma sphingomyelin. Those in the higher quintiles of plasma sphingomyelin tended to be older, female, of African-American or Hispanic origin and lower socioeconomic status. Participants in the higher quintiles also tended to be never smokers and to have higher levels of total serum cholesterol and its subfractions.
Table 1.
Characteristics of MESA Lung Study Participants at Baseline Stratified by Quintiles of Plasma Sphingomyelin Level.
| Quintiles of Sphingomyelin, (range in mg/dL) | |||||
|---|---|---|---|---|---|
| 1st Quintile (19 – 35) |
2nd Quintile (36 – 42) |
3rd Quintile (43 – 49) |
4th Quintile (50 – 59) |
5th Quintile (60 – 125) |
|
|
|
|||||
| n= 799 | n= 778 | n= 746 | n= 773 | n= 744 | |
| Age, mean (SD), years | 61 (10) | 60 (10) | 61 (10) | 62 (10) | 63 (10) |
| Male gender, (%) | 62% | 55% | 48% | 46% | 36% |
| Race/ethnicity, (%) | |||||
| - White (Non-Hispanic) | 43% | 38% | 36% | 33% | 29% |
| - Chinese-American | 15% | 18% | 17% | 17% | 14% |
| - Black (Non-Hispanic) | 22% | 23% | 26% | 27% | 31% |
| - Hispanic | 20% | 22% | 21% | 24% | 26% |
| Education, years, (%) | |||||
| - <High school | 14% | 17% | 15% | 18% | 20% |
| - High school graduate | 18% | 17% | 17% | 20% | 20% |
| - Some college | 28% | 27% | 28% | 27% | 28% |
| - College graduate | 20% | 18% | 18% | 18% | 17% |
| - >Bachelor’s degree | 20% | 21% | 22% | 17% | 15% |
| Height, mean (SD), cm | 169 (9) | 168 (10) | 167 (10) | 166 (10) | 164 (10) |
| Weight, mean (SD), Kg | 79 (16) | 78 (18) | 78 (17) | 78 (17) | 77 (18) |
| Body mass index, mean (SD), kg/m2 | 27.8 (4.9) | 27.9 (5.4) | 28.0 (5.1) | 28.3 (5.4) | 28.4 (5.5) |
| Cigarette smoking,(%) | |||||
| - Never | 40% | 48% | 50% | 51% | 50% |
| - Past | 44% | 38% | 37% | 37% | 38% |
| - Current | 16% | 14% | 13% | 12% | 12% |
|
Cigarettes smoked /day, median (IQR) * |
10 (5, 20) 22902 |
15 (6, 20) 23282 |
10 (3, 18) 22294 |
10 (5, 15) 16711 |
10 (5, 20) 24907 |
| Cotinine, median (IQR), nmol/L * | (7855, 46678) |
(9025, 40197) |
(7407, 37744) |
(5578, 37107) |
(7793, 45997) |
| Pack-years, median (IQR) † | 14 (3, 35) | 16 (4, 34) | 17 (4, 32) | 14 (2, 30) | 17 (6, 34) |
|
Total cholesterol, mean (SD),
mmol/L |
4.79 (0.80) | 4.90 (0.88) | 4.97 (0.83) | 5.05 (0.91) | 5.39 (0.96) |
| Triglycerides, mean (SD), mmol/L | 1.22 (0.52) | 1.31 (0.61) | 1.47 (0.71) | 1.50 (0.87) | 1.85 (1.27) |
| LDL, mean (SD), mmol/L | 2.95 (0.73) | 2.98 (0.80) | 3.00 (0.78) | 3.03 (0.80) | 3.24 (0.83) |
| HDL, mean (SD), mmol/L | 1.30 (0.34) | 1.30 (0.36) | 1.30 (0.36) | 1.32 (0.39) | 1.37 (0.44) |
|
Systolic Blood Pressure, mean (SD),
mmHg |
125 (19) | 123 (19) | 125 (20) | 125 (20) | 126 (21) |
|
Diastolic Blood Pressure, mean (SD),
mmHg |
73 (10) | 72 (10) | 72 (10) | 71 (10) | 71 (10) |
| Family history of emphysema, (%) | 5% | 4% | 5% | 3% | 4% |
| Airflow Limitation, (%) ‡ | 14% | 11% | 10% | 13% | 10% |
| CT scanners at Baseline | |||||
| - Siemens S4+ Volume Zoom | 239 (30%) | 199 (26%) | 172 (23%) | 206 (27%) | 184 (25%) |
| - Imatron C-150 | 426 (53%) | 778 (59%) | 462 (62%) | 459 (59%) | 460 (62%) |
| - GE Light Speed QX/I | 27 (4%) | 21 (3%) | 18 (2%) | 13 (2%) | 12 (2%) |
| - GE Light Speed Plus | 107 (13%) | 98 (13%) | 94 (13%) | 95 (12%) | 88 (12%) |
| CT scanners at Last Follow-Up | |||||
| - Siemens S4+ Volume Zoom | 96 (13%) | 69 (9%) | 56 (8%) | 63 (8%) | 46 (6%) |
| - Siemens Sensation 16 | 72 (9%) | 77 (10%) | 68 (9%) | 83 (11%) | 74 (10%) |
| - Siemens Sensation 64 | 43 (6%) | 57 (8%) | 75 (10%) | 79 (11%) | 104 (15%) |
| - Imatron C-150 | 368 (48%) | 383 (51%) | 369 (51%) | 359 (48%) | 336 (47%) |
| - GE Light Speed Plus | 8 (1%) | 5 (1%) | 3 (0%) | 13 (2%) | 6 (1%) |
| - GE Light Speed Pro 16 | 123 (16%) | 111 (15%) | 107 (15%) | 94 (13%) | 92 (13%) |
| - Toshiba Aquilion 32 | 3 (0%) | 5 (1%) | 3 (0%) | 9 (1%) | 7 (1%) |
| - Toshiba Aquilion 64 | 52 (7%) | 45 (6%) | 38 (5%) | 44 (6%) | 52 (7%) |
Among current smokers at baseline exam.
Pack-years among ever smokers at baseline exam.
Defined by FEV1/FVC ratio < lower limit of normal.
Longitudinal Association of Sphingomyelin with Percent Emphysema
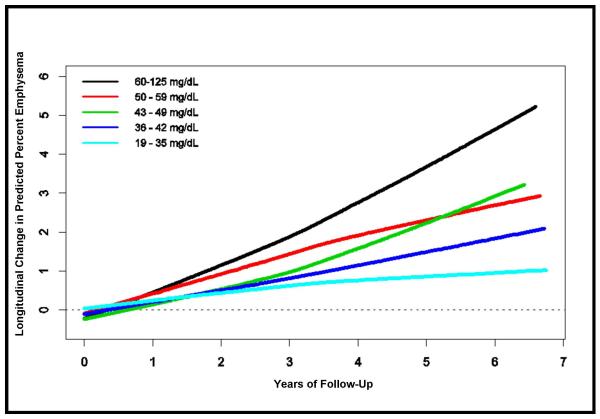
There was a monotonic association between plasma sphingomyelin level and longitudinal change in percent emphysema across quintiles of sphingomyelin (Table 2) in the initial/smaller model. This association remained significant after adjustment for the additional potential confounders listed in Table 2. A one standard deviation increment in baseline sphingomyelin level was associated with a 0.12% greater annual increase in percent emphysema (95% CI: 0.02, 0.22; P=0.019). As shown in Figure 2, these relationships were approximately linear and consistent over about seven years of follow-up.
Table 2.
Mean Annual Rate of Change in Percent Emphysema, by to Baseline Plasma Sphingomyelin Level.
| Quintiles of Plasma Sphingomyelin | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Mean Annual Change in Percent Emphysema per 1 standard deviation increase in baseline SM |
p- Value* |
|
| Model 1 | Ref. | 0.003 | 0.016 | 0.103 | 0.247 | 0.105 (0.015, 0.195) |
0.022 |
| Model 2 | Ref. | 0.031 | 0.034 | 0.152 | 0.263 | 0.119 (0.020, 0.219) |
0.019 |
Model 1 controlled for age, gender, race, height, weight, BMI, scanner model and protocol.
Model 2 included model 1 variables and further controlled for educational attainment, number of cigarettes smoked per day among current smokers, cumulative pack-years, urinary levels of cotinine at baseline exam, family history of emphysema, having asthma before the age of 45, total serum cholesterol, HDL, LDL, triglycerides, intake of lipid lowering agents, systolic and diastolic and blood pressure, and study field center.
P-value for the relationship of mean annual change in percent emphysema to sphingomyelin level expressed as a continuous variable.
Figure 2.
Mean Longitudinal Change in Predicted Percent Emphysema by Quintiles of Plasma Sphingomyelin
The direction and magnitude of the association were similar when the analysis was limited to observations in which the same scanner was used over follow-up, although statistical significance was attenuated given the smaller sample size (0.11%, 95% CI:-0.017, 0.24; P=0.09 among 2,317 participants with 4,786 scans). Results were consistent after stratification by scanner type (0.15%, 95% CI: −0.01, 0.31; P=0.06 for MDCT scanners and 0.11%, 95% CI: −0.017, 0.24; P=0.09 for EBT scanners) and after the exclusion of 313 participants with restrictive spirometry (0.13%, 95% CI: 0.03, 0.24; P=0.014) or the use of Toshiba Aquillion and GE Pro16 scanners (0.13%, 95% CI= 0.02, 0.23; P=0.02) but were attenuated by the use of a −950 HU threshold to define percent emphysema (0.005%, 95% CI: −0.004, 0.013; P=0.23). Additional adjustment by controlling for HAAs yielded consistent, significant results (0.12%, 95% CI: 0.04, 0.21; P= 0.007).
There was no evidence that the association between sphingomyelin level and increase in percent emphysema was modified by smoking status, race/ethnicity or gender (interaction P-values are P=0.31, P=0.76 and P=0.22, respectively; Online Supplement Table E3).
Longitudinal Association of Sphingomyelin with Lung Function
There was no evidence that sphingomyelin level was associated with longitudinal decline in FEV1 or FEV1/FVC ratio (−0.02 mL, 95% CI: −2, 2; P= 0.98 and 0.036%, 95% CI: −0.006, 0.079; P=0.10, respectively) among 3192 participants with repeat measures of spirometry over an interval of six years.
Cross-sectional Associations of Sphingomyelin with Percent Emphysema and Lung Function
Despite the strong, graded association of sphingomyelin levels with longitudinal change in percent emphysema, there was no evidence that sphingomyelin levels were associated with percent emphysema at baseline (multivariate mean difference 0.04%, 95% CI: −0.37, 0.45; P=0.84 - Online Supplement Table E4). Higher baseline levels of sphingomyelin were associated with modestly higher values of the FEV1 but not FEV1/FVC ratio (multivariate mean difference 24, 95% CI: 9, 40; P=0.002 and 0.11, 95% CI: −0.16, 0.39; P=0.42, respectively- Online Supplement Table E5).
DISCUSSION
Higher levels of plasma sphingomyelin, the precursor of ceramide, was associated with greater longitudinal increases in percent emphysema on CT scan in this large prospective cohort study. The association was dose-dependent and was not modified by smoking status. Given the prospective design, the mostly subclinical disease, and the lack of apparent significant confounding, the current findings provide evidence that the ceramide pathway of emphysema development found in animal studies may apply to humans.
Ceramide, as an apoptotic mediator, had been shown to be increased in several clinical scenarios including Alzheimer’s disease,(Filippov et al., 2012) atherosclerosis,(Devlin et al., 2008) and radiation induced tumor lysis.(Hannun and Obeid, 2008) In lungs, ceramide up-regulation is associated with pulmonary epithelial and endothelial cell apoptosis, decreased apoptotic cell clearance, potentiation of inflammatory response, and increased matrix proteolysis leading ultimately to alveolar airspace enlargement and emphysema.(Petrache et al., 2011) Cigarette smoke exposure, the main risk factor for emphysema, increases murine and human pulmonary ceramide levels via oxidative stress and decreased vascular endothelial growth factor (VEGF) signaling.(Petrache et al., 2005, Petrache et al., 2011)
In addition to its de novo synthesis, ceramide is produced from sphingomyelin under the effect of sphingomyelinase enzyme, of which two isoenzymes are mainly found in lung tissue: acid sphingomyelinase (lysosomal: L-aSMase and secretory: S-aSMase) and neutral sphingomyelinase (nSMase). Petrache and colleagues showed that VEGF receptor blockade or instillation of exogenous ceramide increase endogenous lung ceramide level via induction of ceramide synthase and S-aSMase.(Petrache et al., 2005) Thus, abundant plasma sphingomyelin provides copious substrate for activated S-aSMase and ultimately increases local apoptotic ceramide levels.
Although the present study is the first to demonstrate the positive relationship of plasma sphingomyelin to annual increase in percent emphysema in humans, several clinical disease states suggest that this mechanism is biologically plausible. In Nieman-Pick disease, deficiencies of both lysosomal and secretory aSMase result in accumulation of sphingomyelin and, hypothetically, lower levels of ceramide. Lack of ceramide should hinder apoptosis and thus prevent emphysema in those patients. Consistent with this line of reasoning, most patients with Nieman-Pick disease have increased lung mass, predisposition to interstitial lung disease,(Guillemot et al., 2007) and are relatively protected against lung architecture destruction which is the essence of emphysema.(Guillemot et al., 2007) Our findings, however, are not explained by subclinical interstitial lung disease as additional adjustment for %HAA, a measure of subclinical interstitial lung disease, had no impact on the results.
A major strength of this study was its longitudinal design of repeated, precise CT measures in a large sample. Several potential limitations, however, are worthy of comment.
Percent emphysema was assessed on partial lung CT scans that did not include the lung apices; hence inferences are limited for apical emphysema, i.e., classically smoking-related emphysema. However, we have previously validated these scans compared to full-lung CT scans,(Hoffman et al., 2009) the protocol was highly standardized, and the breath-holding technique was similar to recent lung CT studies.(Carr et al., 2005)
A minority of patients was scanned on different scanners during follow-up. This was inevitable given the seven-year duration of the study, yet the total number of scanners used was small, the scanners were very stable over seven years, and analyses restricted to patients scanned on single scanners yielded consistent results. Standardization of CT values using outside air HU density also reduced technical variation between scanners. Furthermore, particular attention was made during analysis to adjust for the effect of scanner change by controlling for the scanner model and scanning mAs.
The use of plasma sphingomyelin level as a surrogate for pulmonary tissue ceramide level may be considered as a limitation. Yet, given that vascular endothelium represents a rich source of sphingomyelinase(Marathe et al., 1998) and since all its isoenzymes share the same principal function of hydrolyzing the sphingomyelin into ceramide, it is plausible to assume that a significant percentage of plasma sphingomyelin will be hydrolyzed by pulmonary endothelium sphingomyelinase upon smoke exposure(Petrache et al., 2005, Filosto et al., 2011) resulting in escalation of the local apoptotic ceramide pool and eventually leading to progression of emphysema.
The study was conducted among a population-based sample with relatively low levels of emphysema and a modest prevalence of airflow limitation, which makes our conclusions applicable to the general population and unlikely to be confounded by medication use for COPD. The generalizability of the results to patients with clinical COPD, however, is not definitely demonstrated in the current study.
In addition, sphingomyelin was associated with increases in percent emphysema but not with accelerated decline in lung function. This specificity matches the animal studies(Reddy et al., 2011) and is consistent with studies suggesting emphysema and spirometric obstruction overlaps less frequently than previously thought.(Soriano et al., 2003)
Confounding can potentially bias observational studies. Major confounders were measured precisely and included in models. In particular, smoking status was verified with cotinine levels at baseline. Smoking history is unlikely to be a confounder as the main association did not change after adjusting for smoking history. The results were consistent in stratified analyses and the association was, if anything, of greater magnitude among never smokers. The observation may be due to misclassification of percent emphysema measures by current smoking, which would be expected to attenuate the relationship among current smokers.
Finally, although we observed a strong and graded longitudinal relationship between sphingomyelin levels and rate of increase in percent emphysema, there was no evidence for a cross-sectional relationship. This situation is not, however, unusual and longitudinal results often conflict with cross-sectional ones, the former being preferable given its superior ascertainment of temporality, alleviation of recall bias, controlling for cohort effect, and limiting error introduced by interindividual variability.(van Belle et al., 2004)
In conclusion, plasma sphingomyelin level was associated positively with higher annual increase in quantitative pulmonary emphysema measures on CT scan in a large prospective cohort study. These findings provide the first support for the endothelial hypothesis of emphysema in a prospective cohort study and also suggest that sphingomyelin may be of utility as a biomarker of emphysema progression.
Supplementary Material
Acknowledgment
Special thanks also are directed to Jaime Madrigano, BE, MPH, ScD (Research Scientist, Earth Institute, Columbia University) for creating the graph of the outside air density by different scanners over the follow up time.
Funding: NIH R01 HL077612, R01 HL075476, N01-HC-95159 through N01-HC-95169, and RR032646.
Role of Funding Source: MESA and MESA Lung Study were funded by the National Institute of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI). The MESA Lung Study was designed by the study investigators. The authors, together with other MESA investigators, collected and analyzed the data, wrote the manuscript for publication. NHLBI staff monitored study performance routinely and participated in the internal review of the manuscript before submission.
Footnotes
- - FSA did literature review, conducted statistical analysis, wrote the paper, and made the tables and graph.
- - RGB designed the study, edited the paper, and supervised statistical analysis.
- - JES constructed the statistical models.
- - XJ measured plasma sphingomyelin.
- - EAH measured CT emphysema values.
- - XJ, JES, EAH, JY, SS, KMB, and RGB critically reviewed the paper.
- - FSA, XJ, JES, JY, SS, KMB declared no conflict of interest.
- - RGB was reimbursed for travel to the Trans Atlantic Airways Conference by Boehringer Ingelheim.
- - EAH has interest in VIDA Diagnostics.
License for publication R. Graham Barr, MD, DrPH, the corresponding author, has the right to grant on behalf of all authors and does grant on behalf of all authors, a full copyright assignment to Biomarkers.
References
- MESA Manual of Operations . Field Center and Laboratory Procedures [Online] University of Washington; Seattle: [Accessed December 23 2009]. Available: http://www.mesa-nhlbi.org/manuals.aspx. [Google Scholar]
- Auerbach O, Hammond E, Garfinkel L, et al. Relation of Smoking and Age to Emphysema - Whole-Lung Section Study. New England Journal of Medicine. 1972;286:853–957. doi: 10.1056/NEJM197204202861601. [DOI] [PubMed] [Google Scholar]
- Bartlett G. Phosphorus Assay in Column Chromatography. The Journal of biological chemistry. 1959;234:466–468. [PubMed] [Google Scholar]
- Bild D, Bluemke D, Burke G, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. American Journal of Epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bligh E, Dyer W. A Rapid Method for Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Carr JJ, Nelson JC, Wong ND, et al. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- Celli B, Macnee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. 2004. 932-46. [DOI] [PubMed] [Google Scholar]
- Coxson HO, Mayo JR, Behzad H, et al. Measurement of Lung Expansion with Computed Tomography and Comparison with Quantitative Histology. Journal of Applied Physiology. 1995;79:1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- Devlin CM, Leventhal AR, Kuriakose G, et al. Acid Sphingomyelinase Promotes Lipoprotein Retention Within Early Atheromata and Accelerates Lesion Progression. Atherosclerosis, Thrombosis, and Vascular Biology. 2008;28:1723–1730. doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Song MA, Zhang K, et al. Increased Ceramide in Brains with Alzheimer’s and Other Neurodegenerative Diseases. Journal of Alzheimer’s Disease. 2012;29:537–47. doi: 10.3233/JAD-2011-111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosto S, Castillo S, Danielson A, et al. Neutral Sphingomyelinase 2: A Novel Target in Cigarette Smoke–Induced Apoptosis and Lung Injury. American Journal of Respiratory Cell and Molecular Biology. 2011;44:350–360. doi: 10.1165/rcmb.2009-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot N, Troadec C, Villemeur TBD, et al. Lung Disease in Niemann-Pick Disease. Pediatric Pulmonology. 2007;42:1207–1214. doi: 10.1002/ppul.20725. [DOI] [PubMed] [Google Scholar]
- Hankinson J, Kawut S, Shahar E, et al. Performance of American Thoracic Society-Recommended Spirometry Reference Values in a Multiethnic Sample of Adults: The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest. 2010;137:138–45. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson J, Odencrantz J, Fedan K. Spirometric Reference Values From a Sample of The General U.S. Population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Hannun Y, Obeid L. The Ceramide-Centric Universe of Lipid-Mediated Cell Regulation: Stress Encounters of The Lipid Kind. The Journal of Biological Chemistry. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of Bioactive Lipid Signalling: Lessons From Sphingolipids. Nature Reviews Molecular Cell Biology. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Haruna A, Muro S, Nakano Y, et al. CT Scan Findings of Emphysema Predict Mortality in COPD. Chest. 2010;138:635–40. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and Validity of Lung Density Measures from Cardiac CT scans—The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Academic Radiology. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojjati M, Jian X. Rapid, Specific, and Sensitive Measurements of Plasma Sphingomyelin and Phosphatidylcholine. Journal of Lipid Research. 2006;47:673–676. doi: 10.1194/jlr.D500040-JLR200. [DOI] [PubMed] [Google Scholar]
- Jiang Xc, Paultre F, Pearson Ta, et al. Plasma Sphingomyelin Level As a Risk Factor for Coronary Artery Disease. Atherosclerosis, Thrombosis, and Vascular Biology. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- Lederer D, Enright P, Kawut S, et al. Cigarette Smoking is Associated with Subclinical Parenchymal Lung Disease: The Multi-Ethnic Study of Atherosclerosis (MESA)-Lung Study. American Journal of Respiratory and Critical Care Medicine. 2009;180:407–14. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levade T, Andrieu-Abadie N, SéGui BA, Nathalie, et al. Sphingomyelin-Degrading Pathways in Human Cells: Role in Cell Signalling. Chemistry and Physics of Lipids. 1999;102:167–178. doi: 10.1016/s0009-3084(99)00085-7. [DOI] [PubMed] [Google Scholar]
- Marathe S, Schisse S, Yellin M, et al. Human Vascular Endothelial Cells are a Rich and Regulatable Source of Secretory Sphingomyelinase. The Journal of Biological Chemistry. 1998;273:4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- Medler TR, Petrusca DN, Lee PJ, et al. Apoptotic Sphingolipid Signaling by Ceramides in Lung Endothelial Cells. American Journal of Respiratory Cell and Molecular Biology. 2008;38:639–646. doi: 10.1165/rcmb.2007-0274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of Spirometry. European Respiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Pauwels R, Buist A, Calverley P, et al. Global Strategy for The Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative Strategies for Lung Function Tests. European Respiratory Journal. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Petrache I, Natarajan V, Zhen L, et al. Ceramide Upregulation Causes Pulmonary Cell Apoptosis and Emphysema-Like Disease in Mice. Nature Medicine. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrache I, Petrusca Dn. The involvement of sphingolipids in chronic obstructive pulmonary diseases. In: Gulbins E, Petrache I, editors. Handbook of Experimental Pharmacology. Springer-Verlag Wien; 2013. [DOI] [PubMed] [Google Scholar]
- Petrache I, Petrusca DN, Bowler RP, et al. Involvement of Ceramide in Cell Death Responses in the Pulmonary Circulation. Proceeding of American Thoracic Society. 2011;8:492–496. doi: 10.1513/pats.201104-034MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaty BM, Lee M, Savage PJ, et al. Assessing The Use of Medications in The Elderly: Methods and Initial Experience in The Cardiovascular Health Study. Journal of Clinical Epidemiology. 1992 Jun;45(Issue 6):683–692. doi: 10.1016/0895-4356(92)90143-b. Volume 45. Pages 683–692. [DOI] [PubMed] [Google Scholar]
- Reddy J, Mccauley K, Goya J, et al. Correlations of Lung Ceramide Expression with Clinical Parameters in COPD. American Thoracic Soceity Annual Meeting; Denver, Colorado. American Jouranl of Respiratory and Critical Care Medicine; 2011. [Google Scholar]
- Reddy SB, Barr Rg BK. Quantitative Evaluation of Emphysema Presence and Distribution Via Coronary Calcium CT Compared with Full Lung Study. The International Conference of the Radiological Society of North America; Chicago, IL. November 27-December 2, 2005; 2005. Abstract presented at. [Google Scholar]
- Reunanen N, Westermarck J, Häkkinen L, et al. Enhancement of Fibroblast Collagenase (Matrix Metalloproteinase-1) Gene Expression by Ceramide is Mediated by Extracellular Signal-Regulated and Stress-Activated Protein Kinase Pathways. The Journal of Biological Chemistry. 1998;273:5137–5145. doi: 10.1074/jbc.273.9.5137. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Jiang R, Johnson WC, et al. The Association of Pipe and Cigar Use with Cotinine Levels, Lung Function and Airflow Obstruction: a Cross-sectional Study. Annals of Internal Medicine. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano J, Davis K, Coleman B, et al. The Proportional Venn Diagram of Obstructive Lung Disease. Chest. 2003;124:474–481. doi: 10.1378/chest.124.2.474. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Yoshida T, Fijalkowka I, et al. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proceedings of the American Thoracic Society. 2006;3:673–9. doi: 10.1513/pats.200605-124SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle G, Fisher L, Heagerty PJ, et al. Biostatistics: A Methodology for the Health Sciences. John Wiley & Sons; 2004. Chapter 18: Longitudinal Data Analysis. [Google Scholar]
- Zhang L, Hoffman EA, Reinhardt J. Atlas-Driven Lung Lobe Segmentation in Volumetric X-Ray CT Images. IEEE Trans Med Imaging. 2006;25:1–16. doi: 10.1109/TMI.2005.859209. [DOI] [PubMed] [Google Scholar]
- Zulueta J, Wisnivesky J, Henschke C, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141:1216–23. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.