Summary
Enteroaggregative Escherichia coli (EAEC) induces release of pro-inflammatory markers and disruption of intestinal epithelial barriers in vitro suggesting an inflammatory aspect to EAEC infection. However, the mechanisms underlying EAEC-induced mucosal inflammatory responses and the extent to which these events contribute to pathogenesis is not well characterized. Employing an established in vitro model we demonstrated that EAEC prototype strain 042 induces migration of polymorphonuclear neutrophils (PMNs) across polarized T84 cell monolayers. This event was mediated through a conserved host cell signaling cascade involving the 12/15-LOX pathway and led to apical secretion of an arachidonic acid-derived lipid PMN chemoattractant, guiding PMNs across the epithelia to the site of infection. Moreover, supporting the hypothesis that inflammatory responses may contribute to EAEC pathogenesis, we found that PMN transepithelial migration promoted enhanced attachment of EAEC 042 to T84 cells. These findings suggest that EAEC-induced PMN infiltration may favor colonization and thus pathogenesis of EAEC.
Keywords: Enteroaggregative Escherichia coli, host-pathogen interactions, inflammatory responses, neutrophils, 12-lipoxygenase
Introduction
Enteroaggregative Escherichia coli (EAEC) are increasingly recognized as a cause of diarrheal illness in diverse populations in both industrialized and developing countries (Nataro, 2005). The pathogen is characterized by its ability to adhere to the laryngeal epithelial cell line HEp-2 in an aggregative adherence (AA) pattern described as a ‘stacked-brick’ formation (Nataro and Kaper, 1998). Symptoms of EAEC disease include inflammatory, watery diarrhea accompanied by vomiting, nausea and fever (Harrington et al., 2006; Huppertz et al., 1997; Steiner et al., 1998). Studies also link EAEC infection to growth impairment in children (Steiner et al., 1998).
EAEC pathogenesis is believed to be initiated by adherence of the bacteria to the intestinal mucosa of the ileum and colon which requires expression of aggregative adherence fimbrae (AAF) encoded on 60-65 MDa EAEC virulence plasmids designated pAA (Czeczulin et al., 1997; Knutton et al., 1992). A bacterial biofilm is then formed and subsequent release of enterotoxins causes mucosal damage and secretion of fluids (Hicks et al., 1996; Nataro, 2005; Sheikh et al., 2001).
Several indications suggest that EAEC infection is associated with inflammation of the gut mucosa. EAEC strains have been shown to induce barrier disruption of intestinal epithelial cells as well as basolateral release of the potent neutrophil chemokine interleukin (IL)-8 (Steiner et al., 1998; Strauman et al., 2010). Furthermore, epidemiological studies have documented increased levels of fecal lactoferrin, IL-8 and IL-1β in infected individuals (Bouckenooghe et al., 2000; Jiang et al., 2002; Steiner et al., 1998). Neutrophil infiltration at the site of insult is a hallmark of the host inflammatory response to infection with several enteric pathogens (Bétis et al., 2003; McCormick et al., 1993; McCormick et al., 1998; Murphy et al., 2011; Savkovic et al., 1996). However, the extent to which inflammatory responses contribute to EAEC pathogenesis is not well understood.
Ongoing studies using a well-established in vitro model for polymorphonuclear neutrophil (PMN) transepithelial migration has led to much insight into the molecular and cellular events underlying PMN infiltration induced by enteric pathogens such as Salmonella enterica serotype Typhimurium (S. Typhimurium) and Shigella flexneri both of which evoke potent inflammatory diarrhea (Bennish et al., 1991; McCormick et al., 1993).
S. Typhimurium-induced PMN transmigration is triggered by the type III secretion system (T3SS) effector protein SipA, which activates a host cell ADP-ribosylation factor 6- and phospholipase D-dependent signaling cascade recruiting cytosolic protein kinase C α (PKC-α) to the apical membrane (Criss et al., 2001, Lee et al., 2000, Silva et al., 2004). In a less understood process activated PKC-α then phosphorylates downstream targets leading to activation of calcium-independent phospholipase A2 (iPLA2) (McCormick, 2007; Mumy et al., 2008).
S. flexneri-associated PMN transmigration is initiated by activation of a mitogen-activated protein kinase (MAPK) signal transduction pathway involving extracellular signal-regulated kinase (ERK1/2) and the upstream ERK kinase (MEK) (Köhler et al., 2002). This activation is facilitated in part by the T3SS effector proteins OspF and OspC1 (Zurawski et al., 2006). Based on these observations, the ERK1/2 signaling cascade is infered to be involved in activation of cytosolic PLA2 (cPLA2) (Mumy et al., 2008; Slomiany and Slomiany, 2006; Su et al., 2004).
The mechanisms underlying S. Typhimurium- and S. flexneri-induced PMN transmigration converge at the point of release of arachidonic acid from cellular membranes by iPLA2- and cPLA2-activity, respectively. Subsequent metabolism of arachidonic acid through a pathway involving 12/15-lipoxygenase (12/15-LOX) leads to synthesis of the eicosanoid hepoxilin A3 (HXA3; 8-hydroxy-11,12-epoxy-eicosatetranenoic acid), a potent PMN chemoattractant (Mrsny et al., 2004; Mumy et al., 2008; Sutherland et al., 2000). The apically restricted efflux ATP-binding cassette (ABC) protein transporter multidrug resistance-associated protein 2 (MRP2) then facilitates secretion of HXA3 to the apical surface from where HXA3 establishes a paracellular chemotactic gradient through the tight junctional complex, guiding PMN movement from the submucosa across epithelia to the luminal site of infection (Chan et al., 2004; Mrsny et al., 2004; Pazos et al., 2008).
The objective of this study was to investigate the role of PMN infiltration in EAEC pathogenesis, employing our in vitro host inflammatory model system and focusing on the non-invasive EAEC prototype strain 042, a proven human pathogen (Nataro et al., 1995). We demonstrate that EAEC 042 induces significant PMN transepithelial migration and that this event is mediated through the conserved signaling cascade involving the 12/15-LOX pathway. Furthermore, we provide data suggesting that host inflammatory responses can be beneficial to EAEC colonization and survival.
Results
Apical infection of intestinal cells by EAEC prototype strain 042 induces basolateral-to-apical directed PMN transepithelial migration
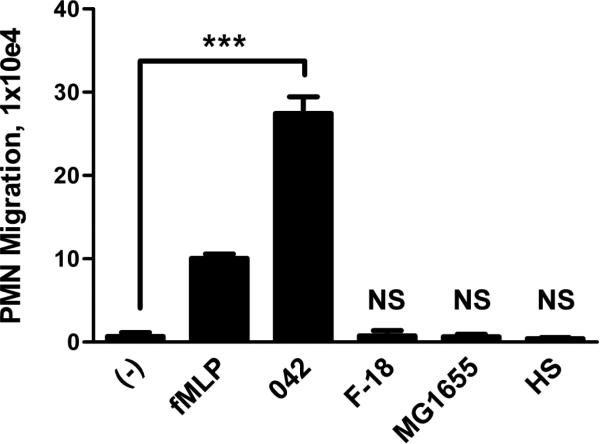
We initially examined EAEC 042 for its ability to induce PMN transepithelial migration in the physiological basolateral-to-apical direction using our in vitro inflammatory model system. Polarized T84 colonic epithelial monolayers were infected apically with 042 or one of the commensal E. coli strains HS, MG1655 or F-18. Imposed gradients of the potent PMN chemoattractant N-formylmethionyl-leucyl-phenylalanine (fMLP) served as the positive control. fMLP-induced PMN transmigration occurs by a mechanism that is independent of pathogen-induced signaling (McCormick et al., 1993). We found that unlike any of the commensal E. coli strains tested, EAEC 042 induced significant PMN transepithelial migration, suggesting a possible role for PMNs in EAEC pathogenesis (Fig. 1).
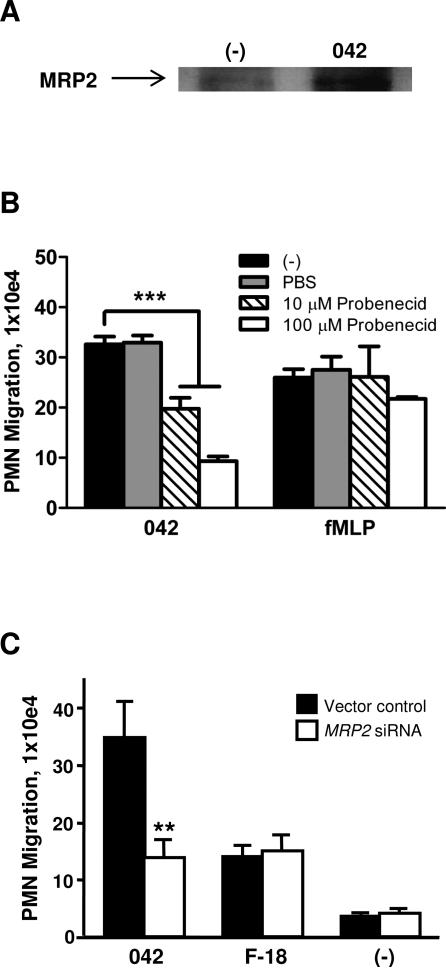
FIG. 1.
EAEC 042 induces PMN transepithelial migration. T84 cell monolayers were infected apically with EAEC 042 or one of the commensal E. coli strains HS, MG1655 or F-18. The ability to induce PMN transepithelial migration was assessed 90 min later (see Experimental procedures). HBSS+ buffer (-) and the PMN chemoattractant fMLP were included as negative and positive controls, respectively. The data are expressed as the mean ± standard deviation (SD) for triplicate samples and represent one of at least three independent experiments performed with similar results. ***, p < 0.001. N.S., not significantly different from buffer control.
EAEC 042-induced PMN transepithelial migration does not involve the MEK/ERK pathway
Having established the PMN in vitro model for EAEC, we next sought to determine the signaling pathways underlying this inflammatory response to EAEC 042 infection. We focused on the MAPK pathways, which constitute three parallel cascades: ERK, p38 and JNK. Pathogenic E. coli are commonly known to induce MAPK-dependent inflammatory responses (Bétis et al., 2003; Savkovic et al., 2001). Notably, EAEC infection of intestinal epithelial cells has been shown to stimulate activation of MAPK pathways, mediating induction of the transcription factors nuclear factor kappa-B (NF-κB) and activator protein 1 (AP-1), resulting in IL-8 production and secretion (Khan et al., 2010). Moreover, ERK1/2 has been demonstrated to play a key role in triggering PMN transmigration induced by S. flexneri as well as by diffusely adherent E. coli (DAEC) and in the latter case, p38 has also been shown to be involved (Bétis et al., 2003; Köhler et al., 2002). We investigated the extent to which ERK1/2 is involved in EAEC 042-associated PMN transmigration and found that 042 promotes a significant increase in phosphorylated ERK1/2 in the Triton X-100-soluble cytosolic fractions compared to an uninfected buffer control (data not shown). However, pretreatment of T84 cells with PD98059 or SB203580, inhibitors selective for MEK and p38, respectively, had no effect on 042-induced PMN transepithelial migration (data not shown), indicating that ERK1/2 and p38 signaling is not involved in this aspect of the inflammatory response to EAEC 042 infection.
PKC-δ is involved in the regulation of EAEC 042-induced PMN transepithelial migration
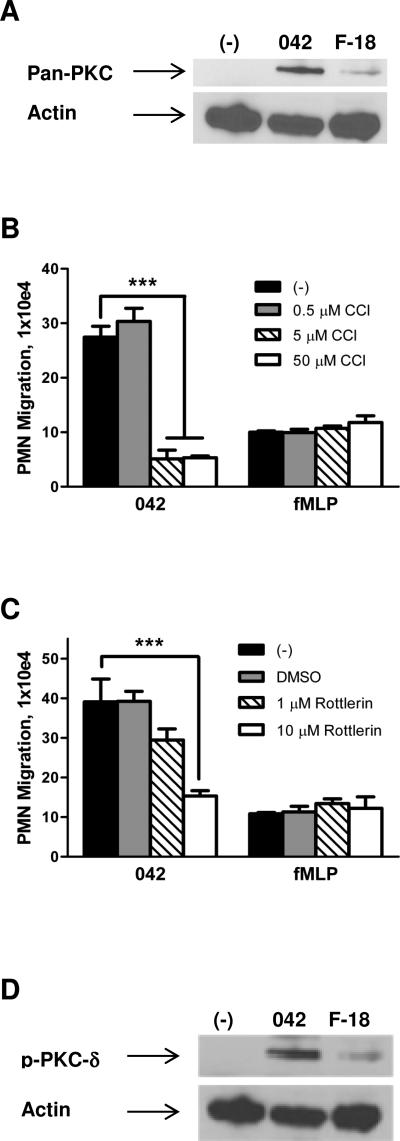
We next examined the possibility that PKC activation is involved in regulating the pathway leading to PMN transepithelial migration. Since activation of PKC has been associated with inflammatory disease, as demonstrated in animal models as well as in response to enteric pathogens (Chang et al., 2000; Jacobsen et al., 1995; Savkovic et al., 2003), we first examined whether EAEC 042 induces translocation of this enzyme to the host cell membrane. As shown in Fig. 2A, 042 promotes a significant increase in PKC in the Triton X-100-insoluble membrane fractions compared to an uninfected buffer control or the commensal E. coli strain F-18. We next evaluated the effect of the pan-PKC inhibitor chelerytherine chloride (CCl) on the ability of EAEC 042 to induce PMN transmigration. We found that pretreatment of T84 cell monolayers for 1 hour with 5 μM CCl significantly attenuated 042-induced PMN transmigration by ~80% while not affecting the fMLP-imposed PMN response (Fig. 2B).
FIG. 2.
PKC-δ is involved in regulation of EAEC 042-induced PMN transepithelial migration. A and D: Polarized T84 cell monolayers were infected apically with EAEC 042 or commensal E. coli strain F-18 for 90 min and then lysed and enriched for Triton X-100-insoluble membrane fractions (see Experimental procedures). HBSS+-treated cells served as a negative control (-). 30 μg protein samples were separated on gradient polyacrylamide gels and immunoblotted using a pan-PKC (A) or phosphorylated PKC-δ, p-PKC-δ, (D) antibody diluted 1:1,000. Detection of actin levels served as an internal control for protein loading conditions. B and C: T84 monolayers were pretreated with the pan-PKC inhibitor chelerytherine chloride, CCl, (B) or the PKC-δ inhibitor rottlerin (C) for 1 hour and assessed for effect on 042-induced PMN transepithelial migration. fMLP was included as a positive control. The data are expressed as the mean ± SD for triplicate samples and represent one of at least three independent experiments performed with similar results. ***, p < 0.001.
Five specific PKC isoforms (α, βII, δ, ε and ζ) are known to be expressed in T84 cells, and PKC-α has been shown to play a pivotal role in triggering S. Typhimurium-induced PMN transmigration (Silva et al., 2004). We assessed the effect of PKC-α, -δ and -ζ inhibition on EAEC 042-induced PMN transmigration by pre-treating T84 cell monolayers for 1 hour with one of the PKC-α inhibitors HBDDE or Gö 6976, the PKC-δ inhibitor rottlerin, or the PKC-ζ inhibitor myristoylated PKC-ζ pseudosubstrate. While none of the PKC-α or PKC-ζ inhibitors had any effect (data not shown), 10 μM of rottlerin significantly reduced 042-induced PMN transmigration without affecting the PMN response to the imposed fMLP gradient (Fig. 2C). Confirming the results from our PKC-δ drug study, we determined that EAEC 042 promotes a significant increase in phosphorylated PKC-δ in the Triton X-100-insoluble membrane fractions compared to controls (Fig. 2D). These results imply that PKC-δ plays a role in mediating the cellular response leading to EAEC-induced PMN transmigration.
An arachidonic acid-derived metabolite generated through the 12/15-LOX pathway plays a role in regulating EAEC 042-induced PMN transepithelial migration
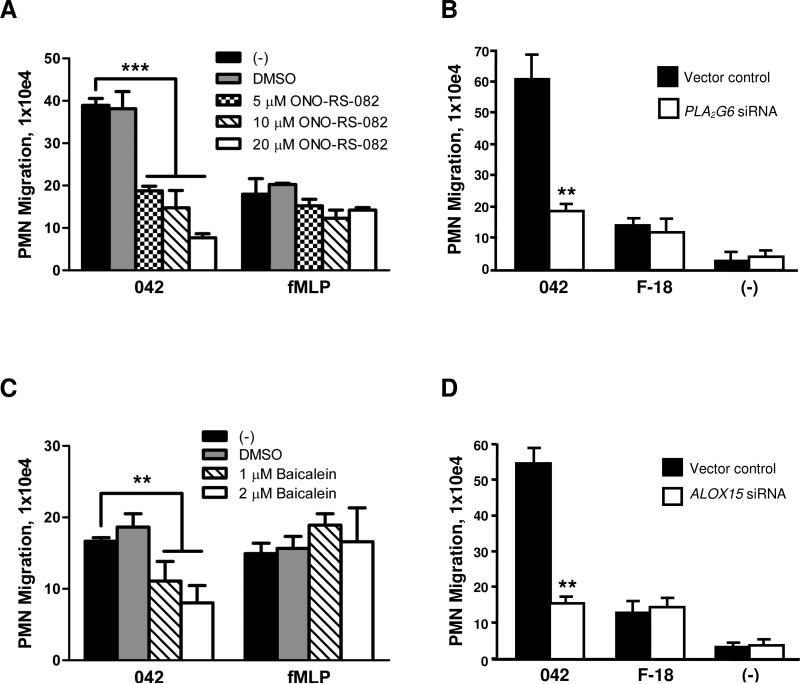
PKC isoforms have been shown to activate PLA2 which in turn plays a key role in inflammatory responses by facilitating release of arachidonic acid from membrane phospholipids (Mumy et al., 2008; van Rossum and Patterson, 2009; Steer et al., 2002). Mobilized arachidonic acid serves as a precursor for the eicosanoid class of lipids with distinct roles as pro-inflammatory or anti-inflammatory mediators (Serhan and Savill, 2005). To determine whether arachidonic acid is also involved in the events underlying inflammatory responses to EAEC 042, we investigated the potential involvement of PLA2 in 042-induced PMN transmigration. We found that a 3 hour pre-treatment of T84 cells with 20 μM of the pan-PLA2 inhibitor ONO-RS-082 significantly reduced 042-induced PMN transmigration by ~80% without affecting fMLP-induced PMN transmigration (Fig. 3A).
FIG. 3.
PLA2 and 12/15-LOX are involved in regulation of EAEC 042-induced PMN transepithelial migration. A and C: T84 monolayers were pretreated with the pan-PLA2 inhibitor ONO-RS-082 for 3 hours (A) or with the 12/15-LOX inhibitor baicalein for 48 hours (C) and assessed for effect on EAEC 042-induced PMN transepithelial migration. fMLP was included as a positive control. B and D: Monolayers of HCT-8 cells transfected with a vector control or a vector modified to generate siRNAs aimed at decreasing the expression of PLA2G6 (B) or ALOX15 (D) were assessed for their effect on EAEC 042- or E. coli F-18-induced PMN transepithelial migration. The data are expressed as the mean ± SD for triplicate samples and represent one of at least three independent experiments performed with similar results. ***, p < 0.001; **, p < 0.01.
We next sought to confirm these findings by a more selective, non-inhibitor-based approach. We employed previously generated HCT-8 cells expressing a plasmid generating small interfering RNAs (siRNAs) against mRNA of PLA2G6, encoding human iPLA2 (Mumy et al., 2008). HCT-8 cells were more suitable for this particular procedure since, similar to T84 cells, they are a polarizing human intestinal transformed cell line and PMNs are able to traverse HCT-8 monolayers in response to infection with low background levels. However, unlike T84 cells, the HCT-8 cell line is easily and highly transfectable by standard methods. Consistent with the drug study results, we found that PMN transmigration in response to EAEC 042 infection was reduced by ~70% across HCT-8 monolayers expressing siRNA against PLA2G6 mRNA in comparison to what was observed for monolayers expressing nonspecific siRNA (Fig. 3B). Although the commensal E. coli strain F-18 did elicit modest levels of PMN migration across the HCT-8 monolayers, this response was not affected by reduction in iPLA2 activity. These findings suggest a regulatory role for iPLA2-released arachidonic acid in the inflammatory response to EAEC 042 infection.
Among the eicosanoids derived from arachidonic acid are the potent PMN chemoattractants leukotriene B4 (LTB4) and HXA3, respectively generated through the 5-LOX and 12/15-LOX pathways (Funk, 2001; Mrsny et al., 2004). Having demonstrated the involvement of arachidonic acid in EAEC 042-induced inflammation, we next investigated the extent to which the specific 5-LOX or 12/15-LOX pathways are involved. We found that pre-treatment of T84 cells with 2 μM of the 12/15-LOX inhibitor baicalein resulted in a significant ~50% reduction in 042-induced PMN transepithelial migration (Fig. 3C) whereas pre-treatment of T84 cells with the 5-LOX inhibitor caffeic acid failed to inhibit this inflammatory response (48.6 ± 12.2 vs. 45.4 ± 16.9 × 104 PMNs for monolayers pre-treated with DMSO or 2 μM caffeic acid, respectively (not significantly different)). In addition, neither inhibitor significantly affected fMLP-induced PMN transmigration.
To substantiate our drug study findings, we took advantage of previously generated HCT-8 cells expressing a plasmid encoding siRNAs against mRNA of ALOX15, encoding human 12/15-LOX (Mumy et al., 2008). There are four enzymes known to possess 12-LOX activity in humans: platelet-type, 12R, epidermal, and 12/15-LOX (Yamamoto et al., 1997; Yamamoto et al., 1999; Yoshimoto and Yamamoto, 1995). Since previous studies using mouse leukocyte type 12-LOX showed that intestinal epithelial cells selectively express epithelial 12-LOX during active states of inflammation (Shannon et al., 1993), we choose to generate siRNA constructs using the mRNA sequence for ALOX15, which encodes for 12/15 LOX, the human homologue of murine leukocyte type 12-LOX (Mumy et al., 2008). We found that decreased expression of 12/15-LOX significantly attenuated (~70% inhibition) EAEC 042-induced PMN transmigration compared to control monolayers (Fig. 3D). The lower levels of PMN transmigration observed by E. coli F-18 infection were not affected by the reduction in 12/15-LOX activity.
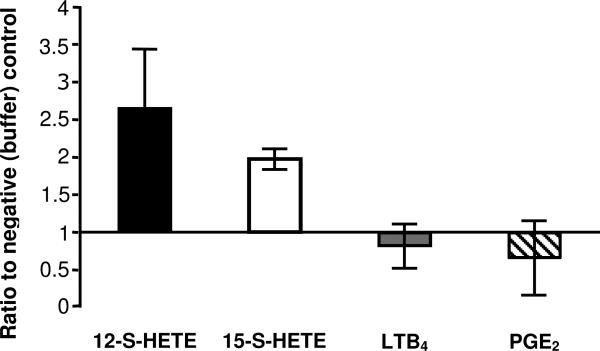
Thus far, our observations suggest that an arachidonic acid-derived lipid generated through the 12/15-LOX pathway is involved in the inflammatory response to EAEC 042 infection. To quantify the relative amounts of various arachidonic acid metabolites in response to EAEC 042 infection we used commercially available ELISA-based assays (Enzo Life Sciences, Inc.). Since 12/15-LOX was found to play a crucial role in EAEC 042-induced PMN transepithelial migration, we examined the relative amounts of 12-S-HETE and 15-S-HETE given that 12/15-LOX-1 is a complex enzyme that produces primarily 15-S-HETE, but can also produce 12-SHETE (Kuhn et al., 2002; Kuhn and Borngraber, 1999). As shown in Fig. 4, we found that levels of 12-S-HETE (used as a biomarker for the activation of the 12-LOX pathway) and 15-S-HETE (used as a biomarker for the activation of the 12/15-LOX pathway) were significantly elevated following infection with EAEC 042 compared to the buffer (HBSS+) control. By contrast, analysis of metabolites involved in prostanoid synthesis (PGE2) or leukotriene synthesis (LTB4) showed no difference when compared to negative controls.
FIG. 4.
Activation of the 12/15-LOX pathway after infection with EAEC 042 as demonstrated by quantification of arachidonic acid metabolites 12-S-HETE, 15-S-HETE, LTB4, and PGE2. ELISA's were performed on cell extracts collected from T84 cells (see Experimental procedures). Data represents the ratio of each metabolite from 042 infected samples to a baseline (set to one) from negative HBSS+ controls. Data are expressed as mean ± SD of duplicate samples from at least three experiments performed. Raw means of each 042 infected metabolite are as follows, 12-S-HETE: 25.2 ng, 15-S-HETE: 16.5 ng, LTB4: 0.232 ng, PGE2: 0.216 ng.
EAEC 042-induced PMN transepithelial migration is facilitated by the MRP2 efflux transporter
Expression of the ABC efflux transporter MRP2, which is localized on the apical surface, is upregulated in states of epithelial inflammation and the 12/15-LOX pathway has been implicated as a requirement for maximal induction of this MRP2 upregulation (Pazos et al., 2008). MRP2 plays a key role in facilitating S. Typhimurium-induced PMN transmigration by functioning as an efflux pump for the vectoral release of HXA3 to the apical surface (Pazos et al., 2008). Having demonstrated the involvement of the 12/15-LOX pathway in PMN inflammatory responses to EAEC 042 infection we sought to determine the extent to which MRP2 also plays a role. We tested whether infection with 042 alters expression levels of MRP2 on the apical surface. Selective cell surface biotinylation of T84 cell monolayers revealed that 042 does indeed upregulate expression of MRP2 to the apical surface compared to an uninfected buffer control (Fig. 5A). To determine the functional relevance of MRP2, we next examined the effect of the MRP2 inhibitor probenecid on the ability of 042 to cause PMN transmigration. We found that pre-treatment of T84 cell monolayers for 24 hours with 100 μM probenecid significantly reduced 042-induced PMN transmigration by ~70% while the fMLP-induced PMN response was unaffected (Fig. 5B). However, inhibition of another intestinal apical efflux transporter, P-glycoprotein, did not result in a corresponding decrease in PMN migration (data not shown). We next applied previously generated HCT-8 cells generating siRNAs against mRNA of MRP2 (Pazos et al., 2008). Validating our MRP2 drug study data, we observed a ~60% reduction in 042-induced PMN transepithelial migration across monolayers with decreased MRP2 expression compared to control monolayers, whereas the lower levels of F-18-induced PMN migration were unaffected by reduced MRP2 expression (Fig. 5C). Together, these results suggest that MRP2 plays a key role in facilitating the inflammatory response to EAEC 042 infection.
FIG.5.
MRP2 expression is upregulated in response to EAEC 042 infection and plays a key role in regulating 042-induced PMN transepithelial migration. A: Following apical infection with EAEC 042, the apical side of polarized T84 cell monolayers was coated with biotin and the cells were then lysed and biotin-coated proteins immunoprecipated with streptavidin-beads (see Experimental procedures). HBSS+-treated cells served as a negative control (-). 25 μg protein samples were separated on gradient polyacrylamide gels and immunoblotted using an MRP2 antibody diluted 1:500. B: T84 monolayers were pretreated with the MRP2 inhibitor probenecid for 24 hours and assessed for effect on 042-induced PMN transepithelial migration. fMLP was included as a positive control. C: Monolayers of HCT-8 cells transfected with a vector control or a vector modified to generate siRNAs aimed at decreasing the expression of MRP2 were assessed for their effect on EAEC 042- or E. coli F-18-induced PMN transepithelial migration. The data are expressed as the mean ± SD for triplicate samples and represent one of at least three independent experiments performed with similar results. ***, p < 0.001; **, p < 0.01.
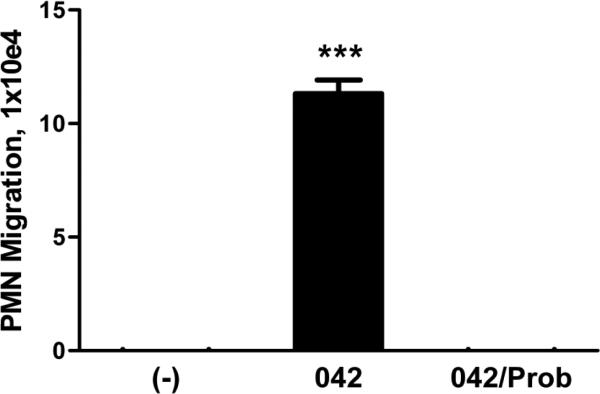
Since we have determined that the potent PMN chemoattractant, HXA3, is a substrate for MRP2 we next investigated the possible involvement of this eicosanoid in EAEC 042-induced PMN transepithelial migration. Using an established protocol (McCormick et al., 1993; Mrnsy et al., 2004), semi-purified fractions of HXA3 were collected from T84 monolayers infected with 042 in the absence (HBSS+) and presence of probenecid treatment (100 μM for 24 hours at 37°C). We assessed the semi-purified HXA3 fractions for bioactivity and found that the control extract collected from 042 infected cells, but not from infected cells pre-treated with probenecid, was able to significantly induce PMN transmigration (Fig. 6). These results not only indicate that EAEC 042 induces the apical release of the PMN chemoattractant HXA3 but also strongly suggest that such secretion is facilitated by MRP2.
FIG. 6.
EAEC 042 induces secretion of PMN-chemoattractive bioactive lipids through MRP2. T84 cell monolayers grown on plastic flasks were pretreated with HBSS+ or 100 μM probenecid (Prob) for 24 hours and then infected with EAEC 042. Uninfected cells served as a negative control (-). Following infection, crude preparations collected from cell culture medium of the monolayers were collected and lipids present in the media extracted from it. The lipids were then added to the apical chambers of polarized T84 cells monolayers and assessed for their ability to induce PMN transepithelial migration. The data are expressed as the mean ± SD for triplicate samples and represent one of at least three independent experiments performed with similar results. ***, p < 0.001.
PMN transepithelial migration promotes enhanced adherence of EAEC 042
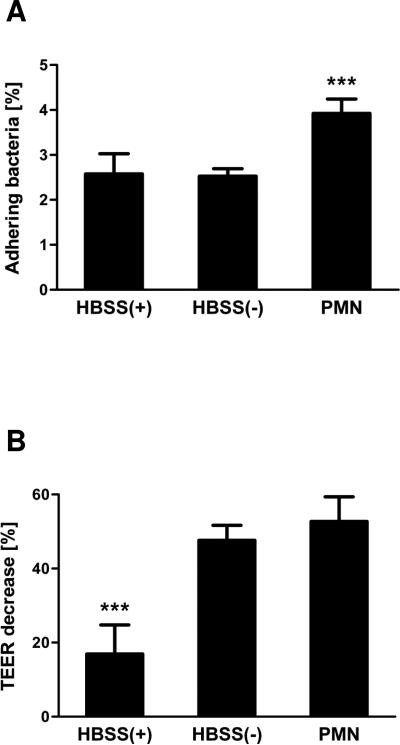
Having established the ability of EAEC to induce PMN transmigration, we next sought to determine if PMN migration is critical to this organism's pathogenic potential. This inquest is founded on studies showing that the adherence of DAEC to intestinal epithelial cells is significantly increased upon PMN infiltration (Bétis et al., 2003). To determine whether a similar effect could be observed for EAEC, we assessed the attachment of EAEC 042 to T84 cell monolayers following PMN transmigration. Treatment of cells with Ca2+-depleted HBSS served as a control for the effect of the loosening of the tight junctions with impairment to transepithelial electrical resistance (TEER), and hence barrier function. We found that fMLP-induced PMN transmigration prior to infection resulted in a significant 50% increase in the number of adhering bacteria compared to control infected cells (Fig. 7A). Moreover, while treatment of cells with Ca2+-depleted HBSS resulted in TEER decreases to the same extent as observed for PMN transmigration (Fig. 7B), this did not affect bacterial attachment. Thus, our data suggest that the increase in bacterial attachment is not due to loss of barrier integrity caused by PMN transmigration but rather a consequence of PMN-mediated modulation of host cell signaling following the migratory event. These results imply that PMN infiltration, itself, can also contribute to EAEC pathogenesis by favoring colonization of the bacteria.
FIG. 7.
PMN transepithelial migration enhances adherence of EAEC 042 to T84 cell monolayers. Inverted T84 cell monolayers were treated with HBSS+, Ca2+-depleted HBSS (HBSS−) or subjected to fMLP-induced PMN transepithelial migration (PMN) for 2½ hours and subsequently incubated for 5 hours. The cells were then infected for 90 min with EAEC 042 followed by cell lysis and quantification of attaching bacteria by colony counting (A). Measurement of TEER was carried out before treatment and again prior to infection and the TEER decreases calculated (B). The data are expressed as the mean ± SD for triplicate samples and represent one of at least three independent experiments performed with similar results. ***, p < 0.001.
Discussion
The role of inflammation in response to infection with the diarrheal pathogen EAEC is an area of ongoing investigation. EAEC strains have been shown to induce release of pro-inflammatory markers such as IL-8 from cultured intestinal epithelial cells suggesting an involvement of PMNs (Steiner et al., 1998). Here, we demonstrate for the first time that EAEC prototype strain 042 induces PMN transepithelial migration across monolayers of polarized T84 colonic epithelial cells, suggesting that host inflammatory responses could be contributing to EAEC pathogenesis.
The objective of this study was to investigate the role of PMNs in response to EAEC infection by probing into the host signal transduction pathways underlying EAEC 042-induced PMN transmigration using an established in vitro model. We discovered that the process of EAEC 042-induced PMN transmigration is mediated, in part, by secretion of HXA3, a potent PMN chemoattractant, from the apical surface of infected model intestinal epithelia. In particular, EAEC 042 utilizes a conserved pathway involving iPLA2-mediated release of arachidonic acid from the host cell membrane. HXA3, an arachidonic acid metabolite produced by the 12/15-LOX pathway, is subsequently released to the apical surface via the membrane transporter MRP2. Secreted HXA3 then forms a chemotactic gradient across the tight junctional complex directing PMN paracellular transit across the epithelia to the luminal site of insult. Applying methods we have used previously to semi-purify HXA3 (Mrsny et al., 2004), we provide evidence to suggest that this eicosanoid is involved in the PMN recruitment response induced by EAEC. Although this fraction did not include LTB4, another PMN chemoattractant, there is still a remote possibility that another lipid-based chemoattractant might be involved in PMN transmigration, and further investigation is ongoing to reconcile this possibility.
Furthermore, we demonstrated the involvement of PKC-δ, the precise role of which remains to be determined. Interestingly, PKC-δ has been suggested to regulate translocation of MRP2, and in addition, PKC isoforms have been shown to activate PLA2 (van Rossum and Patterson, 2009; Schonhoff et al., 2008; Steer et al., 2002). Thus, it is likely that PKC-δ plays a key regulatory role in the process of EAEC-induced PMN transmigration. Moreover, we were able to confirm previous findings that EAEC 042 induces basolateral but not apical secretion of IL-8 (data not shown) (Harrington et al., 2005). Thus, it is expected that IL-8 works in concert with HXA3 by causing recruitment of PMNs through the subepithelial matrix to the subepithelial space upon EAEC infection.
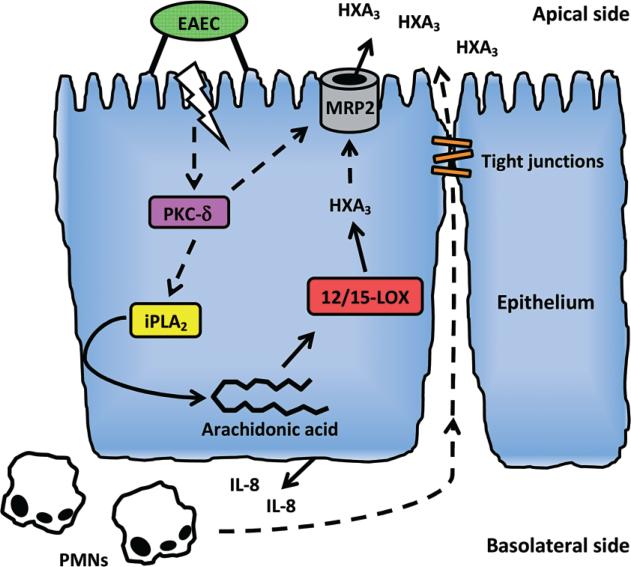
In view of our findings, we propose a model of inflammatory response to EAEC infection in which two distinct PMN chemoattractants, HXA3 and IL-8, acting in concert are required (Fig. 8). Key elements of this signal transduction pathway are conserved among EAEC, S. Typhimurium and S. flexneri as well as Campylobacter species, and have also been observed in alveolar epithelial cells in response to infection with Pseudomonas aeruginosa (Hurley et al., 2004; Hurley et al., 2006; Murphy et al., 2011). Thus, our findings in this study give further strength to our hypothesis that HXA3-dependent inflammatory responses play a significant role in the mucosal inflammatory responses induced by distinct enteric and lung pathogens.
FIG. 8.
Model of inflammatory response to EAEC. Adherence of EAEC to the apical site of the epithelium and possibly other contributing factors triggers activating of PKC-δ which upon translocation to the host cell membrane participates in activation of iPLA2. Upon activation, iPLA2 then facilitates release of arachidonic acid from the membrane to the cytosol from where it is metabolized into HXA3 through the 12/15-LOX pathway. The signal transduction cascade culminates in MRP2-facilitated apical release of HXA3 which forms a chemotactic gradient causing migration of IL-8-recruited PMNs across the epithelium to the site of infection.
An important aspect yet to be determined is how EAEC triggers PMN infiltration. Distinguishing it from S. Typhimurium, PMN transmigration across epithelial monolayers induced by EAEC 042 does not involve PKC-α, nor does it appear to rely on MAPK pathways as is the case for S. flexneri and DAEC. Moreover, EAEC 042 does not possess any of the T3SS-secreted effector proteins SipA or OspF/OspC1 demonstrated to play key roles in initiating inflammatory responses by S. Typhimurium or S. flexneri, respectively (Köhler et al., 2002; Lee et al., 2000). The AAF/II organelle expressed by EAEC 042 has been shown to be required for basolatereal IL-8 secretion as well as for intestinal epithelial barrier disruption induced by this strain (Harrington et al., 2005; Strauman et al., 2010). Epithelial barrier disruption could be contributing to EAEC-induced PMN transmigration as is the case for S. Typhimurium (Köhler et al., 2007). Furthermore, AafA, the major pilin protein of AAF/II, has been shown to bind to fibronectin (Fn) and it is speculated that this binding event could mediate signaling transduction pathways by triggering the Fn receptor integrin α5β1 (Farfan et al., 2008). This could potentially lead to events triggering inflammatory responses. We are currently studying the role of AAF as well as other EAEC virulence factors in our in vitro host inflammatory model system.
Several enteric pathogens have evolved strategies for modulating or utilizing host inflammatory responses to their own benefit. For instance, S. Typhimurium is capable of exploiting a virulence factor-induced electron receptor generated by reactive oxygen species during inflammation, thus gaining a growth advantage over the protective gut microbiota (Winter et al., 2010). In another example, PMN transepithelial migration induced by Afa/Dr-expressing DAEC causes enhanced expression of brush border-associated decay-accelerating factor (DAF/CD55), the receptor for Afa/Dr adhesins, in turn promoting enhanced adherence of these organisms (Bétis et al., 2003). Moreover, Afa/Dr DAEC has been shown to induce an increased rate of apoptosis of PMNs and diminish their phagocytic abilities, thereby increasing bacterial virulence (Brest et al., 2004).
The extent to which host inflammatory responses contribute to EAEC pathogenesis and whether this pathogen exploits such events is an area of ongoing investigation. During active intestinal inflammation, PMNs transmigrate into the lumen and release adenosine 5’-monophosphate (5’-AMP), which can be converted to adenosine by ecto-5’nucleotidase (CD73) expressed on the surface of intestinal epithelial cells (Madara et al., 1993; Strohmeier et al., 1997). Adenosine stimulation of epithelial cells triggers fluid secretion, in turn potentially leading to diarrhea (Madera et al., 1993). In that regard, inflammation could be contributing to enhanced transmission of EAEC via the fecal-oral route. Notably, adenosine stimulation of epithelial cells also triggers secretion of Fn (Walia et al., 2004), and pre-treatment of T84 monolayers with adenosine has been shown to enhance attachment of EAEC 042, likely via enhanced AAF/II-Fn binding (Farfan et al., 2008). Thus, it is hypothesized that EAEC-induced inflammatory responses might favor colonization of the pathogen through binding to Fn or other extracellular matrix proteins (Farfan et al., 2008). Supporting this hypothesis, we demonstrated increased attachment of EAEC 042 to T84 monolayers following fMLP-mediated PMN transmigration. This increase was not due to loss of barrier integrity of the monolayers, suggesting instead that post-transmigratory PMN-mediated events altering host cell signaling are involved. We are currently investigating the possible involvement of PMN-derived 5’-AMP, adenosine and Fn in this regard. Moreover, EAEC 042 secretes Pic, a serine protease autotransporter (SPATE) found commonly among strains of EAEC and Shigella flexneri (Boisen et al., 2009). This mucinolytic protease was recently demonstrated to impair chemotaxis and transendothelial migration of PMNs, induce PMN oxidative burst, and induce apoptosis of activated T cells (Ruiz-Perez et al., 2011). These findings suggest a profound role for Pic in modulation of innate immune responses.
Increasing evidence suggests that the major inflammatory bowel diseases (IBDs), Crohn's disease and ulcerative colitis, are triggered by host responses to intestinal bacteria, particularly mucosa-associated E. coli pathotypes (Rhodes, 2007; Rolhion and Darfeuille-Michaud, 2007; Sepehri et al., 2010). Notably, a recent study demonstrated a high prevalence of aggregative adherent E. coli among IBD patients, suggesting a role for EAEC in IBDs (Thomazini et al., 2011). In this perspective, probing further into the mechanisms underlying EAEC-induced PMN transmigration could potentially contribute to an increased understanding of IBD development. Moreover, a further elucidation of the virulence mechanisms triggering EAEC-induced inflammatory responses will contribute to our overall understanding of EAEC pathogenesis.
Experimental procedures
Bacterial strains and growth conditions
The bacterial strains used in this study are listed in Table 1. Overnight cultures of bacteria grown in Luria-Bertani (LB) broth at 37°C were diluted 1:100 in Dulbecco's Modified Eagle Medium (DMEM) with 4500 mg glucose/L and incubated 4 hours to exponential phase under static conditions at 37°C.
Table 1.
Strains used in this study
| Strain | Description | Reference |
|---|---|---|
| 042 | Wild-type EAEC strain expressing AAF/II | Nataro et al., 1985 |
| HS | Commensal E. coli strain | Levine et al., 1978 |
| MG1655 | Commensal E. coli K-12 strain | Blattner et al., 1997 |
| F-18 | Commensal E. coli strain | Cohen et al., 1983 |
Cell culture
The human colon cancer derived cell lines T84 (passages 57 to 77) and HCT-8 (passages 30 to 40) were maintained in a 1:1 mixture of DMEM (Invitrogen) and Ham's F-12 medium (Invitrogen) supplemented with 15 mM HEPES, 14 mM NaHCO3, 40 μg/ml penicillin, 80 μg/ml ampicillin, 90 μg/ml streptomycin and 7.5 % fetal calf serum. Polarized monolayers were grown on 0.33-, 4.5- or 44.2-cm2 suspended collagen-coated permeable polycarbonated filters (Costar, Cambridge, MA). Inverted monolayers were constructed as previously described (McCormcik et al., 1993; Parkos et al., 1992). Monolayers were utilized after 7 to 14 days, by which time they had reached a confluent, polarized, and differentiated state. For PMN transepithelial migration assays, steady-state TEER was measured using a Millicell ERS voltohmmeter and values of at least 1,200 Ω per cm2 were reached for all monolayers used.
PMN transepithelial migration assay
The PMN transepithelial migration assay was performed as previously described with some modifications (McCormick et al., 1993, Parkos et al., 1992). Briefly, inverted polarized T84 monolayers seeded on 0.33-cm2 filters were apically infected with 25 μl bacterial suspensions at 37°C for 90 min at a multiplicity of infection (MOI) of approximately 100 bacteria per epithelial cell. After infection, the cells were washed and transferred to fresh 24-well plates containing 1 ml of Hank's balanced salt solution (HBSS+) in the bottom chamber (apical side). 100 μl of HBSS+ was placed on the basolateral surface of the monolayers followed by 20 μl of prepared PMNs (1 × 106 PMNs). The monolayers were incubated at 37°C and 5% CO2 for 2½ hours after which the monolayers and non-migrating PMNs were gently removed leaving only those PMNs that had migrated through the monolayers. As a positive control for PMN transmigration 0.2 μM of the potent PMN chemoattractant fMLP (Sigma, St. Louis, MO) was added to the apical chamber. Transmigration was quantified by assaying for the PMN azurophilic granule marker myeloperoxidase as previously described (Parkos et al., 1992).
Inhibitor treatments
Inhibitor treatments used in this study are described in Table 2. For all treatments, T84 monolayers were incubated in the presence of the inhibitor in either HBSS+ or cell culture medium. For inhibitors not diluted in HBSS+, identical monolayers were incubated in the presence of the diluent at the same concentration as during treatment to serve as vehicle controls. Following 5-LOX, 12/15-LOX and MRP2 inhibition treatments, inhibitors/media were thoroughly washed away and monolayers were equilibrated in HBSS+ for 20 min at 37°C prior to infection. For all other treatments the inhibitor remained present until the end of 90 min infection period. All inhibitors were purchased from Enzo Life Sciences.
Table 2.
Inhibitor treatments used in this study
| Target | Inhibitor | Diluent | Incubation period |
|---|---|---|---|
| Pan-PKC | Chelerytherine chloride | HBSS+ | 1 h |
| PKC-α | HBDDE | HBSS+ | 1 h |
| PKC-α | Go 6976 | DMSO | 1 h |
| PKC-δ | Rottlerin | DMSO | 1 h |
| PKC-ζ | Myristoylated PKC-ζ pseudosubstrate | HBSS+ | 1 h |
| MEK | PD98059 | DMSO | 1 h |
| P38 | SB203580 | DMSO | 1 h |
| Pan-PLA2 | ONO-RS-082 | DMSO | 3 h |
| 5-LOX | Caffeic acid | DMSO | 24 h |
| 12/15-LOX | Baicalein | DMSO | 48 h |
| MRP2 | Probenecid | PBS, pH 7 | 24 h |
Adhesion assays
Inverted polarized T84 monolayers seeded on 0.33-cm2 filters were subjected to fMLP-induced PMN transepithelial migration for 2½ hours as described above after which the cells were washed and transferred to fresh plates containing HBSS+ and incubated for 5 hours. After incubation, the cells were infected apically as described above. After infection, non-adhering bacteria were removed by washing with cold HBSS+ and the monolayers transferred to empty plates and lysed with 0.5% Triton X-100 in LB broth for 90 min. Serial dilutions of lysed cells and adhering bacteria were then plated and colony counted the following day.
PKC translocation and ERK1/2 phosphorylation assays
Polarized T84 monolayers seeded on 4.5-cm2 filters were apically infected with 1 ml of bacterial suspensions at 37°C for 90 min at an MOI of approximately 100. Translocation of PKCs to Triton X-100-insoluble membrane fractions and phosphorylation of ERK1/2 in Triton X-100-soluble cytosolic fractions was assayed as previously described (Silva et al., 2004). 30 μg of fractions were separated on 4-20% polyacrylamide gels (Bio-Rad), transferred to nitrocellulose, and immunoblotted for pan-PKC (Santa Cruz), PKC isoforms (Cell Signaling), phosphorylated ERK1/2 (Santa Cruz) or actin (Sigma) and then for horseradish peroxidase (HRP)-conjugated goat-anti-rabbit or -goat-anti-mouse (Santa Cruz). The presented results are representative immunoblots from at least three independent experiments.
MRP2 biotinylation assay
Polarized T84 monolayers seeded on 44.2-cm2 filters were apically infected with 5 ml of bacterial suspensions at 37°C for 90 min at an MOI of approximately 100. Apical cell surface biotinylation was assayed as described by Strohmeier et al., 1997, with some modifications (Strohmeier et al., 1997). After infection, non-adhering bacteria were removed by washing with HBSS+ and the cells were incubated for an additional 60 min. After two biotinylation steps (20 min each at pH 9.0 and pH 8.5, respectively) with 0.5 mg/ml Sulfo-NHS biotin (apical) and 0.5 mg/ml Sulfo-NHS acetate (basolateral; Pierce), the cells were incubated with quenching buffer (50 mM NH4Cl in HBSS+) for 20 min at 4°C. The proteins were extracted following 30 min of incubation at 4°C with lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 8), 5 mM EDTA, 1% Triton X-100, 5 mM Na3VO4, 20 mM NaF, 0.8 mM PMSF and complete protease inhibitor cocktail tablets. The extracted proteins were centrifuged at 14,000 g for 5 min at 4°C, and the supernatant exposed to streptavidin beads (Sigma) pre-washed with high salt buffer (500 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl at pH 7.5 and 0.1 % Triton X-100) and then to lysis buffer. Following overnight incubation at 4°C with slight agitation, the beads were collected by centrifugation and boiled 5 min in tricine sample buffer (Bio-Rad).
25 μl of apical membrane proteins were separated on 4-20% polyacrylamide gels (Bio-Rad), transferred to PVDF membranes, and immunoblotted for MRP2 (Santa Cruz) and then for HRP-conjugated goat anti-rabbit-HRP (Santa Cruz).
Semi-purification of HXA3
T84 monolayers seeded on 162-cm2 flasks were infected with 20 ml of bacterial suspensions at 37°C for 90 min at an MOI of approximately 1. After infection, non-adhering bacteria were removed by washing with HBSS+ followed by 3 hours of additional incubation. The HBSS+ was then collected, acidified to pH 3-4 and passed through a Discovery DSC-18 column (Supelco) primed with HPLC grade methanol and dH2O. The column was then washed with dH2O and the lipids eluted with HPLC grade methanol. This extraction method enriches for the presence of HXA3 in the absence of LTB4.
ELISA Assays
Polarized T84 monolayers seeded on 44.2-cm2 filters were apically infected with 5 ml of bacterial suspensions at 37°C for 90 min at an MOI of approximately 100. After infection, non-adhering bacteria were removed by washing with HBSS+. Following washing, 2 ml HBSS+ was added to the apical side of the filters and they were incubated at 37°C for 2 hours, after which the apical HBSS+ was collected. This was repeated twice more for a total of three collections over 6 hours. The T84 monolayers were then scraped and collected in 10 ml of 66% methanol and placed at −20°C for a minimum of 30 min. Upon completion of the −20°C incubation, the T84 extracts were centrifuged at 4°C for 10 min at 4,000 rpm and the supernatants were collected and diluted 1:10 in dH2O. Both the T84 extracts and the apical HBSS+ samples were acidified to pH 3-4 and passed through a Discovery DSC-18 column (Supelco) primed with HPLC grade methanol and dH2O. The column was then washed with dH2O and eluted with HPLC grade methanol. After elution, the methanol was dried off using compressed nitrogen gas and the samples were resuspended into ELISA assay buffer. ELISA's were carried out for 12-S-HETE, 15-S-HETE, LTB4, and PGE2 as per manufacturers protocol (Enzo Life Sciences, Inc. Farmingdale NY 11735).
Data Presentation
Since variation exists in both transepithelial resistance between groups of monolayers (baseline resistance range (1,200-2,500 ohm × cm2)) and in PMNs obtained from different donors, individual experiments were performed using large numbers of monolayers performed in triplicate and PMNs from single blood donors on individual days. PMN isolation was restricted to 10 different donors (repetitive donations) over the course of these studies. Values are expressed as the mean ± SD of an individual experiment performed in triplicate repeated at least three times. Data were compared by Student's t-test and p values <0.05 were considered statistically significant.
Acknowledgements
We thank Ashley Pollock at Children's Hospital, Boston, for occasionally providing T84 cell monolayers. This work was supported by Danish Council for Strategic Research Grant 2101-07-0023 to Karen A. Krogfelt as well as National Institutes of Health Grants DK56754 and DK33506 to Beth A. McCormick. Beth A. McCormick is also supported by the Crohn's and Colitis Association of America. In addition, travel grants were provided by University of Copenhagen, Denmark, and the Graduate School Program of Basic and Clinical Microbiology (BCM), University of Roskilde, Denmark.
Abbreviations
- ABC
ATP-binding cassette
- AAF
aggregative adherence fimbriae
- DAEC
diffusely adherent E. coli
- EAEC
enteroaggregative E. coli
- ERK1/2
extracellular signal-regulated kinase 1/2
- fMLP
N-formylmethionyl-leucyl-phenylalanine
- Fn
fibronectin
- HBSS
Hank's balanced salt solution
- HXA3
hepoxilin A3
- IL-8
interleukin 8
- 12/15-LOX
12/15-lipoxygenase
- LTB4
leukotriene B4
- MAPK
mitogen-activated protein kinase
- MEK
MAP kinase kinase
- MRP2
multidrug resistance-associated protein 2
- PKC
protein kinase C
- PLA2
phospholipase A2
- PMN
polymorphonuclear neutrophil
- siRNA
small interfering RNA
- T3SS
type III secretion system
- TEER
transepithelial electrical resistance
REFERENCES
- Bennish ML. Potentially lethal complications of shigellosis. Rev Infect Dis 13 Suppl. 1991;4:S319–S324. doi: 10.1093/clinids/13.supplement_4.s319. [DOI] [PubMed] [Google Scholar]
- Betis F, Brest P, Hofman V, Guignot J, Kansau I, Rossi B, Servin A, Hofman P. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infection and Immunity. 2003a;71:1774–1783. doi: 10.1128/IAI.71.4.1774-1783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betis F, Brest P, Hofman W, Guignot J, Bernet-Camard MF, Rossi B, Servin A, Hofman P. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infection and Immunity. 2003b;71:1068–1074. doi: 10.1128/IAI.71.3.1068-1074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, ColladoVides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–&. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- Boisen N, Ruiz-Perez F, Scheutz F, Krogfelt KA, Nataro JP. Short Report: High Prevalence of Serine Protease Autotransporter Cytotoxins among Strains of Enteroaggregative Escherichia coli. American Journal of Tropical Medicine and Hygiene. 2009;80:294–301. [PMC free article] [PubMed] [Google Scholar]
- Bouckenooghe AR, Dupont HL, Jiang ZD, Adachi J, Mathewson JJ, Verenkar MP, Rodrigues S, Steffen R. Markers of enteric inflammation in enteroaggregative Escherichia coli diarrhea in travelers. Am J Trop Med Hyg. 2000;62:711–713. doi: 10.4269/ajtmh.2000.62.711. [DOI] [PubMed] [Google Scholar]
- Brest P, Betis F, Cuburu N, Selva E, Herrant M, Servin A, Auberger P, Hofman P. Increased rate of apoptosis and diminished phagocytic ability of human neutrophils infected with Afa/Dr diffusely adhering Escherichia coli strains. Infect Immun. 2004;72:5741–5749. doi: 10.1128/IAI.72.10.5741-5749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LM, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci. 2004;21:25–51. doi: 10.1016/j.ejps.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Chang Q, Soper BD, Yacyshyn BR, Tepperman BL. Alterations in protein kinase C isoforms in experimentally-induced colitis in the rat. Inflamm Res. 2000;49:27–35. doi: 10.1007/PL00000200. [DOI] [PubMed] [Google Scholar]
- Cohen PS, Rossoll R, Cabelli VJ, Yang SL, Laux DC. Relationship Between the Mouse Colonizing Ability of A Human Fecal Escherichia coli Strain and Its Ability to Bind A Specific Mouse Colonic Mucous Gel Protein. Infection and Immunity. 1983;40:62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss AK, Silva M, Casanova JE, McCormick BA. Regulation of Salmonella-induced neutrophil transmigration by epithelial ADP-ribosylation factor 6. J Biol Chem. 2001;276:48431–48439. doi: 10.1074/jbc.M106969200. [DOI] [PubMed] [Google Scholar]
- Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH, NavarroGarcia F, Nataro JP. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infection and Immunity. 1997;65:4135–4145. doi: 10.1128/iai.65.10.4135-4145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfan MJ, Inman KG, Nataro JP. The major pilin subunit of the AAF/II fimbriae from enteroaggregative Escherichia coli mediates binding to extracellular matrix proteins. Infection and Immunity. 2008;76:4378–4384. doi: 10.1128/IAI.00439-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. Fems Microbiology Letters. 2006;254:12–18. doi: 10.1111/j.1574-6968.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- Harrington SM, Strauman MC, Abe CM, Nataro JP. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cellular Microbiology. 2005;7:1565–1578. doi: 10.1111/j.1462-5822.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- Hicks S, Candy DCA, Phillips AD. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infection and Immunity. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz HI, Rutkowski S, Aleksic S, Karch H. Acute and chronic diarrhoea and abdominal colic associated with enteroaggregative Escherichia coli in young children living in western Europe. Lancet. 1997;349:1660–1662. doi: 10.1016/S0140-6736(96)12485-5. [DOI] [PubMed] [Google Scholar]
- Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- Hurley BP, Williams NL, McCormick BA. Involvement of phospholipase A2 in Pseudomonas aeruginosa-mediated PMN transepithelial migration. Am J Physiol Lung Cell Mol Physiol. 2006;290:L703–L709. doi: 10.1152/ajplung.00390.2005. [DOI] [PubMed] [Google Scholar]
- Jacobson PB, Kuchera SL, Metz A, Schachtele C, Imre K, Schrier DJ. Anti-inflammatory properties of Gö 6850: a selective inhibitor of protein kinase C. J Pharmacol Exp Ther. 1995;275:995–1002. [PubMed] [Google Scholar]
- Jiang ZD, Greenberg D, Nataro JP, Steffen R, Dupont HL. Rate of occurrence and pathogenic effect of enteroaggregative Escherichia coli virulence factors in international travelers. Journal of Clinical Microbiology. 2002;40:4185–4190. doi: 10.1128/JCM.40.11.4185-4190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Konar M, Goyal A, Ghosh S. Enteroaggregative Escherichia coli infection induces IL-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappa B and AP-1 in INT-407 cells. Molecular and Cellular Biochemistry. 2010;337:17–24. doi: 10.1007/s11010-009-0282-3. [DOI] [PubMed] [Google Scholar]
- Knutton S, Shaw RK, Bhan MK, Smith HR, McConnell MM, Cheasty T, Williams PH, Baldwin TJ. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992;60:2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler H, Rodrigues SP, McCormick BA. Shigella flexneri Interactions with the Basolateral Membrane Domain of Polarized Model Intestinal Epithelium: Role of Lipopolysaccharide in Cell Invasion and in Activation of the Mitogen-Activated Protein Kinase ERK. Infect Immun. 2002;70:1150–1158. doi: 10.1128/IAI.70.3.1150-1158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler H, Sakaguchi T, Hurley BP, Kase BJ, Reinecker HC, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007;293:G178–G187. doi: 10.1152/ajpgi.00535.2006. [DOI] [PubMed] [Google Scholar]
- Kuhn H, Borngraber S. Mammalian 15-lipoxygenases - Enzymatic properties and biological implications. Lipoxygenases and Their Metabolites. 1999;447:5–28. [PubMed] [Google Scholar]
- Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases - Structure, function, and biological implications. Prostaglandins & Other Lipid Mediators. 2002;68-9:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- Lee CA, Silva M, Siber AM, Kelly AJ, Galyov E, McCormick BA. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc Natl Acad Sci U S A. 2000;97:12283–12288. doi: 10.1073/pnas.97.22.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Nalin DR, Hornick RB, Bergquist EJ, Waterman DH, Young CR, Sotman S, Rowe B. Escherichia coli Strains That Cause Diarrhea But do Not Produce Heat-Labile Or Heat-Stable Enterotoxins and Are Non-Invasive. Lancet. 1978;1:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Madara JL, Patapoff TW, Gillececastro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5′-Adenosine Monophosphate Is the Neutrophil-Derived Paracrine Factor That Elicits Chloride Secretion from T84 Intestinal Epithelial-Cell Monolayers. Journal of Clinical Investigation. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA. Bacterial-induced hepoxilin A3 secretion as a pro-inflammatory mediator. FEBS J. 2007;274:3513–3518. doi: 10.1111/j.1742-4658.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- McCormick BA, Colgan SP, Delparcher C, Miller SI, Madara JL. Salmonella Typhimurium Attachment to Human Intestinal Epithelial Monolayers - Transcellular Signaling to Subepithelial Neutrophils. Journal of Cell Biology. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA, Siber AM, Maurelli AT. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infection and Immunity. 1998;66:4237–4243. doi: 10.1128/iai.66.9.4237-4243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A(3) in inflammatory events: A required role in neutrophil migration across intestinal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumy KL, Bien JD, Pazos MA, Gronert K, Hurley BP, McCormick BA. Distinct isoforms of phospholipase A(2) mediate the ability of Salmonella enterica serotype Typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils. Infection and Immunity. 2008;76:3614–3627. doi: 10.1128/IAI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H, Cogan T, Humphrey T. Direction of neutrophil movements by Campylobacter-infected intestinal epithelium. Microbes Infect. 2011;13:42–48. doi: 10.1016/j.micinf.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Nataro JP. Enteroaggregative Escherichia coli pathogenesis. Current Opinion in Gastroenterology. 2005;21:4–8. [PubMed] [Google Scholar]
- Nataro JP, Baldini MM, Kaper JB, Black RE, Bravo N, Levine MM. Detection of An Adherence Factor of Enteropathogenic Escherichia coli with A Dna Probe. Journal of Infectious Diseases. 1985;152:560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nataro JP, Deng YK, Cookson S, Cravioto A, Savarino SJ, Guers LD, Levine MM, Tacket CO. Heterogeneity of Enteroaggregative Escherichia coli Virulence Demonstrated in Volunteers. Journal of Infectious Diseases. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clinical Microbiology Reviews. 1998;11:142–+. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Delp C, Arnaout MA, Madara JL. Neutrophil Migration Across A Cultured Epithelial Monolayer Elicits A Biphasic Resistance Response Representing Sequential Effects on Transcellular and Paracellular Pathways. Journal of Cell Biology. 1992;117:757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Siccardi D, Mumy KL, Bien JD, Louie S, Shi HN, Gronert K, Mrsny RJ, McCormick BA. Multidrug Resistance-Associated Transporter 2 Regulates Mucosal Inflammation by Facilitating the Synthesis of Hepoxilin A(3). Journal of Immunology. 2008;181:8044–8052. doi: 10.4049/jimmunol.181.11.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JM. The role of Escherichia coli in inflammatory bowel disease. Gut. 2007;56:610–612. doi: 10.1136/gut.2006.111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflammatory Bowel Diseases. 2007;13:1277–1283. doi: 10.1002/ibd.20176. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez F, Wahid R, Faherty CS, Kolappaswamy K, Rodriguez L, Santiago A, Murphy E, Cross A, Sztein MB, Nataro JP. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1101006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savkovic SD, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infection and Immunity. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savkovic SD, Koutsouris A, Hecht G. PKC zeta participates in activation of inflammatory response induced by enteropathogenic E. coli. Am J Physiol Cell Physiol. 2003;285:C512–C521. doi: 10.1152/ajpcell.00444.2002. [DOI] [PubMed] [Google Scholar]
- Savkovic SD, Ramaswamy A, Koutsouris A, Hecht G. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am J Physiol Gastrointest Liver Physiol. 2001;281:G890–G898. doi: 10.1152/ajpgi.2001.281.4.G890. [DOI] [PubMed] [Google Scholar]
- Schonhoff CM, Gillin H, Webster CRL, Anwer MS. Protein kinase C delta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 2008;47:1309–1316. doi: 10.1002/hep.22162. [DOI] [PubMed] [Google Scholar]
- Sepehri S, Khafipour E, Bernstein CN, Coombes BK, Pilar AV, Karmali M, Ziebell K, Krause DO. Characterization of Escherichia coli Isolated from Gut Biopsies of Newly Diagnosed Patients with Inflammatory Bowel Disease. Inflammatory Bowel Diseases. 2011;17:1451–1463. doi: 10.1002/ibd.21509. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Shannon VR, Stenson WF, Holtzman MJ. Induction of Epithelial Arachidonate 12-Lipoxygenase at Active-Sites of Inflammatory Bowel-Disease. American Journal of Physiology. 1993;264:G104–G111. doi: 10.1152/ajpgi.1993.264.1.G104. [DOI] [PubMed] [Google Scholar]
- Sheikh J, Hicks S, Dall'Agnol M, Phillips AD, Nataro JP. Roles for Fis and YafK in biofilm formation by enteroaggregative Escherichia coli. Molecular Microbiology. 2001;41:983–997. doi: 10.1046/j.1365-2958.2001.02512.x. [DOI] [PubMed] [Google Scholar]
- Silva M, Song C, Nadeau WJ, Matthews JB, McCormick BA. Salmonella typhimurium SipA-induced neutrophil transepithelial migration: involvement of a PKC-alpha-dependent signal transduction pathway. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1024–G1031. doi: 10.1152/ajpgi.00299.2003. [DOI] [PubMed] [Google Scholar]
- Slomiany BL, Slomiany A. Cytosolic phospholipase A2 activation in Helicobacter pylori lipopolysaccharide-induced interference with gastric mucin synthesis. IUBMB Life. 2006;58:217–223. doi: 10.1080/15216540600732021. [DOI] [PubMed] [Google Scholar]
- Steer SA, Wirsig KC, Creer MH, Ford DA, McHowat J. Regulation of membrane-associated iPLA(2) activity by a novel PKC isoform in ventricular myocytes. American Journal of Physiology-Cell Physiology. 2002;283:C1621–C1626. doi: 10.1152/ajpcell.00109.2002. [DOI] [PubMed] [Google Scholar]
- Steiner TS, Lima AAM, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. Journal of Infectious Diseases. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. Enteroaggregative Escherichia coli Disrupts Epithelial Cell Tight Junctions. Infection and Immunity. 2010;78:4958–4964. doi: 10.1128/IAI.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, McClarty G, Dong F, Hatch GM, Pan ZK, Zhong G. Activation of Raf/MEK/ERK/cPLA2 signaling pathway is essential for chlamydial acquisition of host glycerophospholipids. J Biol Chem. 2004;279:9409–9416. doi: 10.1074/jbc.M312008200. [DOI] [PubMed] [Google Scholar]
- Sutherland M, Schewe T, Nigam S. Biological actions of the free acid of hepoxilin A3 on human neutrophils. Biochem Pharmacol. 2000;59:435–440. doi: 10.1016/s0006-2952(99)00345-7. [DOI] [PubMed] [Google Scholar]
- Thomazini CM, Samegima DA, Rodrigues MA, Victoria CR, Rodrigues J. High prevalence of aggregative adherent Escherichia coli strains in the mucosa-associated microbiota of patients with inflammatory bowel diseases. Int J Med Microbiol. 2011;301:475–479. doi: 10.1016/j.ijmm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL. PKC and PLA2: probing the complexities of the calcium network. Cell Calcium. 2009;45:535–545. doi: 10.1016/j.ceca.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Walia B, Castaneda FE, Wang L, Kolachala VL, Bajaj R, Roman J, Merlin D, Gewirtz AT, Sitaraman SV. Polarized fibronectin secretion induced by adenosine regulates bacterial-epithelial interaction in human intestinal epithelial cells. Biochem J. 2004;382:589–596. doi: 10.1042/BJ20040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Baumler AJ. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kishimoto K, Arakawa T, Suzuki H, Nakamura M, Yoshimoto T, Takao T, Shimonishi Y, Tanabe T. Arachidonate 12-lipoxygenases - Catalytic properties and regulation of the enzyme gene. Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury 3. 1997;407:191–196. [PubMed] [Google Scholar]
- Yamamoto S, Suzuki H, Nakamura M, Ishimura K. Arachidonate 12-lipoxygenase isozymes. Lipoxygenases and Their Metabolites. 1999;447:37–44. doi: 10.1007/978-1-4615-4861-4_4. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Yamamoto S. Arachidonate 12-Lipoxygenase. Journal of Lipid Mediators and Cell Signalling. 1995;12:195–212. doi: 10.1016/0929-7855(95)00019-m. [DOI] [PubMed] [Google Scholar]
- Zurawski DV, Mitsuhata C, Mumy KL, McCormick BA, Maurelli AT. OspF and OspC1 are Shigella flexneri type III secretion system effectors that are required for postinvasion aspects of virulence. Infect Immun. 2006;74:5964–5976. doi: 10.1128/IAI.00594-06. [DOI] [PMC free article] [PubMed] [Google Scholar]