Abstract
Current influenza vaccines do not provide good protection against antigenically different influenza A viruses. As an approach to overcome strain specificity of protection, this study demonstrates significantly improved long-term cross protection by supplementing split vaccines with a conserved molecular target, a repeat of the influenza M2 ectodomain (M2e) expressed on virus-like particles (M2e5x VLPs) in a membrane-anchored form. Intramuscular immunization with H1N1 split vaccine (A/California/07/2009) supplemented with M2e5x VLPs induced M2e-specific humoral and cellular immune responses, and shaped the host responses to the vaccine in the direction of T-helper type 1 responses inducing dominant IgG2a isotype antibodies as well as interferon-γ (IFN-γ) producing cells in systemic and mucosal sites. Upon lethal challenge, M2e5x VLP-supplemented vaccination lowered lung viral loads and induced long-term cross protection against H3N2 or H5N1 subtype influenza viruses over 12 months. M2e antibodies, CD4 T cells, and CD8 T cells were found to contribute to improving heterosubtypic cross protection. In addition, improved cross protection by supplemented vaccination with M2e5x VLPs was mediated via Fc receptors. The results support evidence that supplementation with M2e5x VLPs is a promising approach for overcoming the limitation of strain-specific protection by current influenza vaccination.
Introduction
Influenza virus causes respiratory viral diseases in humans, with significant medical and economic burdens, causing ~250,000–500,000 deaths annually worldwide.1,2 The emergence of the 2009 pandemic H1N1 virus is a good example of the generation of a new strain with distinct antigenic properties by triple reassortment.3,4 While antibodies to hemagglutinin (HA) provide strain-specific protection, the current vaccine formulations do not provide good protection against antigenically distinct strains.
Several approaches were previously demonstrated to induce heterosubtypic cross protection by influenza vaccination. Live virus infections were shown to confer heterosubtypic immunity by inducing crossreactive cytotoxic T lymphocytes in mice.5,6,7,8,9,10,11 In contrast to the live virus infection model, inactivated influenza virus is known to be ineffective in inducing heterosubtypic immunity. It was demonstrated that intramuscular immunization with inactivated viral vaccines did not induce heterosubtypic immunity.12,13 Detergent-split vaccine is known to be less protective compared to whole inactivated viral vaccine.14,15,16,17 Therefore, intramuscular immunization with licensed split vaccines has an intrinsic limitation in inducing cross protective immunity.
In contrast to highly variable HA proteins, the ion-channel protein M2 has an extracellular domain of 24 amino acids (M2e) which is a conserved molecular target among human influenza A strains.18 The wild-type M2 presented in virus-like particles (VLPs) was capable of inducing protective anti-M2e antibodies but its incorporation into VLPs and immunogenicity was low.19 Recently, a molecular construct with a tandem repeat of heterologous M2e peptide sequences (M2e5x) was developed in a membrane-anchored form and presented on enveloped VLPs (M2e5x VLPs).20 However, M2 immunity alone provides relatively weak protection.21
In this study, we hypothesized that licensed influenza split vaccine would overcome its strain-specificity of protection by supplementation with M2e5x VLPs as a conserved molecular antigen. This study demonstrates that commercial split vaccine supplemented with M2e5x VLPs provides significantly improved long-term cross protection against lethal challenge with heterosubtypic influenza viruses as shown by 100% protection and prevention of weight loss. In addition, M2e5x VLP supplementation was found to modify the pattern of host responses to the vaccine toward inducing IgG2a antibodies and interferon-γ (IFN-γ)–producing cells as well as to enhance immune response to heterosubtypic viral antigens. A possible mechanism for improving cross protection by supplemented human split vaccines was also investigated, which demonstrates the important roles of Fc receptor molecules in providing nonneutralizing antibody-mediated protection.
Results
M2e5x VLP supplementation enhances M2e and virus-specific responses
We investigated whether these molecularly engineered M2e5x VLPs as a supplement for commercial human influenza split vaccine would enhance the immune responses to M2e and increase the breadth of cross protection against influenza A viruses. Groups of mice were intramuscularly immunized with 2009 H1N1 split vaccine (Split) alone or influenza split vaccine supplemented with M2e5x VLPs (Split+M2e5x). M2e-specific antibodies were induced in the M2e5x VLP-supplemented group at substantially high levels but not in the split vaccine alone group and, after boost, the antibody levels to M2e in mice supplemented with M2e5x VLPs were further increased by ~250-fold (Figure 1a). Levels of antibody responses specific for vaccine strain influenza A/Cali/04/09 (H1N1) virus were slightly higher in split vaccine supplemented with M2e5x VLPs than those in split vaccine alone although these differences between split and M2e5x VLP-supplemented (split+M2e5x) groups were not statistically significant (Figure 1b). Antibody responses specific for different serotypes of influenza A viruses, A/Philippines/2/82 (H3N2), and A/Vietnam/1203/2004 (rgH5N1), were induced at approximately fourfold higher levels in split vaccine supplemented with M2e5x VLPs than those in split vaccine alone after boost vaccination (Figure 1c,d). These results suggest that supplementing influenza split vaccine with M2e5x VLPs as a conserved antigenic target confers increased immune responses to both M2e and heterosubtypic viral antigens.
Figure 1.
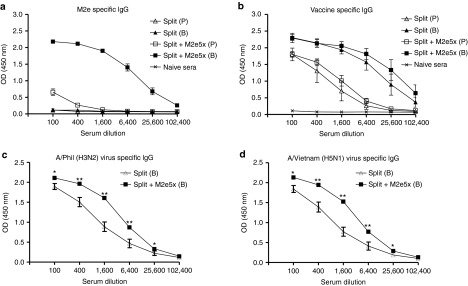
M2e5x VLP-supplemented vaccination induces M2e-specific antibodies and enhanced levels of antibodies to heterosubtypic viral antigens. BALB/c mice (N = 18) were immunized with split vaccine alone (Split) or split vaccine supplemented with M2e5x VLPs (Split+M2e5x). Sera were collected 3 weeks after prime (P) and boost (B) vaccination. The IgG level was detected using M2e peptide or influenza A viruses, A/California/04/09 (A/Cali, H1N1), A/Philippines/2/82 (A/Phil, H3N2), and reassortant A/Vietnam/1203/2004 (rgH5N1) as an ELISA coating antigen for antibody detection. (a) IgG antibody responses to M2e peptide. (b) IgG antibody responses to influenza A/Cali virus. (c) Comparison of IgG antibody responses to influenza A/Phil (H3N2) virus. (d) Comparison of IgG antibody responses to reassortant A/Vietnam/1203/2004 virus (rgH5N1). Asterisk indicates significant differences between sera of split vaccine or split vaccine supplemented with M2e5x VLP (*P < 0.05, **P < 0.01). Error bars indicate mean ± SEM. Split: influenza split vaccine (2009 H1N1 pandemic strain), Split+M2e5x: split vaccine supplemented with M2e5x VLPs. ELISA, enzyme-linked immunosorbent assay; M2e, M2 ectodomain; VLP, virus-like particles.
To further understand types of immune responses, we analyzed IgG isotypes of serum antibodies after boost vaccination (Figure 2). An average of IgG2a antibody levels specific for M2e in the M2e5x VLP-supplemented group was relatively higher than that of IgG1 based on IgG isotype antibody enzyme-linked immunosorbent assay values (Figure 2a). Split vaccine alone showed IgG1 as a dominant isotype antibody for vaccine viral antigen (Figure 2b). In contrast, a reverse pattern of IgG isotypes inducing high levels of vaccine-specific IgG2a antibodies was observed in the M2e5x VLP-supplemented group (Figure 2c). As a result, the ratio of IgG2a/IgG1 in the M2e5x VLP-supplemented vaccine group was the highest among vaccinated groups (Figure 2e). M1 VLP addition also increased the levels of IgG2a antibodies, resulting in high ratios of vaccine-specific IgG2a/IgG1 antibodies compared to those in the split vaccine only group (Figure 2d,e). These results suggest that T helper type 1 (Th1) immune responses such as IgG2a isotype antibodies specific for vaccine can be enhanced by M2e5x VLP-supplemented vaccination or to a less degree by addition of M1 VLP compared to split vaccine alone.
Figure 2.
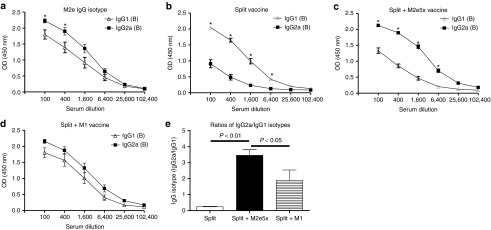
M2e5x VLP-supplemented vaccination modulates virus-specific IgG antibody isotypes. BALB/c mice were immunized with split vaccine alone (Split, N = 18), split vaccine supplemented with M2e5x VLPs (Split+M2e5x, N = 18), or split vaccine supplemented with M1 VLPs (split+M1, N = 8). Sera were collected 3 weeks after boost (B) vaccination. The isotypes of IgG, IgG1, and IgG2a, were detected using M2e peptide or influenza A viruses, A/California/04/09 (A/Cali, H1N1) as an ELISA coating antigen for antibody detection. (a) IgG isotype antibody responses to M2e peptide in M2e5x VLP supplemented split vaccine group. (b) IgG isotype antibody responses to virus in split vaccine group. (c) IgG isotype antibody responses to virus in M2e5x VLP-supplemented split vaccine group. (d) IgG isotype antibody responses to virus in M1 VLP-supplemented split vaccine group. (e) Ratios of IgG2a/IgG1 isotype antibodies. Error bars indicate mean ± SEM. Asterisk (P < 0.05) indicates significant difference between IgG1 and IgG2a isotypes. ELISA, enzyme-linked immunosorbent assay; M2e, M2 ectodomain; VLP, virus-like particles.
M2e5x VLP supplementation provides enhanced cross protection
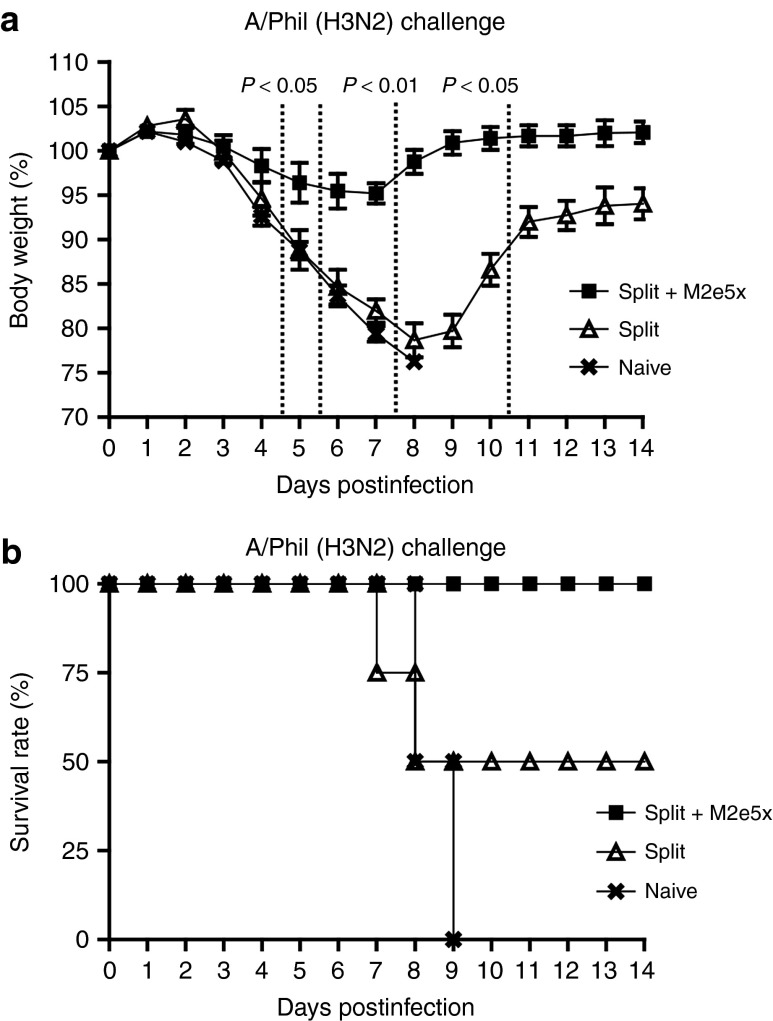
To compare the efficacy of M2e5x or M1 VLP supplementation with split vaccine in conferring cross protection, groups of mice were intramuscularly immunized and challenged with a lethal dose (5xLD50) of heterosubtypic influenza viruses, A/Philippines/2/82 (H3N2 virus), or reassortant A/Vietnam/1203/2004 (rgH5N1) virus, at 6 weeks after boosting (Figure 3). In the H3N2 virus protection experiment, mice that were vaccinated with M2e5x VLP-supplemented split vaccine showed a slight loss (~4%) in body weight postchallenge, resulting in 100% protection (Figure 2a,b). In contrast, mice vaccinated with M1 VLPs supplemented split vaccine showed a significant loss (~14%) in body weight as well as a substantial delay in recovering weight compared to those of M2e5x VLPs supplemented split vaccine (Figure 3a). In addition, mice vaccinated with 2009 H1N1 split vaccine showed a significant loss of over 22% in body weight as well as a substantial delay in recovering weight (Figure 2a). The split vaccine group alone showed 75% survival protection against lethal challenge with H3N2 virus (Figure 2b). In the rgH5N1 virus challenge experiment, mice vaccinated with M2e5x VLP-supplemented split vaccine showed a slight loss (~7%) in body weight postchallenge and 100% protection (Figure 3c,d). In contrast, mice vaccinated with M1 VLP-supplemented split vaccine showed a significant loss (~16%) in body weight as well as a substantial delay in recovering weight comparing to those of M2e5x VLPs supplemented split vaccine (Figure 3a). Mice vaccinated with split vaccine showed a more significant loss of ~22% in body weight and only 50% survival protection against lethal challenge with A/Vietnam H5N1 virus (Figure 3d). These results demonstrate that M2e5x VLP supplementation is superior to M1 VLP addition and split vaccine alone in preventing body weight loss and thus conferring improved cross protection.
Figure 3.
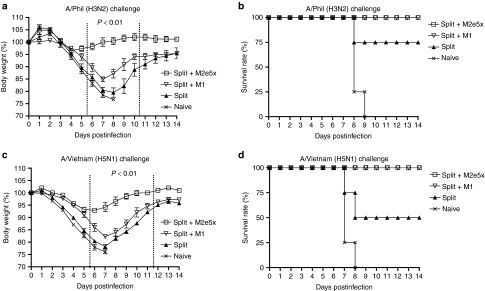
Improved efficacy of cross protection by supplemented vaccine with M2e5x VLPs. Groups of mice (N = 4) that were immunized with split, split+M2e5x, or split+M1 via intramuscular injection were intranasally challenged with a lethal dose (5xLD50) of influenza viruses, A/Philippines/2/82 (H3N2), or rgH5N1, 6 weeks after boost vaccination. (a) Average body weight changes after challenge with A/Philippines/2/82 virus. (b) Survival rates after challenge with A/Philippines/2/82 virus. (c) Average body weight changes after challenge with rgH5N1 virus. (d) Survival rates after challenge with rgH5N1 virus. Body weight and survival rates were monitored for 14 days. P value indicates significant difference between split+M2e5x and split+M1 or split vaccinated groups. Error bars indicate mean ± SEM. LD, lethal dose; M2e, M2 ectodomain; VLP, virus-like particles.
M2e5x VLP supplementation is effective in inducing M2e mucosal antibodies and lowering inflammatory cytokine level and lung viral loads
M2e-specific IgG and IgA antibody responses in bronchoalveolar lavage fluids (BALF) were determined using mice killed at day 4 after viral challenge with H3N2 virus (Figure 4a,b). Significantly, higher levels of IgG and IgA antibody responses specific for M2e were observed in BALF of mice vaccinated with M2e5x VLP-supplemented split vaccine.
Figure 4.
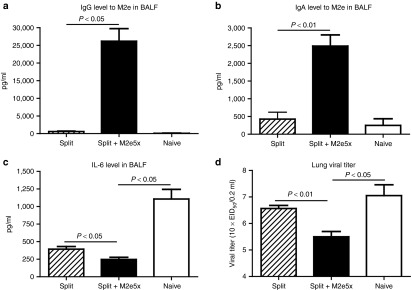
Mucosal antibody responses, IL-6 in BALF, and lung viral titers after boost immunization upon heterosubtypic challenge. Levels of IgG and IgA antibodies were determined from BALF at day 4 postchallenge (N = 4) from the mice after boost vaccination. (a) M2e-specific IgG antibody response. (b) M2e-specific IgA antibody response. IgG or IgA antibody responses were determined by ELISA using human type M2e peptide as a coating antigen (4 µg/ml). Levels of IL-6 cytokine and lung viral titers were determined at day 4 postchallenge (N = 4). (c) IL-6 cytokine in BALF. IL-6 was determined by a cytokine ELISA (N = 4). (d) Lung viral titers. Lung viral titers were determined by an egg inoculation assay day 4 postchallenge (N = 4). P value indicates significant difference between split+M2e5x and split vaccinated or naive groups. Data represent mean ± SEM. BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; M2e, M2 ectodomain; VLP, virus-like particles.
Levels of interleukin-6 were observed at significantly lower in BALF from mice immunized with split vaccine supplemented with M2e5x VLP than those of split vaccine alone or naive mice, indicating lung inflammatory responses probably due to high viral replication after challenge with H3N2 A/Philippines/2/82 virus (Figure 4c). The group of mice immunized with split vaccine supplemented with M2e5x VLP showed ~10-fold and 30-fold lower lung viral titers compared to those in the human split vaccine and naive mouse groups, respectively (Figure 4d). Overall, it is likely that M2e-specific immune responses in systemic and mucosal sites effectively contribute to controlling heterosubtypic virus replication after M2e5x VLP-supplemented immunization.
T cells contribute cross protection by M2e5x VLP-supplemented vaccination
Mice were challenged with H3N2 virus at 8 weeks after boost and cells from spleen or lung samples were collected at day 4 postchallenge to determine IFN-γ producing cellular responses (Figure 5a,b). Over 20-fold higher levels of IFN-γ secreting spleen cells were observed in mice vaccinated with M2e5x VLP-supplemented split vaccine compared to those in the human split vaccine alone or the naive group (Figure 5a). Interestingly, the group of mice that were intramuscularly immunized with split vaccine supplemented with M2e5x VLPs showed substantial levels of IFN-γ secreting cell spots in lung cells (Figure 5a,b). These results provide evidence that M2e5x VLP supplementation to human split vaccine induces an effective recall response of INF-γ secreting T cells specific for M2e.
Figure 5.
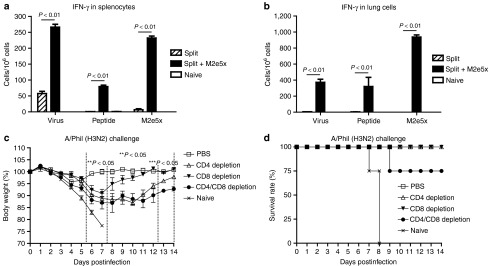
M2e5x VLP-supplemented vaccination induces IFN-γ producing cellular immune responses. Splenocytes and lung cells were isolated from mice at day 4 postchallenge. IFN-γ secreting cells were determined in the presence of A/California /04/09 (H1N1) viral antigen, M2e peptide, or M2e5x VLPs as a stimulator (2 µg/ml). (a) IFN-γ in splenocytes. (b) IFN-γ in lung cells. IFN-γ cytokine-producing cells were counted by enzyme-linked immunosorbent spot (ELISPOT) reader. P-value indicates significant difference between split+M2e5x and split vaccinated groups. At 4 weeks after boost immunization with M2e5x VLP-supplemented split vaccine, groups of four female Balb/c mice were treated with anti-CD4, anti-CD8, anti-CD4/CD8 monoclonal antibodies or PBS as a control on −2 day and +1 day of challenge. All groups were challenged with a lethal dose of A/Philippines/2/82 (H3N2) influenza virus. (c) Average body weight changes. (d) Survival rates. One asterisk indicates significant difference between PBS and CD4, CD8, or CD4/CD8 T-cell–depleted groups. Two asterisks indicate significant difference between PBS and CD4 or CD4/CD8 T-cell–depleted groups. Three asterisks indicate significant difference between CD4 and CD4/CD8 T-cell–depleted groups. Data represent mean ± SEM. IFN, interferon; M2e, M2 ectodomain; PBS, phosphate-buffered saline; VLP, virus-like particles.
To better understand possible contributions of CD4 and CD8 T cells to mediating heterosubtypic protection, mice that had immunized with M2e5x VLP-supplemented split vaccine were treated with phosphate-buffered saline (PBS), CD4, CD8, or both T-cell–depleting antibodies (Figure 5c,d). Mice that were depleted of CD4, CD8, or CD4/CD8 T cells suffered from significant body weight loss or showed less survival rates (Figure 5c, d) whereas PBS-treated control mice showed minimal weight loss (Figure 5c). Mice that were treated with CD8 T-cell-depleting antibodies showed a moderate loss of body weight (~9%) and then fully recovered. Meanwhile, mice with CD4 T-cell-depleting antibodies showed a more substantial body weight loss up to 12% as well as a significant delay in recovering weight (Figure 5c). In addition, mice that received both CD4 and CD8 T-cell–depleting antibodies exhibited a more severe weight loss to ~14% as well as a more extended delay in weight recovery and 75% survival protection (Figure 5c,d). These results suggest that both CD4 and CD8 T cells induced by M2e5x VLP-supplemented vaccination contribute to improving heterosubtypic cross protection with CD4 T cells playing a greater role.
M2e5x VLP supplementation induces long-lasting cross protective immunity
Split vaccine and M2e-specific antibodies in mice vaccinated with split vaccine supplemented with M2e5x VLPs were observed at a substantial level after 1 year postvaccination, indicating that M2e or virus-specific antibody producing plasma cells are long lived (Figure 6a,b). The pattern of IgG isotype still lasted for 12 months (Figure 6c,d) and ratio of IgG2a/IgG1 in M2e5x VLP-supplemented vaccine group (IgG2a/IgG1: 3.3) was 10-fold higher than that of the split vaccine group (IgG2a/IgG1: 0.33; Figure 6e). These results provide evidence that Th1 immune responses such as IgG2a were maintained for over a year in mice with M2e5x VLP-supplemented intramuscular vaccination.
Figure 6.
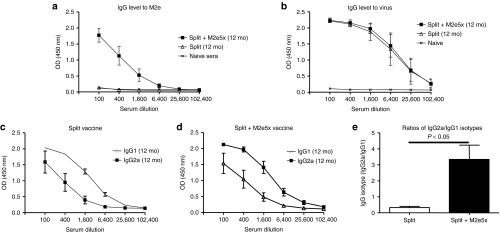
M2e antibodies and a modified pattern of IgG isotypes to vaccine are maintained for over a year. BALB/c mice (N = 4) were immunized with influenza split vaccine alone (Split) or influenza split vaccine supplemented with M2e5x VLPs (Split+M2e5x). Long-term antibody responses were determined in immune sera collected at 12 months after boost vaccination. (a) M2e-specific IgG antibody responses. (b) IgG antibody responses reactive to A/California/04/09 (H1N1) virus. (c,d) IgG1 and IgG2a isotype antibodies were determined using influenza A virus, A/California/04/09 (H1N1) as an ELISA coating antigen. (c) IgG isotypes in split vaccine alone. (d) IgG isotypes in split vaccine supplemented with M2e5x VLP. (e) Ratios of IgG2a/IgG1 isotype antibodies. P-value indicates significant difference between split+M2e5x and split vaccinated groups. Error bars indicate mean ± SEM. ELISA, enzyme-linked immunosorbent assay; M2e, M2 ectodomain; VLP, virus-like particles.
Mice that were vaccinated with split H1N1 vaccine supplemented with M2e5x VLPs showed only 5% weight loss and 100% protection after lethal challenge with H3N2 virus 12 months postvaccination (Figure 7a,b). In contrast, mice vaccinated with split vaccine alone showed a severe weight loss (~22%) and only 50% survival after challenge with H3N2 virus (Figure 7a,b). These results provide evidence that enhanced cross protective immunity by M2e5x VLP supplementation to human split vaccine can be maintained for over 12 months in mice.
Figure 7.
Improved cross protection for over a year is supported by M2e5x VLP-supplemented vaccine. BALB/c mice (N = 4) were immunized with influenza split vaccine alone (Split) or influenza split vaccine supplemented with M2e5x VLPs (Split+M2e5x). Groups of mice were intranasally challenged with a lethal dose (5xLD50) of influenza virus, A/Philippines/2/82 (H3N2), after 12 months of vaccination. (a) Average body weight changes. (b) Survival rates. Body weight and survival rates were monitored for 14 days. P value indicates significant difference between split+M2e5x and split vaccinated groups. Error bars indicate mean ± SEM. M2e, M2 ectodomain; VLP, virus-like particles.
Long-term IFN-γ secreting cellular response by M2e5x VLP supplementation
To better assess the long-term cross protective efficacy, IL-6 inflammatory cytokine levels, and lung viral loads were determined at day 4 postchallenge after 12 months of vaccination (Figure 8a,b). Approximately twofold lower amounts of IL-6 in BALF and 20-fold lower levels of viral titers in lung extracts were detected from the M2e5x VLP-supplemented group compared to the split vaccine only group. IFN-γ–secreting spleen or lung cells were also found to be induced at higher levels even after 12 months of vaccination with split vaccine supplemented with M2e5x VLPs compared to those with split vaccine alone (Figure 8c,d). Taken together, Th1 immune responses (IgG2a isotype, IFN-γ) were shown to be maintained at both systemic and mucosal sites for over a year after M2e5x VLP-supplemented vaccination.
Figure 8.
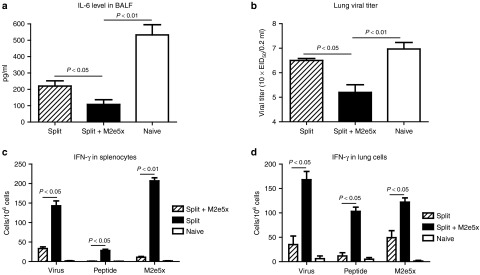
IL-6 in BALF, lung viral titers, and mucosal and systemic IFN-γ secreting cell spots at 12 months after immunization upon heterosubtypic challenge. Levels of IL-6 cytokine and lung viral titers were determined in mice immunized with influenza split vaccine alone (Split) or influenza split vaccine supplemented with M2e5x VLP (Split+M2e5x) day 4 postchallenge with 5xLD50 of A/Philippines/2/82 (H3N2) virus at 12 months after boost vaccination. (a) IL-6 cytokine in BALF. IL-6 was determined by a cytokine ELISA (N = 4). (b) Lung viral titers. Lung viral titers were determined by an egg inoculation assay day 4 postchallenge (N = 4). Splenocytes and lung cells were isolated from mice day 4 postchallenge (N = 4). IFN-γ secreting cells were detected in the presence of A/California/04/09 (H1N1) virus, M2e peptide, or M2e5x VLPs as a stimulator (2 µg/ml). (c) IFN-γ secreting splenocytes. (d) IFN-γ secreting lung cells. IFN-γ– or IL-4 cytokine–producing cells were counted by enzyme-linked immunosorbent spot (ELISPOT) reader. P value indicates significant difference between split+M2e5x and split vaccinated or naive groups. Data represent mean ± SEM. BALF, bronchoalveolar lavage fluid; IFN, interferon; M2e, M2 ectodomain; VLP, virus-like particles.
M2e5x VLP supplementation induces long-lasting antibodies specific fo M2e
Levels of IgG and IgA antibodies in BALF samples were measured at day 4 postchallenge of mice after 12 months of vaccination. Vaccine-specific antibodies were similarly observed in both groups (Figure 9a,c). Significantly, higher levels of IgG (Figure 9b) and IgA (Figure 9d) antibodies specific for M2e were observed in BALF from the group of mice with M2e5x VLP -upplemented vaccination compared to those from split vaccine only control mice. Therefore, M2e5x VLPs mixed with split vaccine are capable of inducing long-term mucosal antibodies specific for M2e, which is likely to contribute to enhanced cross protection.
Figure 9.
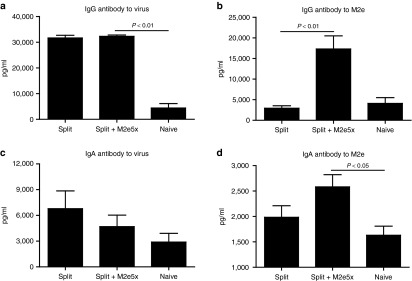
Increased M2e-specific IgG and IgA antibodies in BALF are maintained by M2e5x VLP-supplemented vaccination for over a year. Levels of IgG and IgA antibodies were determined day 4 postchallenge (N = 4) from the mice at the time of 12 months after boost vaccination. (a) IgG antibody level to A/California/04/09 (H1N1) virus. (b) M2e-specific IgG antibody response. (c) IgA antibody level to A/California/04/09 (H1N1) virus. (d) M2e-specific IgA antibody response. IgG or IgA antibody responses were determined by ELISA using inactivated influenza virus or human type M2e peptide as a coating antigen (4 μg/ml). P value indicates significant difference between split+M2e5x and split vaccinated or naive groups. Error bars indicate mean ± SEM. BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; M2e, M2 ectodomain; pg, pictogram; VLP, virus-like particles.
Fc receptors are involved in conferring protection by M2e antibodies
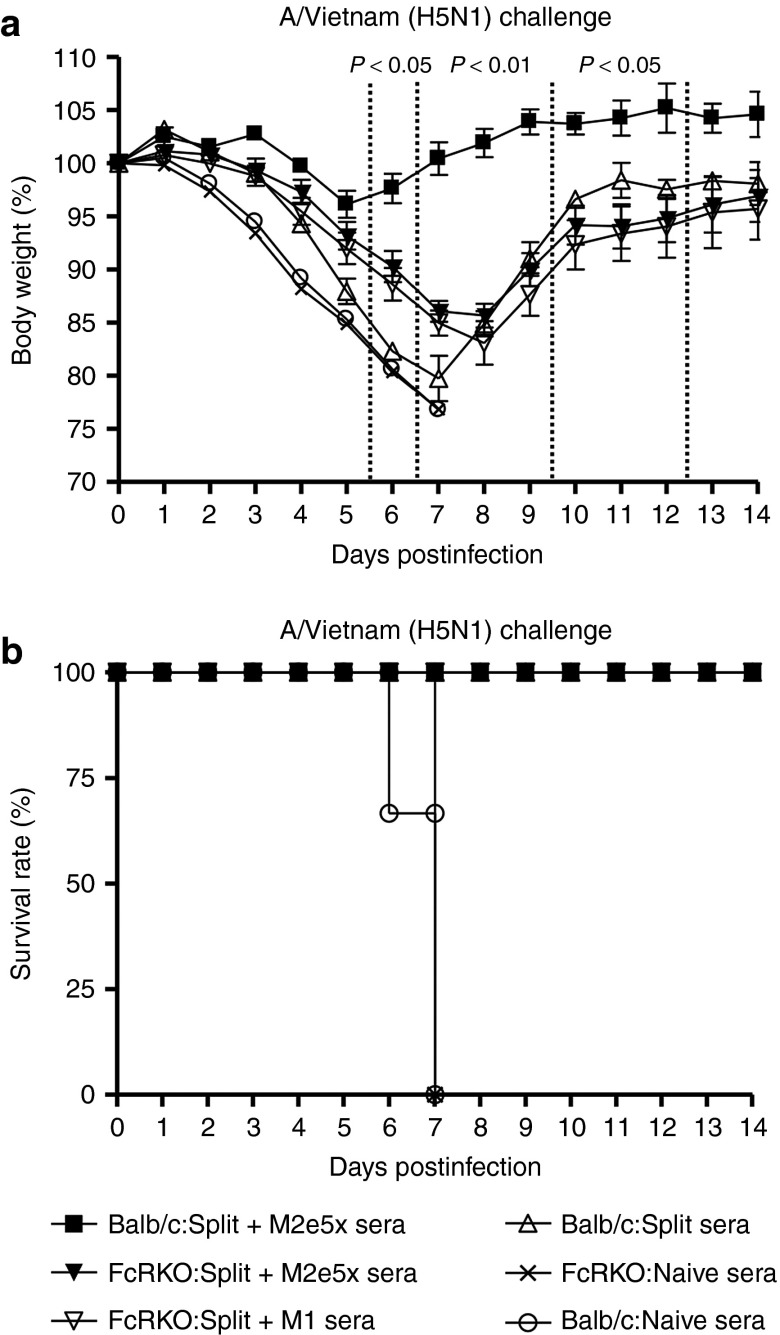
First, we compared the protective efficacy of immune sera of M2e5x VLP-supplemented vaccination and split vaccine immunization in wild-type BALB/c mice (Figure 10a). Naive BALB/c mice that were infected with a lethal dose of rgH5N1 virus with immune sera of M2e5x VLP-supplemented vaccination showed a slight loss (~4%) and then fully recovered to a normal level. However, BALB/c mice that received a lethal dose of rgH5N1 virus with immune sera of the split alone group displayed a severe loss (~20%) and a significant delay in weight recovery (Figure 10a).
Figure 10.
Role of Fc receptor in conferring protection by M2e immune sera. Naive FcR knockout or naive wild-type BALB/c mice (N = 3) were infected with rgH5N1 virus premixed with naive sera, split vaccine, or M1 VLP or M2e5x VLP-supplemented split vaccine immunized sera. (a) Body weight change. (b) Survival rate. Split sera: immune sera from split vaccine immunized mice; Split+M2e5x: immune sera from M2e5x VLP-supplemented mice; Split+M1: immune sera from M1 VLP-supplemented mice; Naive sera: sera from unimmunized mice. Balb/C: wild-type mice; FcRKO: FcR receptor common γ chain knockout mice. P value indicates significant difference between Balb/C and FcRKO mice using immunized sera of split vaccine supplemented with M2e5x VLP. M2e, M2 ectodomain; VLP, virus-like particles.
To determine a possible role of Fc receptors in protection, an FcR knockout mouse model was used with immune sera of M2e5x VLP-supplemented vaccination. FcR knockout mice that received a mixture of rgH5N1 virus with immune sera of M2e5x VLP-supplemented vaccination lost ~15% in body weight at day 8 and then were very slow in weight recovery (Figure 10a). In addition, an FcR knockout mouse model was also used with immune sera of M1 VLP-supplemented vaccination as control. FcR knockout mice that received a mixture of rgH5N1 virus with immune sera of M1 VLP-supplemented vaccination was a little bit more decreased (~17% in body weight at day 8) than that of M2e5x VLP-supplemented vaccination (~15%) but there was no significant difference between M2e5x and M1 VLP-supplemented vaccination (Figure 10a). Therefore, protective efficacy in FcR knockout mice was significantly weaker compared to that observed in wild-type BALB/c mice regardless of M2e-specific IgG2a antibodies (Figure 10a). As negative controls, naive sera mixed with rgH5N1 virus were not protective in both Balb/c and FcR knockout mice and all these mice had to be euthanized (Figure 10a,b). Overall, these results provide evidence that the Fc receptor plays a crucial role in mediating improved cross protection by M2e5x VLP-supplemented vaccination.
Discussion
Current human influenza A vaccines based on the variable HA antigens do not provide effective protection against different serotypes of influenza viruses. In a previous study, we determined the immunogenicity of M2e5x VLP vaccines and demonstrated that M2e5x VLP vaccination alone did not induce good protection against viruses such as 2009 H1N1 pandemic influenza virus.20 In this study, we investigated the effects of supplementing influenza human split vaccine with M2e5x VLPs as a conserved vaccine target antigen. Our study demonstrated that addition of M2e5x VLPs to split vaccine effectively induced M2e-specific humoral and cellular immune responses. Notably, the M2e5x VLP-supplemented 2009 pandemic influenza vaccine conferred long-term cross protection against the distantly related heterosubtypic H3N2 A/Philippines/82 and rgH5N1 A/Vietnam/1203/2004 influenza viruses. It is also worth noting that supplemented vaccination prevented mice from losing body weight, resulting in mitigating illness. Therefore, supplementing human influenza vaccines with M2e5x VLPs can overcome inefficient cross protection induced by current human influenza vaccines.
Developing a truly universal vaccine might not be a realistic goal due to the extremely high genetic and antigenic differences among influenza viruses as well as the relatively low immunogenicity of conserved antigenic targets. To overcome the low immunogenicity of M2e, previous studies used M2e-conjugate vaccines in a range of high doses (40–100 µg per mouse) and multiple immunizations in the presence of potent adjuvants inappropriate for human use.22,23,24,25,26,27,28,29 We found that supplementation with M2e5x VLPs in the absence of adjuvants conferred enhanced M2e-specific immunity as well as higher cross reactivity of immune sera for heterologous and heterosubtypic viral antigens. Interestingly, split vaccine could confer weak and partial cross protection in mice after a low dose of lethal challenge although severe weight loss was accompanied. This weak cross protection might be due to the cross reactivity of HA and neuraminidase as well as the internal virus proteins (NS1, NP, or M1) that are known to be present in split vaccines.30,31,32 Supplementation with conserved target of M2e in a VLP platform was found to be an important correlate in conferring improved cross protection.
Protective immune mechanisms mediated by M2 immunity have not been well defined, probably because anti-M2 antibodies do not directly neutralize the virus.21,33,34 A possible mechanism is that influenza viruses bound to non-neutralizing antibodies are likely to be recognized and removed by opsonophagocytosis.35,36,37,38 The IgG2a isotype antibody is known to interact with Fc receptors by virtue of its Fc domain binding property.35,39,40,41 Furthermore, dendritic and macrophage cells were shown to play an important role in conferring protection by M2 immune sera.34 It is important to note that M2e5x VLP supplementation resulted in inducing IgG2a antibody to a higher level as a dominant isotype. It is important to note that supplementation of M1 VLP could also shape the IgG isotype pattern of vaccine-specific antibodies by increasing IgG2a although it was less in magnitude compared to M2e5x VLP supplementation. Mechanisms by which M1 VLP might have an adjuvant effect on split vaccine remain to be elucidated. These results provide evidence that supplementation of M1 or M2e5x VLP to influenza split vaccine can modify the pattern of host immune responses to the vaccine in a direction of inducing Th1 type immunity. M2e and vaccine-specific Th1 immune responses such as higher levels of IgG2a isotype antibodies and IFN-γ might have contributed to improved cross protection by M2e5x VLP-supplemented vaccination. Therefore, M2e5x VLPs could have a dual role of inducing M2e immunity as well as displaying an adjuvant effect by enhancing and modulating host immune responses to vaccine toward the Th1 responses.
Cellular immunity to influenza virus infection involves CD8 and CD4 T-cell subsets. T-cell depletion experiments prior to challenge suggested that both CD4 and CD8 T cells induced by M2e5x VLP-supplemented vaccination contributed cross protection and, in particular, CD4 T cells appeared to play a greater role than CD8 T cells in conferring cross protection (Figure 5c,d). It is widely believed that CD8 cells promote viral clearance via cytotoxic mechanisms, lysing virus-infected epithelial cells directly using both perforin- and FasL-mediated cytotoxicity.42 In contrast, results in this study indicate that CD4 T cells might have dual roles in conferring cross protection. CD4 T cells induced by M2e5x VLP-supplemented vaccination might have contributed to the production of IFN-γ that is known to be important in lowing influenza virus titers.43 More importantly, consistent with the results in this study, it was suggested that CD4 cells have perforin-mediated cytotoxicity in the lung that may enhance recovery from lethal infection.44 Therefore, M2e5x VLP-supplemented vaccine-induced CD4 T-cell effectors are multifunctional with direct cytotoxic roles in promoting cross protection against influenza.
To better understand the possible roles of Fc receptors in conferring M2e immune–mediated protection, we used mice that are deficient in the common γ-chain of the Fc receptor and observed severe body weight loss in FcR-deficient mice, but not in wild-type mice, after infection with reassortant rgH5N1 virus mixed with immune sera of supplemented vaccination. The common γ-chain is known to be involved in activating several Fc receptors (FcγRI, FcγRIII, and FcγRIV).45 Thus, FcR-mediated clearance of virus–antibody complexes is likely playing an important role in protection by immune sera of M2e5x VLP-supplemented influenza split vaccine.
In summary, we observed the following results: (i) improved cross protection was obtained even after a year of supplemented influenza vaccination with M2e5x VLPs. (ii) Intramuscular systemic immunization with M2e5x VLP-supplemented vaccines induced high levels of humoral and cellular immune responses to M2e at both systemic and mucosal sites. (iii) Enhanced levels of antibodies reactive to antigenically different influenza viruses were induced by supplementing HA-based split vaccines with M2e5x VLPs, which are long-lasting over a year. (iv) M2e5x VLP supplementation modified the pattern of host immune responses resulting in predominant IgG2a isotype antibodies recognizing influenza vaccine virus. (v) CD4 and CD8 T cells were important in mediating cross protection and, in particular, CD4 T cells were found to play a greater role in this cross protection. (vi) Fc receptors are involved in mechanisms by which M2e5x VLP-supplemented influenza split vaccines work for improved cross protection.
Materials and Methods
Viruses, vaccine, and M2e5x VLPs. The A/California/04/2009 (2009 pandemic H1N1 virus; a gift from Dr Richard Webby), A/Philippines/2/1982 (subtype H3N2 virus; a gift from Dr Huan Nguyen), and reassortant A/Vietnam/1203/2004 (rgH5N1; HA and NA were derived from A/Vietnam/1203/2004 and the backbone genes from A/PR/8/34 virus)46 were propagated in a 10-day-old embryonated chicken eggs. Harvested allantoic fluid was clarified by centrifugation (6,000 rpm, 30 minutes) and kept at −80 °C. Purified inactivated A/California/04/2009 (H1N1) virus was produced by treating the virus with formalin at a final concentration of 1:4,000 (v/v) as described previously.47,48 Commercial human influenza split vaccine (Green Flu-S; Green Cross, South Korea) derived from the 2009 pandemic strain of A/California/07/2009 (H1N1) virus, was used in this study. M2e5x VLPs or M1 VLPs were produced as previously described.20,49
Immunization and challenge. For animal experiments, 6- to 8-week-old female BALB/c mice Harlan Laboratories, Indianapolis, IN were intramuscularly immunized with 0.60 µg of human split vaccine proteins (equivalent to 0.24 μg HA) alone (N = 18), human split vaccine (0.60 μg) supplemented with 10 μg of M2e5x VLPs (total protein; N = 18), or split vaccine (0.60 μg) supplemented with 10 μg of M1 VLPs (total protein; N = 8) at weeks 0 and 4. Blood samples were collected at 3 weeks after each immunization or at 12 months after boost vaccination. Immunized mice were then challenged with a lethal dose (5xLD50) of A/Philippines/2/82 or reassortant H5N1 A/Vietnam/1203/2004 influenza viruses at 6 weeks after boost immunization. To determine the long-term protective efficacy, additional groups of mice (N = 4; Harlan Laboratories) were challenged with a lethal dose (5xLD50) of A/Philippines/2/82 influenza virus at 12 months after boost immunization. Mice were monitored daily to record weight changes and mortality. Full details of this study and all animal experiments presented in this manuscript were approved by the Emory University and Georgia State University IACUC review boards (approval numbers 179–2,008 and A11026, respectively). Approved IACUC protocols operate under the federal Animal Welfare Law (administered by the United States Department of Agriculture) and regulations of the Department of Health and Human Services.
Antibody responses. M2e-specific or virus-specific antibody responses were determined by enzyme-linked immunosorbent assay using synthetic M2e peptides or inactivated influenza virus as a coating antigen (4 µg/ml) as previously described.19,20,50
In vivo T-cell depletion. Groups of mice at 4 weeks after boost immunization with M2e5x VLP-supplemented split vaccine were treated with PBS, anti-CD4 (200 ng/mouse, clone GK1.5, Abcam, Cambridge, MA), anti-CD8 (150 ng/mouse, clone 53.6.7, BioXCell, West Lebanon, NH), or CD4/CD8 monoclonal antibodies through intraperiotoneal injection on days −2, +1 relative to the date of challenge. The levels of CD4 and CD8 T-cell depletion were confirmed by flow cytometry of blood samples and over 95% of T cells were depleted. All groups were challenged with a lethal dose (8xLD50) of A/Philippines/2/82 (H3N2) influenza virus. Mice were monitored daily to record weight changes and mortality.
Analysis of antibodies, cytokines in bronchoalveolar lavages, and lung viral loads. BALF were prepared for the analysis of antibody responses and proinflammatory cytokine IL-6 from mice at day 4 after challenge with 5xLD50 of influenza A/Philippines/2/82 (H3N2) virus. BALF samples were obtained by infusing 1 ml of PBS into the lungs via the trachea using a 25-gauge catheter (Exelint International, Los Angeles, CA). Lung extracts were prepared for viral titer using a mechanical tissue grinder with 1.5 ml of PBS per each lung. Embryonated chicken eggs were inoculated with diluted lung extracts, incubated at 37 °C for 3 days, and tested for hemagglutination activity to determine viral titers as described.51
Determination of T-cell responses. At day 4 postchallenge, splenocytes and lung cells were isolated from corresponding tissue samples as described.52 IFN-γ secreting cell spots were determined on Multi-screen 96-well plates (Millipore, Billerica, MA) coated with cytokine specific capture antibodies as described.19,34 Briefly, 0.5 × 106 spleen cells per well or 0.2 × 106 lung cells per well were cultured with inactivated influenza A/California/04/09 virus (2 µg/ml), M2e peptide (2 µg/ml), or M2e5x VLP (2 µg/ml) as an antigenic stimulator. After 36 hours of incubation, the spots of IFN-γ–secreting T cells were counted using an ELISpot reader (BioSys, Miami, FL).
Cross protective efficacy of immune sera from M2e5x VLP-supplemented vaccination. BALB/c mice with a genetic defect in the Fc receptor common γ-chain (FcR KO mice homozygous Fcer1g) were obtained from Taconic Farms (Germantown, NY) to test cross protective efficacy with immune sera from mice that were immunized with supplemented vaccines. In brief, immune and naïve sera were two times diluted and heat-inactivated at 56 °C for 30 minutes. The sera were mixed with twelve lethal doses (12xLD50) of influenza A/Vietnam/1203/2004 (rgH5N1) virus and incubated at room temperature for 30 minutes as described.19,34 The mixture of a lethal infectious dose of A/Vietnam/1203/2004 (H5N1) influenza virus with sera was administered to FcR knockout mice or naive Balb/c mice (N = 3) and body weight and survival rates were daily monitored.
Statistical analysis. To determine the statistical significance, a two-tailed Student's t-test was used when comparing two different conditions. A P value less than 0.05 was considered to be significant.
Acknowledgments
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI093772 (S.M.K.), and AI087782 (S.M.K.) and Animal and Plant Quarantine Agency grant (I-1541781-2012-15-01), Republic of Korea.
References
- Osterholm MT. Preparing for the next pandemic. N Engl J Med. 2005;352:1839–1842. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary Estimates of Mortality and Years of Life Lost Associated with the 2009 A/H1N1 Pandemic in the US and Comparison with Past Influenza Seasons. PLoS Curr. 2010;2:RRN1153. doi: 10.1371/currents.RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Epstein SL, Lo CY, Misplon JA, Lawson CM, Hendrickson BA, Max EE, et al. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997;158:1222–1230. [PubMed] [Google Scholar]
- Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- Moss P. Cellular immune responses to influenza. Dev Biol (Basel) 2003;115:31–37. [PubMed] [Google Scholar]
- Heinen PP, de Boer-Luijtze EA, Bianchi AT. Respiratory and systemic humoral and cellular immune responses of pigs to a heterosubtypic influenza A virus infection. J Gen Virol. 2001;82 Pt 11:2697–2707. doi: 10.1099/0022-1317-82-11-2697. [DOI] [PubMed] [Google Scholar]
- Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–1038. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol. 2001;158:119–130. doi: 10.1016/s0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan RJ, Cauley LS, Ely KH, Cookenham T, Roberts AD, Brennan JW, et al. Long-term maintenance of virus-specific effector memory CD8+ T cells in the lung airways depends on proliferation. J Immunol. 2002;169:4976–4981. doi: 10.4049/jimmunol.169.9.4976. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003;21:3212–3218. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- Cox RJ, Hovden AO, Brokstad KA, Szyszko E, Madhun AS, Haaheim LR. The humoral immune response and protective efficacy of vaccination with inactivated split and whole influenza virus vaccines in BALB/c mice. Vaccine. 2006;24:6585–6587. doi: 10.1016/j.vaccine.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Miyaki C, Quintilio W, Miyaji EN, Botosso VF, Kubrusly FS, Santos FL, et al. Production of H5N1 (NIBRG-14) inactivated whole virus and split virion influenza vaccines and analysis of immunogenicity in mice using different adjuvant formulations. Vaccine. 2010;28:2505–2509. doi: 10.1016/j.vaccine.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Matsuoka S, Takenaka N, Haredy AM, Tanimoto T, Gomi Y, et al. Intranasal immunization with a formalin-inactivated human influenza A virus whole-virion vaccine alone and intranasal immunization with a split-virion vaccine with mucosal adjuvants show similar levels of cross-protection. Clin Vaccine Immunol. 2012;19:979–990. doi: 10.1128/CVI.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu SZ, Dong SS, Dong XP, Zhang WL, Lu M, et al. Safety and immunogenicity of adjuvanted inactivated split-virion and whole-virion influenza A (H5N1) vaccines in children: a phase I-II randomized trial. Vaccine. 2010;28:6221–6227. doi: 10.1016/j.vaccine.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Liu W, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005;7:171–177. doi: 10.1016/j.micinf.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, et al. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS ONE. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, et al. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–5605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerging Infect Dis. 2007;13:426–435. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Liang X, Horton MS, Perry HC, Citron MP, Heidecker GJ, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Denis J, Acosta-Ramirez E, Zhao Y, Hamelin ME, Koukavica I, Baz M, et al. Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine. 2008;26:3395–3403. doi: 10.1016/j.vaccine.2008.04.052. [DOI] [PubMed] [Google Scholar]
- Fu TM, Grimm KM, Citron MP, Freed DC, Fan J, Keller PM, et al. Comparative immunogenicity evaluations of influenza A virus M2 peptide as recombinant virus like particle or conjugate vaccines in mice and monkeys. Vaccine. 2009;27:1440–1447. doi: 10.1016/j.vaccine.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Wu F, Yuan XY, Li J, Chen YH. The co-administration of CpG-ODN influenced protective activity of influenza M2e vaccine. Vaccine. 2009;27:4320–4324. doi: 10.1016/j.vaccine.2009.04.075. [DOI] [PubMed] [Google Scholar]
- De Filette M, Min Jou W, Birkett A, Lyons K, Schultz B, Tonkyro A, et al. Universal influenza A vaccine: optimization of M2-based constructs. Virology. 2005;337:149–161. doi: 10.1016/j.virol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Heinen PP, Rijsewijk FA, de Boer-Luijtze EA, Bianchi AT. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J Gen Virol. 2002;83 Pt 8:1851–1859. doi: 10.1099/0022-1317-83-8-1851. [DOI] [PubMed] [Google Scholar]
- Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W. A “universal” human influenza A vaccine. Virus Res. 2004;103:173–176. doi: 10.1016/j.virusres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, Subbarao K, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerging Infect Dis. 2002;8:796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day EB, Zeng W, Doherty PC, Jackson DC, Kedzierska K, Turner SJ. The context of epitope presentation can influence functional quality of recalled influenza A virus-specific memory CD8+ T cells. J Immunol. 2007;179:2187–2194. doi: 10.4049/jimmunol.179.4.2187. [DOI] [PubMed] [Google Scholar]
- Richards KA, Chaves FA, Alam S, Sant AJ. Trivalent inactivated influenza vaccines induce broad immunological reactivity to both internal virion components and influenza surface proteins. Vaccine. 2012;31:219–225. doi: 10.1016/j.vaccine.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci USA. 2011;108:757–761. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13:981–990. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006;352:418–426. doi: 10.1016/j.virol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger MS, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–1016. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- Heusser CH, Anderson CL, Grey HM. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977;145:1316–1327. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86:6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Song JM, Kim YC, Barlow PG, Hossain MJ, Park KM, Donis RO, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010;88:244–247. doi: 10.1016/j.antiviral.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, Kang SM. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology. 2012;430:127–135. doi: 10.1016/j.virol.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee JS, Kwon YM, O E, Lee YJ, Choi JG, et al. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res. 2013;99:328–335. doi: 10.1016/j.antiviral.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen HS, Wang SP. Mouse adaptation of the Asian influenza virus. J Infect Dis. 1959;105:9–17. doi: 10.1093/infdis/105.1.9. [DOI] [PubMed] [Google Scholar]
- Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]