Abstract
Intracellular cations are essential for the physiology of all living organisms including bacteria. Cations such as potassium ion (K+), sodium ion (Na+) and proton (H+) are involved in nearly all aspects of bacterial growth and survival. K+ is the most abundant cation and its homeostasis in Escherichia coli and Salmonella is regulated by three major K+ transporters: high affinity transporter Kdp and low affinity transporters Kup and Trk. Previous studies have demonstrated the roles of cations and cation transport in the physiology of Escherichia coli; their roles in the virulence and physiology of pathogenic bacteria are not well characterized. We have previously reported that the Salmonella K+ transporter Trk is important for the secretion of effector proteins of the type III secretion system (TTSS) of Salmonella pathogenicity island 1 (SPI-1). Here we further explore the role of Salmonella cation transport in virulence in vitro and pathogenesis in animal models. Impairment of K+ transport through deletion of K+ transporters or exposure to the chemical modulators of cation transport, gramicidin and valinomycin, results in a severe defect in the TTSS of SPI-1, and this defect in the TTSS was not due to a failure to regulate intrabacterial pH or ATP. Our results also show that K+ transporters are critical to the pathogenesis of Salmonella in mice and chicks and are involved in multiple growth and virulence characteristics in vitro, including protein secretion, motility and invasion of epithelial cells. These results suggest that cation transport of the pathogenic bacterium Salmonella, especially K+ transport, contributes to its virulence in addition to previously characterized roles in maintaining homeostasis of bacteria.
Introduction
Living organisms must fulfil basic physiological needs in order to grow and replicate. Critical among them is the need to maintain a stable intracellular environment, usually within a strict range that is unique to each organism. Bacteria, like any other living organism, maintain a homeostasis of pH, osmolarity and ion concentrations. The physiologically important cations for bacteria include potassium ions (K+), sodium ions (Na+) and protons (H+), and their intracellular concentrations are controlled by multiple transporters and channels. Among the intracellular cations, K+ is the most abundant and its concentration is regulated by K+ efflux pumps and transporters (Kem & Trachewsky, 1983). K+ transport is best characterized in the Gram-negative bacterium Escherichia coli, which has three major K+ transport systems – Trk, Kdp and Kup. Trk is a low affinity, rapid transport system that is the main K+ transporter at neutral or alkaline pH (Bossemeyer et al., 1989; Epstein, 2003; Trchounian & Kobayashi, 2000). Kdp is a high affinity K+ transport system that is induced in low K+ environments of 5 mM or less (Epstein, 2003; Frymier et al., 1997). Kup has similar affinity to K+ as Trk, and is believed to be the major K+ transport system under acidic conditions (Trchounian & Kobayashi, 1999; Zakharyan & Trchounian, 2001).
A subset of Gram-negative bacteria are important human pathogens, such as pathogenic E. coli and Salmonella species (Murray, 2003). K+ transport in the pathogenic species of Gram-negative bacteria is expected to be important but has not been characterized extensively so far. Pathogenic bacteria face many more challenges than their non-pathogenic counterparts. Not only do they need to carry out the activities necessary for all living organisms, such as metabolism and reproduction, they additionally need to produce virulence factors to enable them to invade, survive and proliferate in their hosts (Gal-Mor & Finlay, 2006; Groisman & Ochman, 1997; Jones & Falkow, 1996). Whether and how cations and cation transport are involved in pathogenesis has not been extensively characterized. We have previously reported that the K+ transporter Trk is involved in regulating the expression and secretion of the effector proteins SipA, SipC and SopB of the type III secretion system (TTSS) of Salmonella pathogenicity island 1 (SPI-1) of Salmonella, suggesting the existence of a link between the physiological state of Salmonella and its virulence characteristics (Su et al., 2009). Here we report the characterization of roles of the three major K+ transporter systems, Kdp, Kup and Trk, in the pathogenesis of Salmonella. We present evidence that modulation of Salmonella K+ transport, either through deletion of K+ transporters or through exposure to chemical modulators of cation transport, leads to lowered secretion of effector proteins of the TTSS of SPI-1 and reduced virulence in animal models of infection.
Methods
Reagents.
All chemicals were purchased from Sigma-Aldrich unless otherwise indicated. Reagents for Western blot analyses were from Bio-Rad and reagents for PCR were from Invitrogen. Restriction and modifying enzymes for recombinant DNA were obtained from New England Biolabs. Custom oligonucleotides used for generating and complementing Salmonella mutants were from Sigma Genosys. Bacteria and cell culture media were obtained from BD Diagnostics and Invitrogen, respectively.
Bacterial strains, culture conditions and growth curves.
Salmonella enterica serovar Enteritidis isolate SE2472 (clinical isolate) was used as the WT parental strain for all experiments (Table 1) (Lu et al., 1999, 2002, 2003). SE2472 was virulent in mouse and chick infection, can survive in macrophages and was resistant to a number of stress conditions including reactive oxygen and nitrogen intermediates and the antimicrobial activities of chicken egg albumin (Lu et al., 1999, 2002, 2003). E. coli DH5α was used for constructing recombinant plasmid DNA (Table 1). Unless otherwise stated, all bacteria were cultured in Luria–Bertani (LB) broth (BD Diagnostics) at 37 °C with shaking at 225 r.p.m. (Ausubel et al., 1997). LB broth contains 8 mM K+ as measured by a K+-selective electrode (Denver Instruments). A minimal K+ medium (MKM) consisting of 6.78 g Na2HPO4 l−1, 2.9587 g NaH2PO4 . H2O l−1, 10 g NaCl l−1, 1 g NH4Cl l−1, 4 g glucose l−1, 2 mM MgSO4 and 0.2 mM CaCl2 (pH 7) was used to examine the role of K+ in bacterial growth. Although no K+ was added to MKM, it contained approximately 0.2 mM K+ as measured by a K+-selective electrode, likely from K+ contaminants in the salts used (Su et al., 2009). Antibiotics were added as appropriate.
Table 1. Bacterial strains and plasmids.
| Bacterial strain | Characteristics | Source or reference |
| Strains | ||
| E. coli | ||
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA–argF)U169deoRrecA1endA1 | Invitrogen |
| hsdR17(rK− mK+) phoAsupE44λ− thi-1gyrA96relA1 | ||
| Salmonella enterica serovar Enteritidis | ||
| SE2472 | Clinical isolate | Lu et al. (1999) |
| ΔtrkA | SE2472 ΔtrkA : : kan | Su et al. (2009) |
| ΔtrkA-comp | SE2472 ΔtrkA : : kan transformed with pRB3-trkA | Su et al. (2009) |
| ΔkdpA | SE2472 ΔkdpA : : cm | This study |
| Δkup | SE2472 Δkup : : cm | This study |
| ΔkdpA/Δkup | SE2472 ΔkdpA : : cm Δkup | This study |
| ΔkdpA/ΔtrkA | SE2472 ΔkdpA : : cm ΔtrkA : : kan | This study |
| Δkup/ΔtrkA | SE2472 Δkup : : cm ΔtrkA : : kan | This study |
| SipA(HF) | SE2472 sipA : : His-FLAG, SipA+ | Su et al. (2009) |
| SipC(HF) | SE2472 sipC : : His-FLAG, SipC+ | Su et al. (2009) |
| SipA(HF)ΔtrkA | SipA(HF) ΔtrkA : : kan | Su et al. (2009) |
| SipA(HF)ΔtrkA-comp | SipA(HF) ΔtrkA : : kan transformed with pRB3-trkA | Su et al. (2009) |
| SipC(HF)ΔtrkA | SipC(HF) ΔtrkA : : kan | Su et al. (2009) |
| SipC(HF)ΔtrkA-comp | SipC(HF) ΔtrkA : : kan transformed with pRB3-trkA | Su et al. (2009) |
| SipA(HF) ΔkdpA/Δkup | SipA(HF) ΔkdpA : : cm Δkup | This study |
| SipC(HF) ΔkdpA/Δkup | SipC(HF) ΔkdpA : : cm Δkup | This study |
| SipA(HF) ΔkdpA/ΔtrkA | SipA(HF) ΔkdpA : : cm ΔtrkA : : kan | This study |
| SipC(HF) ΔkdpA/ΔtrkA | SipC(HF) ΔkdpA : : cm ΔtrkA : : kan | This study |
| SipA(HF) Δkup/ΔtrkA | SipA(HF) Δkup : : cm ΔtrkA : : kan | This study |
| SipC(HF) Δkup/ΔtrkA | SipC(HF) Δkup : : cm ΔtrkA : : kan | This study |
| Plasmids | ||
| pRB3-273C | Apr, low to medium copy number plasmid for Salmonella | Berggren et al. (1995) |
| pRB3-trkA | Derivative of pRB3-273C containing trkA | Su et al. (2009) |
| pLN | Derivative of pRB3-273C containing pHluorin | This study |
| pKD3 | Apr Cmr, oriRγ | Datsenko & Wanner (2000) |
| pKD4 | Apr Kanr, oriRγ | Datsenko & Wanner (2000) |
| pKD46 | Apr, containing the Red recombinase of λ phage | Datsenko & Wanner (2000) |
| pCP20 | Apr, cat cl857 λPr flp pSC101 oriTS | Datsenko & Wanner (2000) |
Growth curves of Salmonella in various media were determined by plating as previously described (Lu et al., 1999). Salmonella was cultured overnight in LB broth and was diluted 1 : 100 in LB broth, MKM or MKM supplemented with 100 mM KCl. Diluted cultures were incubated at 37 °C with shaking at 225 r.p.m.; an aliquot was collected after various incubation periods and serial dilutions of each bacterial culture were plated onto LB agar plates to determine the bacterial concentration (c.f.u. ml−1). Growth curves were constructed by plotting bacterial concentration against the incubation period.
Construction of K+ transporter mutants and complementation of the mutants.
Single K+ transporter mutants of Salmonella were generated using the one-step mutagenesis method of Datsenko and Wanner (Datsenko & Wanner, 2000). The deletion mutant ΔtrkA of Salmonella was reported previously, in which the coding sequence of trkA was replaced by a kanamycin resistance cassette (KanR) (Su et al., 2009). The deletion mutants ΔkdpA and Δkup were generated similarly using oligonucleotides listed in Table 2. The mutagenesis primer pairs (kdp5KO/kdp3KO and kup5KO/kup3KO) were used to amplify the chloramphenicol resistance cassette (CmR) from plasmid pKD3 (Table 1). The PCR products were then used to generate deletion mutations in kdpA and kup, respectively, through homologous recombination. Each mutant was verified with the corresponding verification primer pair (kdpA5/kdpA3 or kup5/kup3) in combination with primers in the CmR cassette. All mutations were transduced into fresh Salmonella by general transduction using phage P22, and phage-free colonies were used for further analysis (Maloy et al., 1996). Double mutants were generated by transducing a second deletion mutation into single mutants using general transduction by phage P22. Since CmR was used as the selection marker for both the ΔkdpA and the Δkup mutants, the CmR cassette was first removed from the chromosome of the Δkup mutant by transformation of plasmid pCP20 before the ΔkdpA mutation was transduced into the Δkup mutant to generate the ΔkdpA/Δkup double mutant (Datsenko & Wanner, 2000). The ΔtrkA mutant of Salmonella was complemented by the plasmid pRB3-trkA (Su et al., 2009). Vector pRB3-273C was also transformed into the mutants as a control for any possible effect of plasmid transformation alone and no effect was observed in any assay (Lu et al., 2002).
Table 2. Oligonucleotides used for mutagenesis and complementation of mutants.
| Oligonucleotide | Used for | Sequence (5′→3′) |
| kdpA5KO | Mutagenesis of kdpA | caaggatttttactgatcgccagttttttactgattttattggtactggcgaaaccgttgggttcgggcctggcaaggctcatcgccgccgttccgttgcgtgtaggctggagctgcttc |
| kdpA3KO | Mutagenesis of kdpA | taccgggccaagcgccagggcaggaataaacgtcagcgcgccgaccagcagtaccgtgccgatgagcagcccaataaatagcgcgccgtgggtcgccagccatatgaatatcctccttag |
| kup5KO | Mutagenesis of kup | ataataagcaatcgttgcctgcgattaccctcgcagctattggggttgtctacggtgatattggtaccagcccgctttatacgcttcgtgaatgtttgtcgtgtaggctggagctgcttc |
| kup3KO | Mutagenesis of kup | taactcaatcacgcggttaggcgggatctcgaactgatccggcgcgcgcagggcgttgcgttgcaacaacaaatacagcttgccacgcagacgcaaataccatatgaatatcctccttag |
| kdpA5 | Confirmation of kdpA mutation | tatgccctgattaatgcgga |
| kdpA3 | Confirmation of kdpA mutation | atcaatgtactccgcatcgc |
| kup5 | Confirmation of kup mutation | gtgtataaacgaaagatgag |
| kup3 | Confirmation of kup mutation | tgttgatgggaggttaaatc |
| LNHd5 | Expression of pHluorin | agaccaggaagcttgatgagtaaaggagaagaactt |
| LNHd3 | Expression of pHluorin | atagcaacaagcttttatttgtatagttcatccat |
Quantification of protein levels of culture supernatant.
Salmonella strains were cultured in LB broth overnight at 37 °C with shaking at 225 r.p.m. Antibiotics were added to mutant Salmonella strains as appropriate. An aliquot of each culture was serially diluted and plated onto LB agar plates to determine the bacterial c.f.u. ml−1. Three millilitres of each bacterial culture was then spun down at 13 000 r.p.m. for 10 min and 2.4 ml of culture supernatant was transferred to a fresh tube without disturbing the bacterial pellet. Proteins were purified from culture supernatant by TCA precipitation as described by Komoriya et al. (1999). Briefly, 2.4 ml of culture supernatant was mixed with 0.8 ml 25 % TCA and incubated on ice for 30 to 60 min. After incubation, the culture supernatant and TCA mix was spun down at 13 200 r.p.m. for 10 min at 4 °C and the supernatant was then removed. The protein pellet was washed three times vigorously with 2 ml acetone, dried briefly and resuspended in a urea sample buffer (8 M urea, 2 % CHAPS and 10 mM Tris pH 8.0). Protein concentration from each Salmonella strain was determined by DC protein assay (Bio-Rad) and normalized against the bacterial concentration of the culture (c.f.u. ml−1).
Analysis of protein levels of SipA and SipC in culture supernatant and whole-cell lysate.
Salmonella strains containing tandem epitope tags of FLAG and six histidines (6×His) in SipA or SipC were constructed and described previously (Su et al., 2009). The resulting strains, SipA(HF) and SipC(HF), were used to monitor the levels of the respective protein in the culture supernatant and the bacterial lysate (Table 1). All bacterial strains were cultured in LB broth at 37 °C overnight with shaking. The OD600 of bacterial cultures was measured and the cultures of all bacterial strains were adjusted to the same density before being used for analysis of SipA and SipC expression and secretion. Bacterial whole-cell lysate was prepared by spinning down bacterial cultures, washing bacteria twice in ice-cold PBS, resuspending bacterial pellets in an SDS sample buffer (50 mM Tris/HCl pH 6.8, 100 mM DTT, 2 % SDS, 10 % glycerol, 0.1 % bromophenol blue) followed by boiling for 10 min (Sambrook & Russell, 2001). Secreted proteins were prepared by using TCA precipitation as described by Komoriya et al. (1999) and protein concentrations were determined by DC protein assay. Five micrograms of secreted proteins from SipA-tagged strains or 15 µg of secreted proteins from SipC-tagged strains were used for Western blot analysis to compare the relative level of SipA or SipC in the total secreted proteins. Fifteen micrograms of secreted proteins were used for Western blot analysis for SipC because the SipC level in secreted proteins was much lower than the SipA level. The equal loading of protein samples was further confirmed by Coomassie blue staining of SDS-PAGE gels following electrophoresis. Following electrophoresis, SipA or SipC was detected by Western blot analysis as described previously (Su et al., 2009). A monoclonal anti-FLAG antibody (Sigma-Aldrich) was used at 1 : 1000 dilution as the primary antibody and a horseradish peroxidase-conjugated sheep anti-mouse IgG (GE Healthcare) was used at 1 : 2000 dilution as the secondary antibody. Hybridization signal was visualized by the enhanced chemiluminescence method (GE Healthcare). The exposure time was adjusted according to the signal level for each protein.
Exposure of Salmonella to cation transport modulators.
Cation transport modulators gramicidin, valinomycin and nigericin were obtained from Sigma-Aldrich. All modulators were dissolved in DMSO and subsequently diluted in bacterial culture to the concentrations indicated. To determine the effect of the cation transport modulators on the growth and the TTSS of Salmonella, overnight culture of Salmonella in LB broth was diluted 1 : 100 in 3 ml of fresh LB broth supplemented with various concentrations of cation transport modulators and cultured for 16 to 20 h at 37 °C with shaking. The levels of SipA and SipC in the culture supernatant and in bacterial cells were analysed as described above.
Measurement of intrabacterial pH.
The pH inside bacteria was measured using a pH-sensitive green fluorescent protein, pHluorin, that displays a pH-dependent ratio of fluorescence emission at 510 nm with excitation at 375 nm to emission at 510 nm with excitation at 405 nm (Miesenböck et al., 1998). Primers LNHd5 5′-AGACCAGGAAGCTTGATGAGTAAAGGAGAAGAACTT-3′ and LNHd3 5′-ATAGCAACAAGCTTTTATTTGTATAGTTCATCCAT-3′ were used to amplify the pHluorin sequence from plasmid pGEX2T (gift of Dr Sabine Ehrt, Weill Cornell Medical College) by PCR. Amplified DNA was digested with HindIII and cloned into the HindIII site of pRB3-273C (Berggren et al., 1995). The resulting plasmid, pLN, contained pHluorin under the control of the lac promoter. The plasmid pLN was transformed into Salmonella strains for measuring intrabacterial pH as described by Vandal et al. (2008). Bacterial culture was spun down and the pellet was resuspended in PBS at 1/10 volume of the original culture. Three hundred microlitres of resuspended bacteria was transferred to a 96-well black plate (USA Scientific) and the fluorescence was detected with a SpectraMax II plate reader (Molecular Devices). Fluorescence was detected at emission of 510 nm (Em510) with excitation of 375 nm (Ex375 nm) or 405 nm (Ex405 nm), and the ratio of excitation of Em510-Ex375 nm to Em510-Ex405 nm was calculated.
For each experiment, a standard curve of pHluorin was prepared for determining the intrabacterial pH of Salmonella. Whole-cell lysate of Salmonella transformed with plasmid pLN was prepared and diluted to 50 µg ml−1 in a series of sodium phosphate buffers at defined pH from 6.6 to 8.0. Em510 was measured with a SpectraMax II plate reader at Ex375 nm or Ex405 nm and the ratio of Em510-Ex375 nm to Em510-Ex405 nm was plotted against the pH of the phosphate buffer. The intrabacterial pH of whole-cell Salmonella was determined by plotting the ratio of Em510-Ex375 nm to Em510-Ex405 nm on the standard curve prepared for each experiment.
Measurement of bacterial ATP and K+.
For measuring bacterial ATP, Salmonella strains were grown overnight at 37 °C with shaking at 225 r.p.m. in LB broth with appropriate antibiotics. Overnight cultures were diluted 1 : 100 in fresh LB or LB supplemented with various concentrations of cation transport modulators. Aliquots were harvested at various time points and serially diluted bacterial cultures were plated to determine bacterial concentration (c.f.u. ml−1) after overnight incubation. Bacteria were then collected by centrifugation at 13 200 r.p.m. for 5 min and bacterial extracts were prepared by perchloric acid extraction (Bagnara & Finch, 1972). Two hundred microlitres of bacterial culture was mixed with 100 µl 1.2 M perchloric acid pre-cooled on ice and vortexed for 10 s. The mixture was incubated on ice for 15 min and spun down at 132 000 r.p.m. for 5 min at 4 °C. Two hundred microlitres of supernatant was transferred to a fresh tube and mixed with 100 µl neutralizing solution containing 0.72 M KOH and 0.16 M KHCO3. Neutralized extract was spun down at 132 000 r.p.m. for 5 min and supernatant was transferred to a fresh tube for ATP assay using a BacTiter-Glo microbial cell viability assay (Promega) following the manufacturer’s procedure.
Luminescence was read in a SpectraMax M2 plate reader (Molecular Devices). A standard curve was prepared in each assay using ATP standard solutions prepared from adenosine 5-triphosphate disodium salt hydrate (A2383; Sigma-Aldrich) and ATP quantity in bacterial samples was determined by comparing to the standard curve.
Bacteria K+ content was determined as described by Yan et al. (1996) with modifications. Salmonella strains were grown overnight at 37 °C with shaking at 225 r.p.m. in LB broth with appropriate antibiotics. An aliquot of each culture was serially diluted and plated to determine bacterial concentration (c.f.u. ml−1) after overnight incubation. Overnight culture of Salmonella was spun down at 10 000 r.p.m. for 2 min and the supernatant was removed. Bacterial pellet was resuspended in an equal volume of 1 % nitric acid and the mixture was incubated at room temperature for 1 h to lyse bacteria. The reaction mixture was spun down at 13 200 r.p.m. for 5 min and supernatant containing lysed bacteria was neutralized with 10 M NaOH. The K+ concentration in neutralized bacterial lysate was measured using a K+-selective electrode and calculated by comparing to a K+ standard curve. Each measurement was carried out three times and the mean was used for data analysis.
Invasion of HeLa cells by Salmonella.
HeLa cell invasion assay was performed as described (Lu et al., 1999). All Salmonella strains were cultured in LB broth overnight at 37 °C without shaking. Antibiotics were added as appropriate. HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco by Life Technologies) supplemented with 10 % FBS that has a K+ concentration of approximately 5 mM as measured by a K+-selective electrode. Overnight culture of bacteria was added to HeLa cells at an m.o.i. of 5 : 1 to 10 : 1, and intracellular bacteria were quantified after one or two hours of invasion followed by an incubation period in the presence of 50 µg gentamicin ml−1 to kill extracellular bacteria. The invasiveness of Salmonella was measured by the ratio of intracellular bacteria to the input bacteria, which was calculated as (number of intracellular bacteria/number of input bacteria)×100 %.
Motility of the K+ transporter mutants of Salmonella.
Salmonella strains were grown overnight at 37 °C with shaking at 225 r.p.m. in LB broth with appropriate antibiotics. Motility agar plates were prepared using 25 ml motility agar (BD Diagnostics) in each Petri dish. Overnight culture of bacteria was inoculated in the centre of a Petri dish using an inoculation needle and each strain was analysed in triplicate. After overnight incubation at 37 °C, the diameter of the zone of bacterial growth on each Petri dish was measured. To study the effect of K+ or Na+ on the motility of the K+ transporter mutants of Salmonella, Salmonella strains were grown in LB broth supplemented with 100 mM KCl or NaCl and assayed on motility agar plates supplemented with the same concentration of the corresponding salt.
Mouse and chick infection of Salmonella.
Salmonella cultured overnight in LB broth at 37 °C with shaking at 225 r.p.m. was used in all animal infection experiments. Six- to eight-week-old BALB/c mice (Jackson Laboratory) were infected intragastrically and the LD50 was determined by infecting groups of five mice with 10-fold dilutions of bacteria in PBS (Lu et al., 1999; Reed & Muench, 1938).
For infection of chicks, specific pathogen-free eggs were obtained from Charles River SPAFAS (North Franklin, CT) and incubated in an incubator/hatcher (G.F.Q. Manufacturing) with automatic turning. After 18 days of incubation, eggs were moved to the hatching tray of the incubator until hatched. Newly hatched chicks were left in the incubator to dry for 4–6 h and then moved to a cage. Within 24 or 36 h after hatching, 109 c.f.u. of Salmonella suspended in 250 µl sterile PBS was administered to the crop of each chick via a feeding tube. Infected chicks (in groups of 10) were monitored twice daily for 14 days and survival was recorded.
Results
Construction of K+ transporter mutants of Salmonella
Since K+ is the most abundant cation in all living cells and plays an important role in maintaining the electrical gradient in bacteria, we generated mutations in K+ transporters and determined if K+ transporters are important for the pathogenesis of Salmonella. We have previously reported the construction and analysis of a ΔtrkA mutant of Salmonella that contained a deletion mutation of the low affinity K+ transport system Trk (Su et al., 2009). The ΔtrkA mutant was found to be defective in the expression and secretion of the effector proteins of the TTSS of SPI-1, invasion of epithelial cells and virulence in mouse infection (Su et al., 2009). In this analysis, we constructed additional mutants of the low affinity K+ transport system Kup and the high affinity K+ transport system Kdp in the virulent clinical Salmonella strain SE2472 (Lu et al., 1999). We generated double mutants ΔkdpA/Δkup, ΔkdpA/ΔtrkA and Δkup/ΔtrkA for in vitro and in vivo analyses (Table 1).
All K+ transporter mutants of Salmonella displayed the same colony morphology as the WT Salmonella on LB agar, except that the ΔtrkA-containing mutants (ΔtrkA, ΔkdpA/ΔtrkA and Δkup/ΔtrkA) formed smaller colonies (data not shown). The growth properties of the K+ transporter mutants were determined in LB broth, MKM and MKM supplemented with a high concentration (100 mM) of KCl (Fig. 1). Using a K+-selective electrode, the concentration of K+ in LB broth was determined to be approximately 8 mM. The MKM was prepared without any K+ salt; however, it contained approximately 0.2 mM K+, likely from K+ contamination in salts used for the medium. In LB broth, all mutants grew to the same concentration after 24 h of incubation (Fig. 1a). In the MKM, mutants with deletions in Kdp failed to reach the same concentration as those strains with an intact Kdp system (Fig. 1b) (P = 0.00008 and 0.00007 for ΔkdpA/Δkup and ΔkdpA/ΔtrkA, respectively at 24 h, Student’s t-test). The double mutant ΔkdpA/ΔtrkA was especially severely affected and displayed little or no growth over 24 h of incubation (Fig. 1b). In the presence of a high K+ concentration (100 mM), the growth of all K+ transporter mutants was relatively normal and reached the same bacterial concentration after 24 h of incubation as the WT parental strain (Fig. 1c). The ΔtrkA-containing double mutants displayed a slight growth delay during mid- to late-exponential phase (P = 0.002 for both ΔkdpA/ΔtrkA and Δkup/ΔtrkA at 6 h, Student’s t-test), but eventually reached the same bacterial concentration at the stationary phase as the WT Salmonella (Fig. 1c).
Fig. 1.
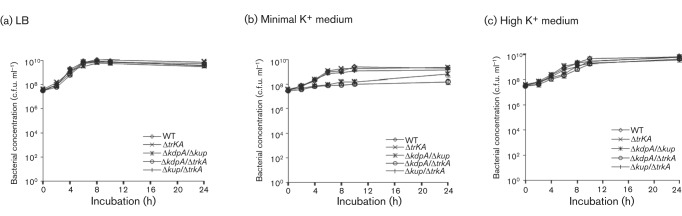
Growth properties of the K+ transporter mutants of Salmonella in culture media. The WT Salmonella (◊) and ΔtrkA (×), ΔkdpA/Δkup (*), ΔkdpA/ΔtrkA (○) and Δkup/ΔtrkA (|) mutants were cultured in LB broth (a), minimal K+ medium (b) and minimal K+ medium supplemented with 100 mM KCl (c). Bacterial concentrations were determined by plating. At least three experiments were performed and results from a representative experiment performed in triplicate are shown. Error bars indicate sd.
K+ transporters are important for the secretion of the effector proteins of the TTSS of SPI-1
Since the K+ transporter Trk was shown to be involved in the secretion of effector proteins of the TTSS of SPI-1 (Su et al., 2009), we tested how other transporters, Kdp and Kup, affect the secretion of the effector proteins SipA and SipC of the TTSS of SPI-1, factors that have been previously shown to play important functions in host-cell invasion and in the modulation of the host cytoskeleton to facilitate Salmonella infection (Jepson et al., 2001; Kaniga et al., 1995; Raffatellu et al., 2005). We used tagged strains SipA(HF) and SipC(HF) that contained a tandem tag of 6×His and FLAG epitope fused in-frame to the C-terminus of the corresponding protein in the chromosome (Su et al., 2009; Uzzau et al., 2001). The ΔkdpA, Δkup and ΔtrkA mutations were transduced into each tagged strain either singularly or in combinations (Table 1) and the levels of the tagged SipA or SipC protein in the whole bacterial lysate and the culture supernatant were analysed by Western blot using an anti-FLAG antibody (Fig. 2).
Fig. 2.
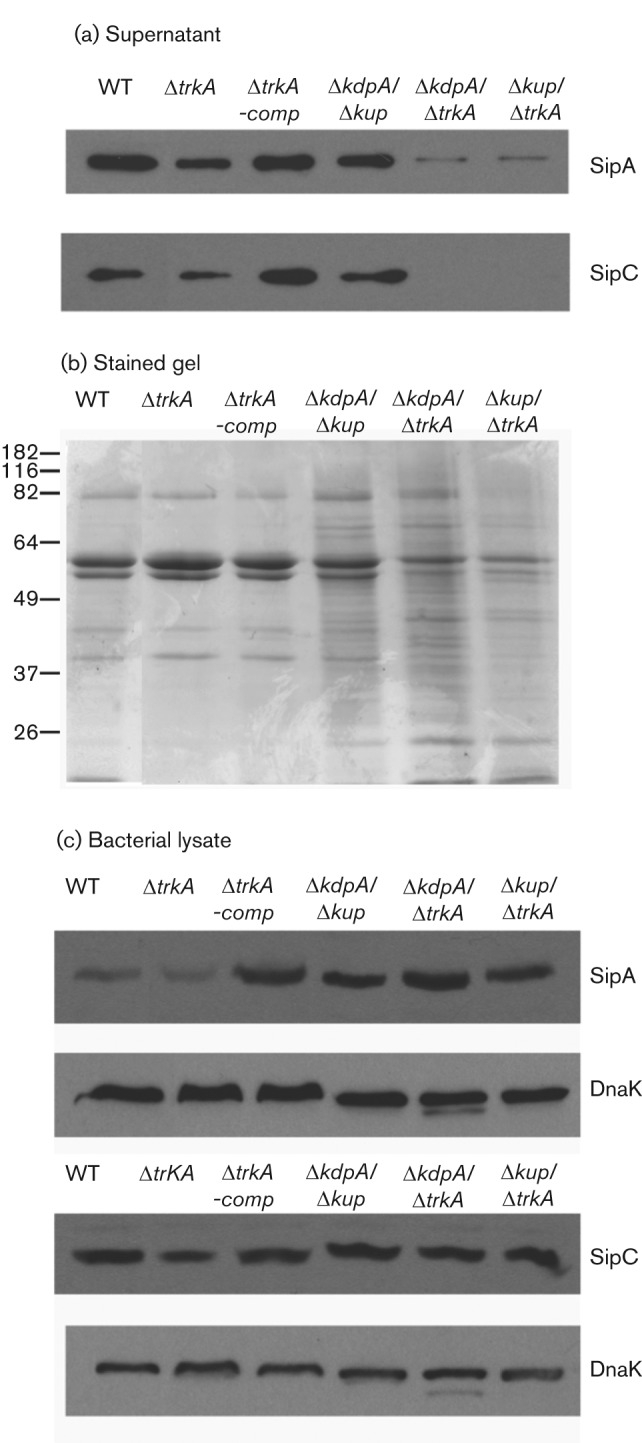
Secretion and expression of effector proteins SipA and SipC of the SPI-1-encoded TTSS by K+ transporter mutants of Salmonella. (a) Western blot analysis of SipA and SipC levels in culture supernatant. Epitope-tagged strains SipA(HF) and SipC(HF) with the WT K+ transporters, one or two K+ transporter mutant alleles (ΔtrkA, ΔkdpA/Δkup, ΔkdpA/ΔtrkA or Δkup/ΔtrkA), or complemented single mutant ΔtrkA-comp were cultured in LB broth. Secreted proteins were purified from culture supernatant by TCA precipitation, quantified by DC protein assay and equal quantities of secreted proteins from each strain were used for each gel. SipA and SipC levels in the culture supernatant were determined by Western blot analysis. The bacterial strain used for each sample is labelled above each lane. The effector protein analysed in each panel is labelled on the right. (b) A duplicate gel of secreted proteins from SipA-tagged strains was included to show the total secreted proteins from each strain. (c) Western blot analysis of SipA and SipC levels in whole-cell lysates. DnaK was used as a control for equal loading. The effector protein analysed in each panel or DnaK control is labelled on the right. At least three experiments were performed and results from a representative experiment are shown.
Secreted proteins were purified from culture supernatant of the WT and K+ transporter mutant Salmonella and equal quantities of secreted proteins from each strain were used for Western blot analysis (Fig. 2a). Since no secreted protein has been shown to be constitutive and can thus serve as an internal control, a duplicate gel of secreted proteins from SipA-tagged K+ transporter mutant strains was stained to visualize the protein content in the culture supernatant (Fig. 2b). The ΔkdpA/ΔtrkA and Δkup/ΔtrkA double mutants were severely defective in SipA and SipC secretion (Fig. 2a) and also had altered compositions of total secreted proteins (Fig. 2b). Compared with the double mutants, the ΔtrkA single mutant had a less severe defect in SipA and SipC secretion and the defect was rescued by complementation with plasmid pRB3-trkA, which carries a WT allele of the trkA gene (Fig. 2a). The SipA and SipC levels in the ΔkdpA/Δkup mutant were close to those in the WT Salmonella (Fig. 2a).
In contrast to changes of the levels of SipA and SipC in culture supernatant of K+ transporter mutants, the levels of SipA and SipC in bacterial lysate were not significantly affected by the deletion of any of the K+ transporters (Fig. 2c), suggesting that the lowered levels of SipA and SipC in the culture supernatant of K+ transporter mutants were not due to a lack of their expression. The levels of SipA and SipC were slightly lower in the ΔtrkA mutant (Fig. 2c), but not in the ΔtrkA-containing double mutants (ΔkdpA/ΔtrkA and Δkup/ΔtrkA), possibly because more SipA and SipC were retained in bacteria in the double mutants instead of being secreted (Fig. 2c).
Cation transport modulators affect the TTSS of Salmonella
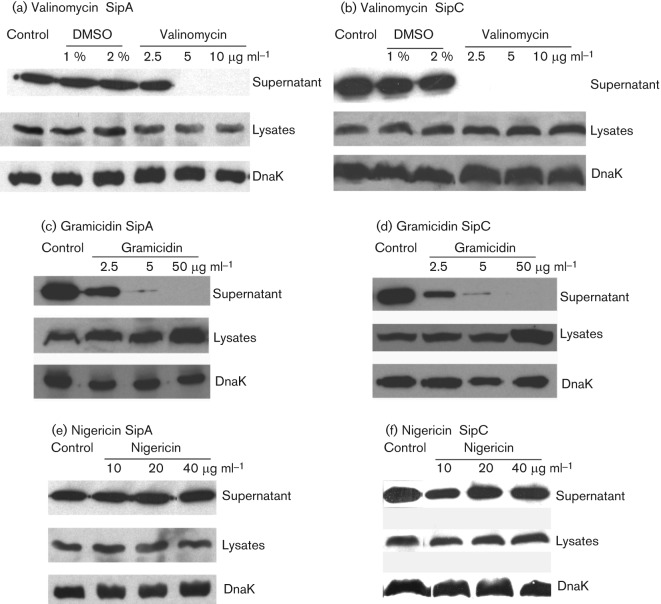
Since analysis of K+ transporter mutants indicated that K+ transport may be involved in the TTSS, we next tested if chemical reagents that modulate cation transport (including K+ transport) would affect the TTSS of Salmonella. The cation transport modulators we used included valinomycin, nigericin and gramicidin. Valinomycin is an ionophore for K+ and allows transport of K+ along the K+ gradient. Gramicidin increases permeability to all cations instead of specifically to K+. Nigericin mediates the exchange of K+ and H+. The cation transport modulators were first tested for their effect on the growth of Salmonella. None was found to have any effect on the growth of Salmonella at the highest soluble concentrations tested (data not shown).
Salmonella was treated with the cation transport modulators to determine if they affect the TTSS using SipA and SipC proteins as markers. Salmonella strains with the FLAG epitope tag at SipA or SipC were exposed to various concentrations of the cation transport modulators and the levels of SipA and SipC in the culture supernatant and the bacterial cell lysates were determined by Western blot analysis (Fig. 3). Valinomycin and gramicidin inhibited the secretion of both SipA and SipC, while nigericin had no effect at all concentrations tested (Fig. 3). None of the cation transport modulators inhibited the expression of SipA or SipC and the levels of both proteins were comparable to those in the WT Salmonella (Fig. 3). DMSO was used as a solvent for all cation transport modulators and it had no effect on the expression or secretion of SipA or SipC (Fig. 3).
Fig. 3.
Secretion and expression of effector proteins SipA and SipC of the SPI-1-encoded TTSS in Salmonella exposed to cation transport modulators. Epitope-tagged strains SipA(HF) and SipC(HF) were grown in LB broth and exposed to various concentrations of the cation transport modulators valinomycin (a, b), gramicidin (c, d) or nigericin (e, f). DMSO was used as a solvent for all cation modulators and was included as a control. The levels of SipA (a, c, and e) and SipC (b, d, and f) in the culture supernatant and whole-cell lysates were determined by Western blot analysis and compared to those of untreated Salmonella. Proteins were isolated from culture supernatant by TCA precipitation and quantified by DC protein assay. Equal quantities of proteins from culture supernatant were loaded in each lane in each experiment. DnaK was used as a control for equal loading of bacterial lysates. At least three experiments were performed and results from a representative experiment are shown.
The cation transport modulator gramicidin increases the permeability of bacterial cell membranes to cations and exposure to gramicidin inhibits the TTSS in Salmonella (Fig. 3c, d). We reasoned that the inhibition of the TTSS by gramicidin could be due to a loss of intrabacterial K+ along the gradient since intracellular K+ concentration is much higher than that in the medium (>100 mM vs 8 mM). To test this hypothesis, we exposed the WT Salmonella to a low concentration of gramicidin in the presence or absence of 100 mM KCl or NaCl, two of the most physiologically relevant cations. The partial inhibition of SipA and SipC secretion by a low concentration of gramicidin was relieved by KCl supplementation but not by NaCl (Fig. 4a). This result suggests that the rescue of gramicidin inhibition of the TTSS by KCl is not simply due to the osmotic pressure KCl provides since NaCl provides the same osmotic pressure as KCl but failed to restore the TTSS in gramicidin-treated Salmonella (Fig. 4a).
Fig. 4.
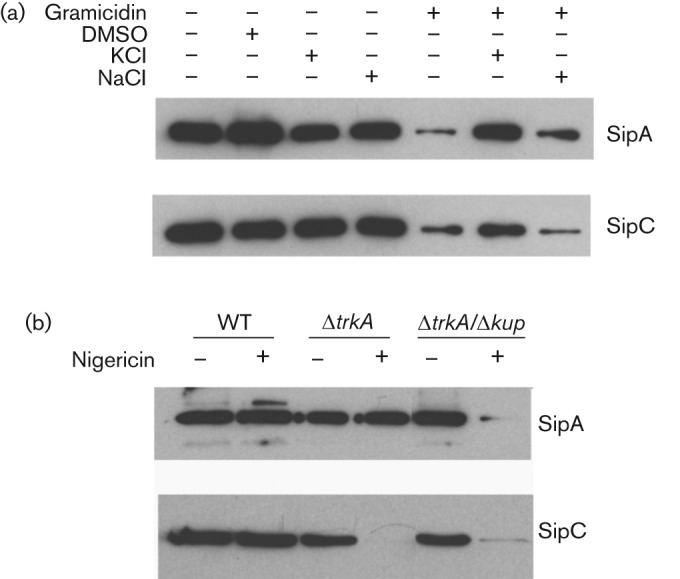
Ions, cation transport modulators and K+ transporters interact to affect the TTSS of SPI-1. (a) Epitope-tagged strains SipA(HF) and SipC(HF) were grown in LB broth in the presence of the cation transporter modulator gramicidin and cation supplementation as marked. (b) Epitope-tagged strains SipA(HF) and SipC(HF) containing mutations in K+ transporters were cultured in the presence or absence of the cation transport modulator nigericin. Proteins were isolated from culture supernatant and equal quantities of proteins from culture supernatant were loaded in each lane. The levels of SipA and SipC were determined by Western blot analysis. At least three experiments were performed and results from a representative experiment are shown.
The cation transport modulator nigericin does not affect the TTSS of the WT Salmonella (Fig. 3e, f). We next treated the K+ transport mutants of Salmonella with nigericin to determine if this affects the TTSS when K+ transport is impaired. Similar to that observed with the WT Salmonella, exposure to nigericin did not affect the growth and survival of the single or double K+ transporter mutants (data not shown). Only the single mutant ΔtrkA and the double mutant ΔkdpA/Δkup were included in the analysis since the double mutants ΔkdpA/ΔtrkA and Δkup/ΔtrkA were defective in SipA and SipC secretion without nigericin treatment (Fig. 3). As shown in Fig. 4(b), SipA secretion was inhibited in the nigericin-treated ΔkdpA/Δkup double mutant, and SipC secretion was inhibited in both the ΔtrkA mutant and the ΔkdpA/Δkup double mutant treated with nigericin. Nigericin treatment affected SipC more than SipA in the ΔtrkA mutant likely because the SipC level is much lower than SipA in secreted proteins and is thus more easily disturbed.
TTSS defect observed in the K+ transport mutant Salmonella and Salmonella treated with cation transport modulators was not due to a failure in maintaining intrabacterial pH
We have shown above that cation transport is important for the TTSS and interference with K+ and H+ transport compromises the TTSS of SPI-1. Since both K+ and H+ are important intrabacterial cations that are major components of proton motive force (PMF), it is possible that K+ transport affects the TTSS because the disturbance of K+ transport causes a distubance in PMF. To evaluate this possibility, we measured the intrabacterial pH in the K+ transporter mutant or cation transport modulator-treated Salmonella to determine if the suppression of the TTSS correlates with a failure in regulating intrabacterial pH. We took advantage of the pH-sensitive green fluorescent protein, pHluorin, to measure the intrabacterial pH (Miesenböck et al., 1998; Vandal et al., 2008). A plasmid, pLN, was constructed that expresses pHluorin in Salmonella and transformed into the WT or K+ transport mutant Salmonella. Overnight culture of Salmonella was diluted in LB broth and intrabacterial pH was measured after 3 or 16 h of growth. The ΔtrkA single mutant and ΔtrkA-containing double mutants displayed a generally lower intrabacterial pH as they grew from log to stationary phase (Fig. 5a). The difference between the ΔtrkA-containing single or double mutants and the WT Salmonella was small, but statistically significant (P = 0.01, 0.009 and 0.005 at 16 h for ΔtrkA, ΔkdpA/ΔtrkA and Δkup/ΔtrkA, respectively, Student’s t-test). However, the intrabacterial pH in the ΔtrkA single mutant was indistinguishable from those of the ΔkdpA/ΔtrkA and Δkup/ΔtrkA double mutants, suggesting that intrabacterial pH did not correlate with the TTSS level as SipA and SipC secretion was much lower in the ΔtrkA-containing double mutants compared to the ΔtrkA single mutant (Fig. 2a). Salmonella mutant ΔyafD (Lu et al., 2003), a deletion mutant of the yafD gene that was shown to be involved in the survival of Salmonella in egg albumin and has no known role in the TTSS, was included as a control for possible influence of mutagenesis or antibiotic resistance markers on intrabacterial pH. The intrabacterial pH of the ΔyafD mutant was similar to that of the WT Salmonella, suggesting that the slightly lowered pH observed in the ΔtrkA mutants was not due to the mutagenesis procedure itself (Fig. 5a).
Fig. 5.
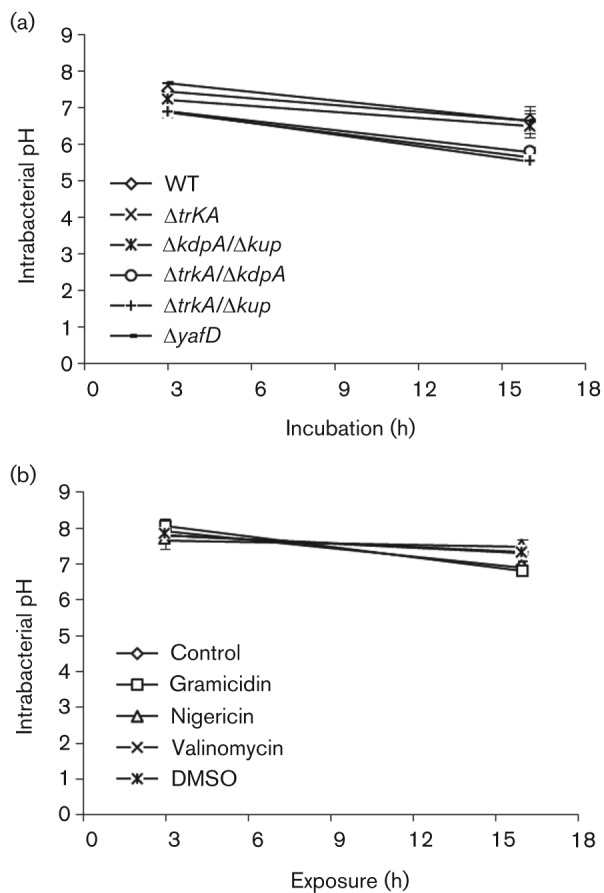
Intrabacterial pH of K+ transporter mutants of Salmonella and Salmonella exposed to cation transport modulators. (a) WT and K+ transporter mutants of Salmonella transformed with PHluorin-expressing plasmid pLN were cultured in LB broth. (b) Salmonella transformed with pLN was cultured in LB broth with or without exposure to cation transport modulators. The fluorescence from each culture was measured and intrabacterial pH was determined by comparing to a standard curve. At least three experiments were performed, and results from a representative experiment performed in triplicate are shown. Error bars indicate sd.
The intrabacterial pH of Salmonella exposed to cation transport modulators was determined as described for K+ transport mutants. WT Salmonella transformed with pLN was exposed to gramicidin (25–100 µg ml−1), nigericin (10–40 µg ml−1) or valinomycin (2.5–10 µg ml−1) and bacteria were harvested after 3 or 16 h of exposure. The intrabacterial pH of treated Salmonella was determined and compared to the untreated Salmonella or Salmonella treated with the solvent DMSO. The results of the highest concentrations tested are shown in Fig. 5b. Treatment with gramicidin, nigericin or valinomycin did not affect the intrabacterial pH, as pH in the treated Salmonella was indistinguishable from that of the untreated Salmonella or Salmonella treated with the solvent DMSO (Fig. 5b).
TTSS defect observed in the K+ transport mutant Salmonella or cation transport modulator-treated Salmonella was not due to a failure in maintaining intrabacterial ATP level
Intracellular ATP levels were measured in the K+ transporter mutants to determine if the defect in the TTSS was due to a lack of intracellular ATP. All strains were cultured in fresh medium and aliquots of each culture were harvested after various incubation periods to quantify bacterial concentration (c.f.u. ml−1) and total ATP levels. The total ATP levels were normalized against bacterial concentration and plotted against the incubation period (Fig. 6a). ATP levels in all bacterial cultures were high during early exponential phase of culture (at 3 h) and subsequently decreased with incubation period. Compared with the WT Salmonella, the K+ transporter mutants displayed comparable or higher ATP levels, suggesting that the defect in the TTSS cannot be explained by a lack of intracellular ATP (Fig. 6a).
Fig. 6.
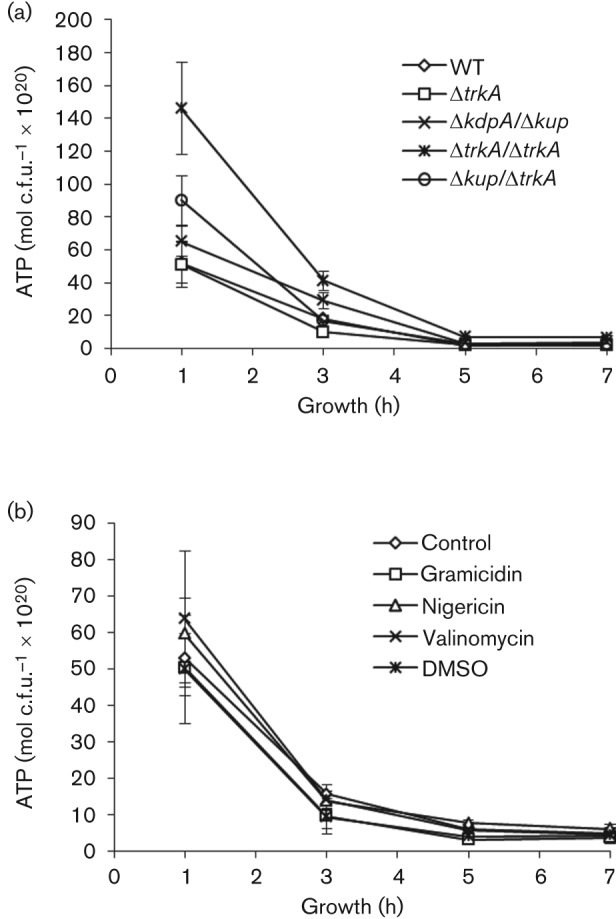
Intrabacterial ATP level of K+ transporter mutants of Salmonella and Salmonella exposed to cation transport modulators. (a) ATP level of the WT and K+ transporter mutants of Salmonella. Salmonella was cultured in LB broth. (b) ATP level of Salmonella exposed to cation transport modulators. Salmonella was cultured in LB broth supplemented with gramicidin (100 µg ml−1), nigericin (40 µg ml−1), valinomycin (10 µg ml−1) or the solvent DMSO. ATP level was determined at various time points of incubation and normalized against bacterial c.f.u. ml−1. At least three experiments were performed, and results from a representative experiment performed in triplicate are shown. Error bars indicate sd.
We next determined the energy level of cation modulator-exposed Salmonella by measuring the ATP level in the treated bacteria. Overnight culture of Salmonella was diluted in fresh LB, or LB supplemented with the solvent DMSO, gramicidin (25–100 µg ml−1), nigericin (10–40 µg ml−1) or valinomycin (2.5–10 µg ml−1). Bacterial culture was harvested after various periods of incubation and bacterial concentration and the ATP level in each sample were determined. Results from treatment with the highest concentration of gramicidin (100 µg ml−1), nigericin (40 µg ml−1) or valinomycin (10 µg ml−1) are shown in Fig. 6(b). No difference was observed between Salmonella treated with cation transport modulators and untreated or DMSO-treated Salmonella. The lack of effect of cation transport modulators on intrabacterial ATP levels suggests that the inhibition by gramicidin and valinomycin of the TTSS of SPI-1 was not likely due to a lack of intracellular ATP.
K+ transporters are necessary for protein secretion and motility of Salmonella and its invasion of epithelial cells
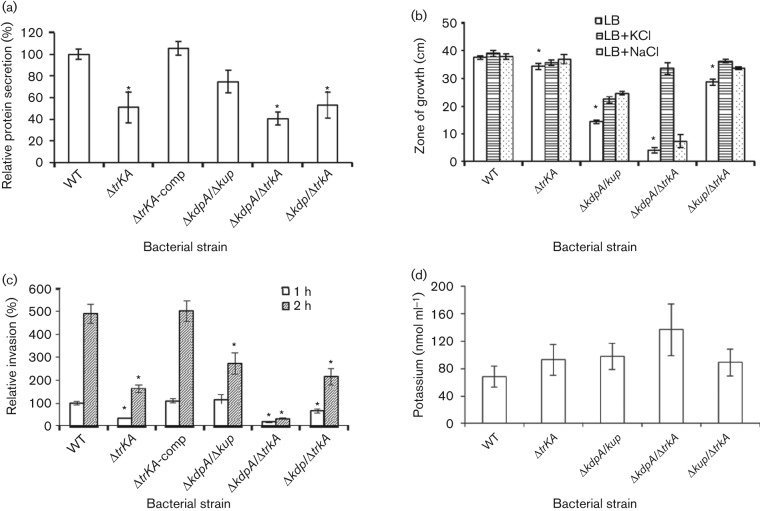
We examined the K+ transporter mutants for their protein secretion, motility and invasion of epithelial cells to determine how K+ transporters are involved in these processes. We first determined if the K+ transporters affect the overall protein secretion in addition to their effects on the TTSS of SPI-1 (Fig. 2). An equal volume of culture supernatant was collected from each of the single mutant ΔtrkA and the double mutants ΔkdpA/Δkup, ΔkdpA/ΔtrkA and Δkup/ΔtrkA, and total proteins in the culture supernatant were isolated, quantified and normalized against their bacterial c.f.u. ml−1. The quantity of total proteins from the culture supernatant of the WT Salmonella was set as 100 % and the quantity of proteins from each mutant was expressed as a relative value to that of the WT Salmonella (Fig. 7a). Mutation in Trk decreased protein secretion by approximately 50 % compared to that of the WT Salmonella and the protein secretion was restored in the complemented mutants (Fig. 7a). The difference between the ΔtrkA mutant and the WT Salmonella was statistically significant (P = 0.04, Student’s t-test). Consistent with this result, the double mutants ΔkdpA/ΔtrkA and Δkup/ΔtrkA also displayed lower protein levels in culture supernatant compared to the WT Salmonella (P = 0.001 and 0.002, respectively, Student’s t-test). The double mutant ΔkdpA/Δkup secretes less protein than the WT Salmonella; however, the difference was not statistically significant (P>0.05, Student’s t-test) (Fig. 7a).
Fig. 7.
K+ transporter mutants of Salmonella display defective phenotypes in characteristics related to pathogenesis. WT Salmonella, K+ transporter ΔtrkA mutant, complemented ΔtrkA mutant (ΔtrkA-comp) and double K+ transporter mutants ΔkdpA/Δkup, ΔkdpA/ΔtrkA and Δkup/ΔtrkA were analysed for total protein secretion (a), motility (b) and epithelial cell invasion (c). (a) Protein secretion by the WT and K+ transporter mutants of Salmonella. Salmonella strains were grown in LB broth. Secreted proteins were purified from culture supernatant, quantified by DC protein assay and normalized against the c.f.u. ml−1 of each bacterial culture. The level of protein secretion from the WT Salmonella was set as 100 % and the level of protein secretion from each K+ transporter mutant Salmonella strain was expressed as a relative value to that of the WT Salmonella. (b) Motility of the WT and K+ transporter mutants of Salmonella. Overnight LB broth culture of each Salmonella strain was inoculated in the centre of a motility agar plate with or without supplement of KCl or NaCl. The zone of growth on each plate was measured after overnight incubation and plotted. (c) Epithelial cell invasion of the WT and K+ transporter mutants of Salmonella. Salmonella strains were growth in LB broth and used to infect HeLa cells and the ratio of intracellular bacteria to the input bacteria was determined for each Salmonella strain. The ratio of intracellular bacteria for the WT Salmonella at 1 h post infection was set as 100 % and the ratio for the other samples was expressed as a relative value. All results are the mean of three independent experiments. Error bars indicate sd. *P<0.05 compared to the WT, Student’s t-test. (d) K+ content of Salmonella strains. WT and K+ transporter mutants of Salmonella were cultured overnight in LB broth and total K+ level in each strain was determined. At least three experiments were performed and results from a representative experiment performed in triplicate are shown. Error bars indicate sd.
Since some of the K+ transporter mutants of Salmonella have altered protein secretion compared to the WT parental strain, we tested the motility of the mutants because flagellar proteins involved in motility are exported through the flagellar secretion apparatus and may be affected in the K+ transporter mutants (Aizawa, 1996; Fraser & Hughes, 1999; Journet et al., 2005). The overnight culture of each single and double K+ transporter mutant was inoculated onto motility agar plates and the diameter of the zone of growth for each strain was measured after overnight incubation. All mutants displayed a significant defect in motility (P<0.05, Student’s t-test) and Salmonella lacking two of the K+ transporters, Kdp, Kup or Trk, were more severely affected than that lacking only Trk (Fig. 7b). The results suggest that at least two of the three major K+ transporters, Kdp, Kup and Trk, are needed for a relatively normal level of motility. Supplementation of KCl or NaCl partially restored the motility of the double mutants (Fig. 7b). The rescue was especially effective for the ΔkdpA/ΔtrkA double mutant supplemented with KCl. KCl supplementation restored the motility of the mutant to close to the level of the WT parental strain. In contrast, the same concentration of NaCl only slightly increased the motility of the ΔkdpA/ΔtrkA double mutant (Fig. 7b).
We then tested the importance of the K+ transporters in epithelial cell invasion. The invasiveness of the single K+ transporter mutant ΔtrkA and the double mutants ΔkdpA/Δkup, ΔkdpA/ΔtrkA and Δkup/ΔtrkA was determined in a HeLa cell invasion assay and the ratio of intracellular bacteria to the input bacteria after one or two hours of invasion was measured for all mutant strains and compared to the WT Salmonella. The ratio of intracellular bacteria for the WT Salmonella at 1 h post infection was set as 100 % and the ratio of intracellular bacteria for the other samples was expressed as a relative value. All mutants, with the exception of ΔkdpA/Δkup at 1 h post infection, displayed a significant defect in invasion (P<0.05, Student’s t-test) (Fig. 7c).
Since intracellular K+ plays important physiological functions and K+ transporters are responsible for maintaining K+ balance, it is possible that K+ transporter mutants are defective in the TTSS because of a K+ deficiency that affects their basic physiology. We measured the total K+ content and bacterial c.f.u. ml−1 in the overnight culture of each Salmonella strain and quantified intracellular K+ levels per c.f.u. (Fig. 7d). The results showed that K+ transporter mutants did not have K+ deficiency. Instead, the ΔkdpA/ΔtrkA and Δkup/ΔtrkA double mutants appeared to have a higher K+ content than the WT Salmonella and the ΔkdpA/Δkup mutant (Fig. 7d).
K+ transporters of Salmonella are involved in infection of mice and chicks
As we have shown that K+ transporters contribute to several virulence characteristics of Salmonella (e.g. protein secretion, motility and epithelial cell invasion), we reasoned that K+ transporters may contribute to the pathogenesis in a host. We tested the virulence of the single K+ transporter mutant ΔtrkA and the double mutants ΔkdpA/Δkup, ΔkdpA/ΔtrkA and Δkup/ΔtrkA in the mouse model of Salmonella infection. Each strain of Salmonella was used for intragastric infection and the LD50 was measured. All mutants displayed a significant attenuation compared to the WT Salmonella (Table 3; P<0.05, Student’s t-test). The single mutant ΔtrkA displayed an attenuation in virulence of approximately 10-fold and the attenuation was rescued by a plasmid expressing trkA. The double mutants ΔkdpA/Δkup, ΔkdpA/ΔtrkA and Δkup/ΔtrkA displayed a much higher degree of attenuation, up to 100 000-fold (Table 3).
Table 3. LD50 of the WT and K+ transporter mutants of Salmonella in intragastric infection of mice.
Results are the means±sd of three experiments.
| Strain | LD50 (c.f.u.) |
| WT | 2.4±1.0×103 |
| ΔtrkA | 2.0±0.9×104* |
| ΔtrkA-comp | 1.4±0.8×103 |
| ΔkdpA/ΔkupA | 1.2±0.5×106* |
| ΔkdpA/ΔtrkA | 2.4±1.1×107* |
| Δkup/ΔtrkA | 2.8±1.4×108* |
P<0.05, Student’s t-test.
In addition to the mouse model of Salmonella infection, the K+ transporter mutant Salmonella strains were also used to infect chicks, a natural host of Salmonella that is important for its transmission to humans. Day-old chicks were infected orally with Salmonella and the survival of infected chicks was monitored for 2 weeks. Similar to what was observed in the mouse infection, the virulence of the K+ transporter mutants was attenuated in the chick infection (Fig. 8; P<0.05, Kaplan–Meier analysis).
Fig. 8.
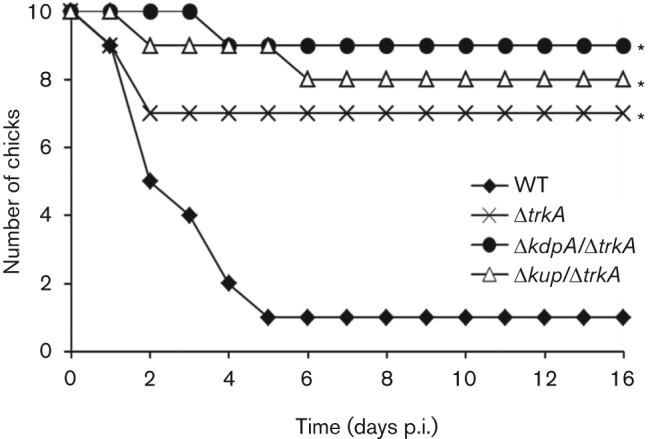
Virulence of the WT and K+ transporter mutants of Salmonella in infection of chicks. Day-old chicks were orally inoculated with overnight culture of the WT Salmonella (⧫) or K+ transporter mutants ΔtrkA (×), ΔkdpA/ΔtrkA (•) and Δkup/ΔtrkA (▵) grown in LB broth. The number of surviving chicks was recorded for each group for two weeks following infection. The experiment was performed twice and results from a representative experiment are presented. *P<0.05 compared with the WT Salmonella (Kaplan–Meier analysis).
Discussion
Cations and cation transport have long been recognized as essential for maintaining the normal physiology of all living organisms including bacteria. In this report, we provide evidence that cation transport is also important for virulence characteristics of pathogenic Salmonella. Chemical modulators of cation transport inhibit the TTSS of SPI-1 (Fig. 3). Mutations in transporters of the most abundant cation, K+, led to deficiencies in the TTSS of SPI-1 (Fig. 2) as well as protein secretion, motility and epithelial cell invasion (Fig. 7). In addition, K+ transporter mutants of Salmonella are attenuated in infection of both mice and chicks (Table 3 and Fig. 8). These results suggest that cation transport plays important roles beyond maintaining the physiology of Salmonella.
K+ is reported to play important functions in many basic cellular processes, such as maintaining cell turgor and homeostasis, adaptation of cells to osmotic conditions and activation of cytoplasmic enzymes (Bossemeyer et al., 1989; Epstein, 2003). K+ transporters are responsible for maintaining intracellular K+ within the normal range regardless of the extracellular K+ concentration. K+ transporter mutants of Salmonella grow relatively normally except at extreme K+ concentrations (Fig. 1); however, they are impaired in carrying out activities contributing to virulence in vitro and are attenuated in mice and chicks (Fig. 8, Table 3). The defects of the K+ transporter mutants were not due to a K+ deficiency since intracellular K+ levels were at or above that of the WT Salmonella (Fig. 7d). Therefore Salmonella is capable of maintaining intracellular K+ levels necessary for growth and survival without one or more of the K+ transporters, although other processes such as those involved in pathogenesis can be severely affected. These results suggest that active cation transport, especially K+ transport, is important for Salmonella as a pathogenic bacterium to carry out its full range of activities contributing to host colonization and transmission.
The TTSSs of Salmonella pathogenicity islands have been extensively studied since their discovery more than a decade ago. However, their energy source has not been identified. In their report on the TTSS of flagella, Paul et al. (2008) proposed that the TTSS of flagella was driven by the electrical component of the PMF. They showed that the TTSS of flagella was independent of intracellular ATP levels, similar to what we observed for the TTSS of SPI-1. They had also demonstrated that at an external pH of 7, the TTSS of flagella was not affected by acetate treatment that eliminates the proton concentration across the cell membrane (Paul et al., 2008), which is consistent with our findings that intracellular pH does not correlate with the TTSS of SPI-1 (Figs 5, 6). Taken together, these results suggest that the TTSS of SPI-1 is likely driven by the electrical potential across the bacterial cell membrane.
Many conditions, such as contact with host cells, salts (Mizusaki et al., 2008; Su et al., 2009), acidic pH (Bajaj et al., 1996), oxygen (Bajaj et al., 1996; Ibarra et al., 2010) and metabolic products (Van Immerseel et al., 2004a, b), have been shown to regulate the TTSS. The regulation pathways of these systems are extremely complex. Using the TTSS of SPI-1 as an example, research by several groups has demonstrated that SPI-1 is controlled by a large number of protein factors including HilA (Bajaj et al., 1995, 1996; Boddicker et al., 2003; Thijs et al., 2007), HilC (Akbar et al., 2003; Golubeva et al., 2012; Schechter & Lee, 2001), HilD (Akbar et al., 2003; Boddicker et al., 2003; Schechter & Lee, 2001), HilE (Baxter et al., 2003; Ellermeier & Slauch, 2007), RtsA (Ellermeier & Slauch, 2003, 2004), PhoP/Q (Aguirre et al., 2006; Behlau & Miller, 1993; Pegues et al., 1995), InvF (Darwin & Miller, 1999, 2000; Eichelberg & Galán, 1999), Dam (López-Garrido & Casadesús, 2010), RcsC/RcsD/RcsB phosphorelay system (Arricau et al., 1998; Lin et al., 2008; Winter et al., 2009), AcrA, AcrB and TolC (Webber et al., 2009), Lon (Boddicker & Jones, 2004), CsrA (Altier et al., 2000; Lawhon et al., 2003) and SirA/SirC (Mizusaki et al., 2008; Rakeman et al., 1999) (reviewed by Golubeva et al., 2012). The best-characterized direct regulators of the TTSS of SPI-1 are HilA (Bajaj et al., 1995, 1996; Boddicker et al., 2003; Thijs et al., 2007) and InvF (Darwin & Miller, 1999, 2000; Eichelberg & Galán, 1999), while other protein factors directly or indirectly exert their activities through these factors. We have shown that in addition to protein factors, HilE is regulated by the small RNA (sRNA) isrM encoded in SPI-1 (Gong et al., 2011). It is not clear though how Salmonella senses diverse environmental signals and induces the complex network of regulatory factors for the TTSS in response. We propose that the physiological and energetic state of Salmonella serves as an important signal to activate the TTSS including that of SPI-1. Disparate TTSS-inducing signals which include contact with host cells, salts, acids, oxygen and organic acids are all capable of altering the membrane potential of Salmonella, and it is possible that altered membrane potential is the direct signal that activates the TTSS instead of an individual stimulus. This would explain how Salmonella activates the TTSS of SPI-1 in response to seemingly disparate signals. We recognize that our methods of measuring intrabacterial pH and ATP levels most likely measured the mean pH and ATP levels inside bacteria and we cannot rule out the possibility that a redistribution of protons and/or ATP in the K+ transporter mutants and cation transport modulator-treated Salmonella was the reason for the inhibition of the TTSS of SPI-1. Currently there is no method available to measure local distribution of protons and ATP because of the small size of bacteria.
Type III secretion systems are widely used by a variety of pathogenic bacteria to secrete virulence factors to facilitate the infection and colonization of their hosts. Many pathogenic bacteria infect multiple hosts ranging from cold-blooded animals, such as flies and turtles, to warm-blooded animals such as birds, rodents and humans. The combination of individual pathogens, their diverse hosts and the conditions the pathogens encounter is so large that it is difficult to speculate how pathogens manage to regulate their TTSS in response to each combination. Our hypothesis that pathogenic bacteria regulate the TTSS through their physiological state helps to explain how these bacteria activate the TTSS in response to external environments. Since K+ and K+ transporters play important roles in the physiological state of bacteria, they are likely involved in the pathogenesis of other pathogenic bacteria in addition to Salmonella. Indeed, K+ transporters and channels have been shown to be important for stress resistance and pathogenesis of a number of bacterial species such as Pseudomonas aeruginosa (Ueda & Wood, 2008), Vibrio vulnificus (Chen et al., 2004), Mycobacterium tuberculosis (Cholo et al., 2006) and Staphylococcus aureus (Xue et al., 2011). Further analysis using genetic and physiological tools will elucidate the interaction of physiological state of pathogenic bacteria and their virulence characteristics including the secretion of virulence factors through the TTSS.
Acknowledgements
We thank Dr Sabine Ehrt of Weill Cornell Medical College for the pHluorin plasmid. This work was supported by USDA CALR-2005-01892 (to S. L.).
Abbreviations:
- PMF
proton motive force
- SPI-1
Salmonella pathogenicity island 1
- TTSS
type III secretion system
References
- Aguirre A., Cabeza M. L., Spinelli S. V., McClelland M., García Véscovi E., Soncini F. C. (2006). PhoP-induced genes within Salmonella pathogenicity island 1. J Bacteriol 188, 6889–6898. 10.1128/JB.00804-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa S. I. (1996). Flagellar assembly in Salmonella typhimurium. Mol Microbiol 19, 1–5. 10.1046/j.1365-2958.1996.344874.x [DOI] [PubMed] [Google Scholar]
- Akbar S., Schechter L. M., Lostroh C. P., Lee C. A. (2003). AraC/XylS family members, HilD and HilC, directly activate virulence gene expression independently of HilA in Salmonella typhimurium. Mol Microbiol 47, 715–728. 10.1046/j.1365-2958.2003.03322.x [DOI] [PubMed] [Google Scholar]
- Altier C., Suyemoto M., Lawhon S. D. (2000). Regulation of Salmonella enterica serovar typhimurium invasion genes by CsrA. Infect Immun 68, 6790–6797. 10.1128/IAI.68.12.6790-6797.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arricau N., Hermant D., Waxin H., Ecobichon C., Duffey P. S., Popoff M. Y. (1998). The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol 29, 835–850. 10.1046/j.1365-2958.1998.00976.x [DOI] [PubMed] [Google Scholar]
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (1997). Current Protocols in Molecular Biology. New York: Wiley. [Google Scholar]
- Bagnara A. S., Finch L. R. (1972). Quantitative extraction and estimation of intracellular nucleoside triphosphates of Escherichia coli. Anal Biochem 45, 24–34. 10.1016/0003-2697(72)90004-8 [DOI] [PubMed] [Google Scholar]
- Bajaj V., Hwang C., Lee C. A. (1995). hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol 18, 715–727. 10.1111/j.1365-2958.1995.mmi_18040715.x [DOI] [PubMed] [Google Scholar]
- Bajaj V., Lucas R. L., Hwang C., Lee C. A. (1996). Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol 22, 703–714. 10.1046/j.1365-2958.1996.d01-1718.x [DOI] [PubMed] [Google Scholar]
- Baxter M. A., Fahlen T. F., Wilson R. L., Jones B. D. (2003). HilE interacts with HilD and negatively regulates hilA transcription and expression of the Salmonella enterica serovar Typhimurium invasive phenotype. Infect Immun 71, 1295–1305. 10.1128/IAI.71.3.1295-1305.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlau I., Miller S. I. (1993). A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol 175, 4475–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren R. E., Wunderlich A., Ziegler E., Schleicher M., Duke R. C., Looney D., Fang F. C. (1995). HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J Acquir Immune Defic Syndr Hum Retrovirol 10, 489–495. [PubMed] [Google Scholar]
- Boddicker J. D., Jones B. D. (2004). Lon protease activity causes down-regulation of Salmonella pathogenicity island 1 invasion gene expression after infection of epithelial cells. Infect Immun 72, 2002–2013. 10.1128/IAI.72.4.2002-2013.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddicker J. D., Knosp B. M., Jones B. D. (2003). Transcription of the Salmonella invasion gene activator, hilA, requires HilD activation in the absence of negative regulators. J Bacteriol 185, 525–533. 10.1128/JB.185.2.525-533.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossemeyer D., Borchard A., Dosch D. C., Helmer G. C., Epstein W., Booth I. R., Bakker E. P. (1989). K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J Biol Chem 264, 16403–16410. [PubMed] [Google Scholar]
- Chen Y. C., Chuang Y. C., Chang C. C., Jeang C. L., Chang M. C. (2004). A K+ uptake protein, TrkA, is required for serum, protamine, and polymyxin B resistance in Vibrio vulnificus. Infect Immun 72, 629–636. 10.1128/IAI.72.2.629-636.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholo M. C., Boshoff H. I., Steel H. C., Cockeran R., Matlola N. M., Downing K. J., Mizrahi V., Anderson R. (2006). Effects of clofazimine on potassium uptake by a Trk-deletion mutant of Mycobacterium tuberculosis. J Antimicrob Chemother 57, 79–84. 10.1093/jac/dki409 [DOI] [PubMed] [Google Scholar]
- Darwin K. H., Miller V. L. (1999). InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J Bacteriol 181, 4949–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin K. H., Miller V. L. (2000). The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol Microbiol 35, 949–960. 10.1046/j.1365-2958.2000.01772.x [DOI] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberg K., Galán J. E. (1999). Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect Immun 67, 4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier C. D., Slauch J. M. (2003). RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J Bacteriol 185, 5096–5108. 10.1128/JB.185.17.5096-5108.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier C. D., Slauch J. M. (2004). RtsA coordinately regulates DsbA and the Salmonella pathogenicity island 1 type III secretion system. J Bacteriol 186, 68–79. 10.1128/JB.186.1.68-79.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier J. R., Slauch J. M. (2007). Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10, 24–29. 10.1016/j.mib.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Epstein W. (2003). The roles and regulation of potassium in bacteria. Prog Nucleic Acid Res Mol Biol 75, 293–320. 10.1016/S0079-6603(03)75008-9 [DOI] [PubMed] [Google Scholar]
- Fraser G. M., Hughes C. (1999). Swarming motility. Curr Opin Microbiol 2, 630–635. 10.1016/S1369-5274(99)00033-8 [DOI] [PubMed] [Google Scholar]
- Frymier J. S., Reed T. D., Fletcher S. A., Csonka L. N. (1997). Characterization of transcriptional regulation of the kdp operon of Salmonella typhimurium. J Bacteriol 179, 3061–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-Mor O., Finlay B. B. (2006). Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell Microbiol 8, 1707–1719. 10.1111/j.1462-5822.2006.00794.x [DOI] [PubMed] [Google Scholar]
- Golubeva Y. A., Sadik A. Y., Ellermeier J. R., Slauch J. M. (2012). Integrating global regulatory input into the Salmonella pathogenicity island 1 type III secretion system. Genetics 190, 79–90. 10.1534/genetics.111.132779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H., Vu G. P., Bai Y., Chan E., Wu R., Yang E., Liu F., Lu S. (2011). A Salmonella small non-coding RNA facilitates bacterial invasion and intracellular replication by modulating the expression of virulence factors. PLoS Pathog 7, e1002120. 10.1371/journal.ppat.1002120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Ochman H. (1997). How Salmonella became a pathogen. Trends Microbiol 5, 343–349. 10.1016/S0966-842X(97)01099-8 [DOI] [PubMed] [Google Scholar]
- Ibarra J. A., Knodler L. A., Sturdevant D. E., Virtaneva K., Carmody A. B., Fischer E. R., Porcella S. F., Steele-Mortimer O. (2010). Induction of Salmonella pathogenicity island 1 under different growth conditions can affect Salmonella–host cell interactions in vitro. Microbiology 156, 1120–1133. 10.1099/mic.0.032896-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson M. A., Kenny B., Leard A. D. (2001). Role of sipA in the early stages of Salmonella typhimurium entry into epithelial cells. Cell Microbiol 3, 417–426. 10.1046/j.1462-5822.2001.00124.x [DOI] [PubMed] [Google Scholar]
- Jones B. D., Falkow S. (1996). Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol 14, 533–561. 10.1146/annurev.immunol.14.1.533 [DOI] [PubMed] [Google Scholar]
- Journet L., Hughes K. T., Cornelis G. R. (2005). Type III secretion: a secretory pathway serving both motility and virulence (review). Mol Membr Biol 22, 41–50. 10.1080/09687860500041858 [DOI] [PubMed] [Google Scholar]
- Kaniga K., Trollinger D., Galán J. E. (1995). Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol 177, 7078–7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem D. C., Trachewsky D. (1983). Potassium metabolism. In Potassium: its Biologic Significance, pp. 25–35. Edited by Whang R. Boca Raton, FL: CRC Press. [Google Scholar]
- Komoriya K., Shibano N., Higano T., Azuma N., Yamaguchi S., Aizawa S. I. (1999). Flagellar proteins and type III-exported virulence factors are the predominant proteins secreted into the culture media of Salmonella typhimurium. Mol Microbiol 34, 767–779. 10.1046/j.1365-2958.1999.01639.x [DOI] [PubMed] [Google Scholar]
- Lawhon S. D., Frye J. G., Suyemoto M., Porwollik S., McClelland M., Altier C. (2003). Global regulation by CsrA in Salmonella typhimurium. Mol Microbiol 48, 1633–1645. 10.1046/j.1365-2958.2003.03535.x [DOI] [PubMed] [Google Scholar]
- Lin D., Rao C. V., Slauch J. M. (2008). The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J Bacteriol 190, 87–97. 10.1128/JB.01323-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Garrido J., Casadesús J. (2010). Regulation of Salmonella enterica pathogenicity island 1 by DNA adenine methylation. Genetics 184, 637–649. 10.1534/genetics.109.108985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Manges A. R., Xu Y., Fang F. C., Riley L. W. (1999). Analysis of virulence of clinical isolates of Salmonella enteritidis in vivo and in vitro. Infect Immun 67, 5651–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Killoran P. B., Fang F. C., Riley L. W. (2002). The global regulator ArcA controls resistance to reactive nitrogen and oxygen intermediates in Salmonella enterica serovar Enteritidis. Infect Immun 70, 451–461. 10.1128/IAI.70.2.451-461.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Killoran P. B., Riley L. W. (2003). Association of Salmonella enterica serovar enteritidis yafD with resistance to chicken egg albumen. Infect Immun 71, 6734–6741. 10.1128/IAI.71.12.6734-6741.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Stewart V. J., Taylor R. K. (1996). Genetic Analysis of Pathogenic Bacteria: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Miesenböck G., De Angelis D. A., Rothman J. E. (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195. 10.1038/28190 [DOI] [PubMed] [Google Scholar]
- Mizusaki H., Takaya A., Yamamoto T., Aizawa S. (2008). Signal pathway in salt-activated expression of the Salmonella pathogenicity island 1 type III secretion system in Salmonella enterica serovar Typhimurium. J Bacteriol 190, 4624–4631. 10.1128/JB.01957-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. (2003). Manual of Clinical Microbiology, 8th edn Washington, DC: American Society for Microbiology. [Google Scholar]
- Paul K., Erhardt M., Hirano T., Blair D. F., Hughes K. T. (2008). Energy source of flagellar type III secretion. Nature 451, 489–492. 10.1038/nature06497 [DOI] [PubMed] [Google Scholar]
- Pegues D. A., Hantman M. J., Behlau I., Miller S. I. (1995). PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol 17, 169–181. 10.1111/j.1365-2958.1995.mmi_17010169.x [DOI] [PubMed] [Google Scholar]
- Raffatellu M., Wilson R. P., Chessa D., Andrews-Polymenis H., Tran Q. T., Lawhon S., Khare S., Adams L. G., Bäumler A. J. (2005). SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype typhimurium invasion of epithelial cells. Infect Immun 73, 146–154. 10.1128/IAI.73.1.146-154.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakeman J. L., Bonifield H. R., Miller S. I. (1999). A HilA-independent pathway to Salmonella typhimurium invasion gene transcription. J Bacteriol 181, 3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L. J., Muench H. (1938). A simple method of estimating fifty percent endpoints. Am J Hyg 27, 493–497. [Google Scholar]
- Sambrook J., Russell D. W. (2001). Molecular Cloning: a Laboratory Manual, 3rd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Schechter L. M., Lee C. A. (2001). AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol Microbiol 40, 1289–1299. 10.1046/j.1365-2958.2001.02462.x [DOI] [PubMed] [Google Scholar]
- Su J., Gong H., Lai J., Main A., Lu S. (2009). The potassium transporter Trk and external potassium modulate Salmonella enterica protein secretion and virulence. Infect Immun 77, 667–675. 10.1128/IAI.01027-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs I. M., De Keersmaecker S. C., Fadda A., Engelen K., Zhao H., McClelland M., Marchal K., Vanderleyden J. (2007). Delineation of the Salmonella enterica serovar Typhimurium HilA regulon through genome-wide location and transcript analysis. J Bacteriol 189, 4587–4596. 10.1128/JB.00178-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trchounian A., Kobayashi H. (1999). Kup is the major K+ uptake system in Escherichia coli upon hyper-osmotic stress at a low pH. FEBS Lett 447, 144–148. 10.1016/S0014-5793(99)00288-4 [DOI] [PubMed] [Google Scholar]
- Trchounian A., Kobayashi H. (2000). K+ uptake by fermenting Escherichia coli cells: pH dependent mode of the TrkA system operating. Biosci Rep 20, 277–288. 10.1023/A:1026493024066 [DOI] [PubMed] [Google Scholar]
- Ueda A., Wood T. K. (2008). Potassium and sodium transporters of Pseudomonas aeruginosa regulate virulence to barley. Appl Microbiol Biotechnol 79, 843–858. 10.1007/s00253-008-1483-5 [DOI] [PubMed] [Google Scholar]
- Uzzau S., Figueroa-Bossi N., Rubino S., Bossi L. (2001). Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A 98, 15264–15269. 10.1073/pnas.261348198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Boyen F., Bohez L., Pasmans F., Volf J., Sevcik M., Rychlik I., Haesebrouck F., Ducatelle R. (2004a). Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl Environ Microbiol 70, 3582–3587. 10.1128/AEM.70.6.3582-3587.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., De Smet I., Pasmans F., Haesebrouck F., Ducatelle R. (2004b). Interactions of butyric acid- and acetic acid-treated Salmonella with chicken primary cecal epithelial cells in vitro. Avian Dis 48, 384–391. 10.1637/7094 [DOI] [PubMed] [Google Scholar]
- Vandal O. H., Pierini L. M., Schnappinger D., Nathan C. F., Ehrt S. (2008). A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med 14, 849–854. 10.1038/nm.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber M. A., Bailey A. M., Blair J. M., Morgan E., Stevens M. P., Hinton J. C., Ivens A., Wain J., Piddock L. J. (2009). The global consequence of disruption of the AcrAB-TolC efflux pump in Salmonella enterica includes reduced expression of SPI-1 and other attributes required to infect the host. J Bacteriol 191, 4276–4285. 10.1128/JB.00363-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S. E., Winter M. G., Thiennimitr P., Gerriets V. A., Nuccio S. P., Rüssmann H., Bäumler A. J. (2009). The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Mol Microbiol 74, 175–193. 10.1111/j.1365-2958.2009.06859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T., You Y., Hong D., Sun H., Sun B. (2011). The Staphylococcus aureus KdpDE two-component system couples extracellular K+ sensing and Agr signaling to infection programming. Infect Immun 79, 2154–2167. 10.1128/IAI.01180-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Ikeda T. P., Shauger A. E., Kustu S. (1996). Glutamate is required to maintain the steady-state potassium pool in Salmonella typhimurium. Proc Natl Acad Sci U S A 93, 6527–6531. 10.1073/pnas.93.13.6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyan E., Trchounian A. (2001). K+ influx by Kup in Escherichia coli is accompanied by a decrease in H+ efflux. FEMS Microbiol Lett 204, 61–64. 10.1111/j.1574-6968.2001.tb10863.x [DOI] [PubMed] [Google Scholar]