Abstract
Human Immunodeficiency Virus-1 (HIV) infection of the central nervous system may cause a neurological syndrome termed HIV-associated neurocognitive disorder (HAND) which includes minor neurocognitive disorders or a more severe form of motor and cognitive impairments. Although treatment with highly active antiretroviral agents decreases the load of HIV in the brain, the prevalence of mild forms of HAND is actually increased due to longer life. Therefore, adjunctive and combined therapies must be developed to prevent and perhaps reverse the neurologic deficits observed in individuals with HAND. Key to developing effective therapies is a better understanding of the molecular and cellular mechanisms by which the virus causes this disorder. A number of HIV proteins has been shown to be released from HIV-infected cells. Moreover, these proteins have been shown to possess neurotoxic properties. This review describes new evidence of a direct interaction of the HIV protein gp120 with neurons, which might play a role in the etiopathology of HAND.
Keywords: Autophagy, BDNF, chemokine receptors, gp120, HIV-1-associated dementia, Tat
Introduction
Human immunodeficiency virus-1 (HIV) enters the central nervous system (CNS) and causes neurological impairments. The most significant of the primary HIV-associated CNS disorders include HIV-associated dementia (HAD) and its often antecedent syndrome, minor neurocognitive disorder (MND), which have recently been collectively termed HIV-associated neurocognitive disorder (HAND) (McArthur et al., 2010). The course of HAND is highly variable from patient to patient, and can present as an abrupt onset in neurocognitive decline, behavioral abnormalities and motor dysfunction over a few weeks or a protracted course over several months (Vivithanaporn et al., 2010). HAD is the most severe neurological acquired immune deficiency syndrome (AIDS) illness. Approximately 20–40% of untreated AIDS patients acquire the diagnosis, while in populations receiving highly active antiretroviral treatment (HAART) the estimated prevalence of HAD is less (~10%). Nevertheless, recent studies suggest that despite HAART, neurocognitive impairments progress (Price and Spudich, 2008; Cysique and Brew, 2009) or are not fully eliminated (Joska et al., 2010). Moreover, as a larger proportion of AIDS patients treated with HAART survive longer, neurocognitive impairments increase when compared to younger controls (Valcour et al., 2004). In addition, the brain pathology of these subjects may be complicated by co-morbidities including chronic substance abuse, previous head injury, or prior opportunistic infection of the CNS (McArthur et al., 2010).
The brains of HAND-affected individuals exhibit axonal injury or aberrant sprouting of synaptodendritic connections (Ellis et al., 2007). Although there is no consensus on the relationship between cellular dysfunction and mild neurological effects, the degree of synaptic degeneration appears to correlate with the severity of cognitive neurological impairment seen in the late stage of the disease (Ellis et al., 2007). In HAD, prior to HAART, neurons showed apoptotic features in several brain areas (James et al., 1999; Petito et al., 1999; Garden et al., 2002), which may explain cerebral atrophy and white matter abnormalities, as well as cell loss in subcortical regions (Albright et al., 2003; McArthur, 2004; Everall et al., 2005). Moreover, a number of researchers have reported pathological alterations of the basal ganglia in HAD, including neuronal loss in the putamen (Everall et al., 1995) and the globus pallidus (Fox et al., 1997), loss of nigro-striatal dopamine (DA) neurons (Reyes et al., 1991; Itoh et al., 2000), and dysfunctional DAergic transport (Wang et al., 2004). Consequently, it is not surprising to find clinical features of HAD resembling Parkinson’s disease, such as loss of postural stability, involuntary movements, bradykinesia, and impairment in fine motor skills (Berger and Arendt, 2000; Koutsilieri et al., 2002; McArthur, 2004; Nath and Berger, 2004). Thus, it appears that HIV could reduce the innate ability of the CNS to cope with a neurotoxic environment. The exact mechanisms by which HIV promotes neurotoxicity and causes the pathology of HAD are not completely understood. Here we will present and discuss new theories about an interaction of HIV proteins with membrane associated receptors that leads to synaptic simplification and neuronal loss.
Viral proteins and neurons
Despite the blood brain barrier, HIV invades the brain soon after systemic infection and infects primarily microglia and microphages (Stevenson and Gendelman, 1994; He et al., 1997; Ghorpade et al., 1998; Albright et al., 1999). Three major pathways have been proposed for HIV infection of brain cells: (1) transport of HIV into the brain by infected cells (“Trojan horse” hypothesis); (2) passage of cell-free virus into the brain; and (3) release of virus into the brain by infected endothelial cells (Albright et al., 2003). Moreover, it has been shown that few HIV-infected astrocytes can propagate infection and toxicity through a gap junction mechanism (Eugenin et al., 2011). HAART cannot totally eliminate HIV infection because the blood brain barrier limits the penetration of the therapeutics into the CNS. Moreover, despite the CNS surveillance by T cells, on a direct weight to weight comparison, the levels of immunological surveillance is far below that occurring in other tissues (Hickey, 2001). Thus, the CNS becomes a reservoir for HIV to hide and replicate (Kramer-Hammerle et al., 2005).
Macrophages and microglia cells express on their surface a major HIV receptor, CD4, an immunoglobulin receptor, and various HIV co-receptors (chemokine receptors). These receptors are known to promote attachment of the virus through gp160, a glycoprotein complex present on the HIV membrane which is comprised of two subunits, gp120 and gp41. Such binding allows fusion of viral and cellular membranes, leading to entry of the virus into the cell [reviewed in (Berger et al., 1999)]. Two chemokine receptors, CXCR4 and CCR5, appear to play a crucial role in HIV entry; CXCR4 receptor mediates the entry of the virus into lymphocytes, CCR5 into monocytes and macrophages (Deng et al., 1996; Dittmar et al., 1997) Nevertheless, CCR5 and CXCR4 using viral strains can co-exist in a single host (Koot et al., 1996).
How can HIV cause synaptic injury and neuronal loss? Neurons do not express CD4 and thus are not capable of productive infection; therefore, the effect of HIV must occur via an indirect mechanism. Many possibilities exist for indirect mechanisms. Infected non-neuronal cells may release host cell-derived factors, including cytokines and chemokines (Kaul et al., 2001). Such neurotoxic factors are likely to affect a diverse range of neuronal populations in different CNS structures, as well as in the white matter. Moreover, several HIV proteins could interact directly with neurons or glial cells and promote toxicity. These proteins include the envelope glycoprotein gp120 (Hesselgesser et al., 1998; Meucci et al., 1998), the transcription factor Tat (Nath, 2002; Maragos et al., 2003), Nef, the regulatory protein that promotes both viral replication and immune evasion of HIV (van Marle et al., 2004), the viral transcription protein Vpr (Patel et al., 2000; Jones et al., 2007), and others (Mattson et al., 2005). These proteins can be actively or passively released from infected non-neuronal cells such as microglia or macrophages. It is also possible that HIV may shed proteins after fusion with the host cell membrane or be released from cells. In either case, HIV would then shed gp120 which would interact with neurons. In addition, although astrocytes are only capable of restricted productive infection (Tornatore et al., 1994a), they can release viral proteins (Brack-Werner, 1999). HIV proteins that are not released from astrocytes, such as Nef (Tornatore et al., 1994b), could also alter astrocyte (Bezzi et al., 2001) or oligodendrocyte function (Radja et al., 2003) and disrupt their supportive and neurotrophic role. Free HIV proteins may also cross the blood brain barrier if that barrier’s integrity is compromised in HIV positive subjects.
Neuronal chemokine receptors and gp120
Gp120 is one of the most potent HIV viral neurotoxins with lethal activities in the picomolar range. Transgenic mice expressing gp120 exhibit neuronal loss and dendritic simplification (Toggas et al., 1994), pathological features seen in postmortem brains of HAD subjects. Debate exists on whether gp120 induces neuronal damage through inflammatory responses (indirect mechanism) or by directly interacting with neurons. Indirect mechanisms could occur through a variety of avenues. HIV infection causes HIV encephalitis which is characterized by neuroinflammation, astrogliosis and microgliosis, resulting in an overall production and release of pro-apoptotic chemokines, such as Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNFα). It was demonstrated that gp120 promotes the release of these cytokines, as well as other neurotoxic factors such as glutamate (Kaul and Lipton, 1999; Bezzi et al., 2001), which, in turn, evoke neuronal apoptosis. Therefore, gp120 may potentially exacerbate the toxic action of neuroinflammation. On the other hand, independent experiments have shown that gp120 can be neurotoxic even in the absence of glutamate receptor activation (Bachis and Mocchetti, 2004) or pro-inflammatory cytokines (Bachis et al., 2006). Thus, it appears that the indirect mechanism alone cannot explain the neurotoxic effect of gp120.
Direct mechanisms may go farther to explain the neurotoxic effect of gp120. The envelope protein may promote neuronal loss via activation of chemokine receptors. In vitro, gp120 binds to CXCR4 or CCR5, even without CD4, in both neurons (Hesselgesser et al., 1998) and astrocytes (Liu et al., 2004). X4- and R5-gp120s bind to CXCR4 and CCR5, respectively. Intriguingly, these receptors are highly expressed in vivo throughout the brain (Klein et al., 1999; van der Meer et al., 2000; Tran et al., 2007; Trecki et al., 2010; Avdoshina et al., 2011). Several lines of independent investigations have shown that gp120 binding to these receptors promotes an apoptotic signal both in vitro (Hesselgesser et al., 1998; Meucci et al., 1998; Zheng et al., 1999; Biard-Piechaczyk et al., 2000) and in vivo (Bagetta et al., 1996; Bansal et al., 2000; Acquas et al., 2004). Apoptosis can be prevented by the CXCR4 antagonist AMD3100 (Donzella et al., 1998) both in vitro (Lazarini et al., 2000) and in vivo (Bachis et al., 2006). Conversely, DAPTA, a CCR5 antagonist (Polianova et al., 2005) prevents R5-gp120 neurotoxicity (Bachis and Mocchetti, 2005). Therefore, the cellular mechanism of how gp120 transmits a toxic signal through CXCR4 or CCR5 remains to be established.
CXCR4 and CCR5 are G protein coupled receptors. Several signaling molecules are associated with these receptors including inositol triphosphate and phospholipase C, and inhibition of adenylyl cyclase. CXCR4 activation by gp120 has been shown to increase cytosolic free Ca2+ (Holden et al., 1999; Zheng et al., 1999) and extracellular receptor kinase (ERK) (Lazarini et al., 2000). CXCR4-mediated increases in ERK and Ca2+ signal transduction have been shown to promote neuronal migration and differentiation (Lazarini et al., 2000). Further, ERK tyrosine phosphorylation has also been shown to promote neuronal survival (Volosin et al., 2006) and to be the main mediator of neurotrophic factor-induced neuritic morphogenesis (Naska et al., 2006). Therefore, it is unlikely that this pathway is the key in promoting the toxic effect of gp120, although the involvement of CXCR4-mediated activation of ERK and Ca2+ in gp120-mediated apoptosis may differ among cell types and context. Similarly, gp120 toxicity may depend upon activation of p38/mitogen-activated protein kinase (p38/MAPK) and c-Jun terminal kinase (JNK) in cerebrocortical cultures (Kaul and Lipton, 1999) or striatal neurons (Singh et al., 2005) but not in other cells (Meucci et al., 1998; Biard-Piechaczyk et al., 2000). Activation of JNK signaling cascade generally results in p53-mediated apoptosis, a key feature observed also in HIV subjects (Garden et al., 2002). Conversely, pharmacological inhibition of the JNK pathway prevents gp120-mediated apoptosis (Bodner et al., 2004). Overall these data suggest that gp120 exhibits a CXCR4-mediated intrinsic neurotoxic property that does not necessarily involve production of inflammatory cytokines. Nevertheless, M-tropic gp120 has been shown to induce the pro-inflammatory chemokine CXCL10 that promotes apoptosis through CXCR3 (Sui et al., 2004) or IL-1β (Bachis et al., 2010). Thus, gp120 may activate potential pathways for neurotoxicity based upon its affinity for different chemokine receptors.
Tat promotes apoptosis
Tat is the protein that mediates HIV transcription and is important for viral replication. As mentioned above, Tat can be released from HIV-infected cells (Ensoli et al., 1993) and alter the blood brain barrier (Toborek et al., 2003). Virion-free Tat interacts with different membrane receptors, such as integrins (Albini et al., 1996) and low density lipoprotein receptor-related protein (LPR) (Liu et al., 2000). Integrins are a family of glycoproteins that are expressed on neuronal membranes and mediate the adhesion of neurons to the extracellular matrix (Cavallaro and Dejana, 2011). Thus, Tat can alter neuronal adhesion to extracellular matrix. Whether this effect is crucial for Tat neurotoxicity is still unclear because binding of Tat to integrins activates the protein kinase p125FAK (Milani et al., 1998) which is considered neuroprotective (Ivankovic-Dikic et al., 2000) and essential for dendritic morphology (Beggs et al., 2003).
Binding of Tat to LPR results in its internalization into neurons (Liu et al., 2000). Once inside, Tat is transported to the nucleus (Bruce-Keller et al., 2003). Whether nuclear Tat is neurotoxic is unclear; indeed, a nuclear effect of Tat is more consistent with its function as a transcriptional regulator (Zhou et al., 2004) rather than with its neurotoxic property (see later). There are also data showing that Tat binds to the N-methyl-D-aspartate (NMDA) receptor at the allosteric zinc site (Song et al., 2003) or its polyamine sensitive site (Prendergast et al., 2002). Tat may also potentiate glutamate overactivation of NMDA receptors (Haughey et al., 2001). Consistent with this hypothesis, blockade of NMDA receptor by MK801, a potent antagonist (Eugenin et al., 2003), or by memantine, an antagonist with moderate affinity for this receptor (Nath et al., 2000) reduces significantly Tat toxicity. However, additional experiments using different combinations of various NMDA receptor subunits are warranted to prove the relative affinity of Tat for this receptor.
It was recently demonstrated that after binding of Tat to LPR, a complex is formed between LRP and the NMDA receptor, mediated by an intracellular scaffolding protein, PSD-95 (Eugenin et al., 2007). PSD-95 contains many protein-binding domains, facilitating interaction with diverse synaptic and signaling proteins (Kim and Sheng, 2004). Thus, it is possible that Tat activates NMDA receptor through the cross-activation of several signaling pathways. Nevertheless, the effect of Tat on production of pro-inflammatory cytokines or other inflammatory response mediators cannot be overlooked (Nath et al., 1999; Pu et al., 2003). In fact, Tat promotes cellular events such as nitric oxide and reactive oxygen species production (Toborek et al., 2003), and mitochondrial membrane depolarization (Li et al., 2009) that are induced by cytokines and are known to be implicated in neuronal cell death. Thus, it can be concluded that the neurotoxic effect of Tat includes multiple mechanisms.
Diversity of mechanisms: microtubular transport and neurotrophic support
A number of cellular effects that are believed to participate in the neurotoxic effects of Tat and gp120 are similar. However, Tat and gp120 can promote neuronal loss through different mechanisms. Learning more about the differences of Tat and gp120 in their interaction with cellular signaling may shed light onto how HAND develops. For instance, in striatal neurons both Tat and gp120 activate caspase-3 (Singh et al., 2004). Depending upon experimental conditions and cell types used, both Tat and gp120 promote cytochrome C and endonuclease G (Singh et al., 2004; Melli et al., 2006), which are components of the apoptotic cascades. Nevertheless, p38 MAPK activation alone mediates gp120-induced neurite dystrophy and cell death, while neither p38 MAPK nor JNK inhibition is sufficient to prevent Tat-induced neurite losses or neuronal death (Singh et al., 2005). Thus, while apoptotic neuronal loss is crucial for the neurotoxic effect of these two viral proteins, their mechanisms of action are different.
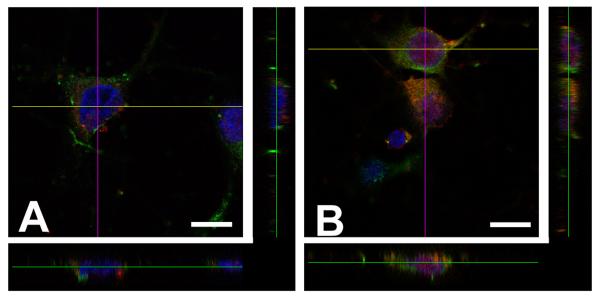
Another feature that may be important for Tat and gp120 toxic effect is their ability to be endocytosed by neurons (Bruce-Keller et al., 2003; Bachis et al., 2006). Once inside, gp120 localizes in late endosomes (Bachis et al., 2006) and remains perinuclearly, Tat escapes from endosomes and migrates to the nucleus (Fig. 1). In the nucleus, Tat may alter transcription of survival factors such as p35 (Darbinian et al., 2008), a neuron-specific activator of cyclin-dependent kinase 5 (Cdk5), an enzyme with multiple neuronal effects implicated in synaptic plasticity. In fact, the induction of p35/Cdk5 kinase activity is critical for neurite outgrowth and survival (Song et al., 2005; Fu et al., 2007). These data suggest that Tat may be neurotoxic by altering both intranuclear and cytoplasmic (i.e. calcium dysregulation) events. As noted above, gp120 does not enter the neuronal nucleus but interacts directly with synaptic vesicles, microtubules and lysosomes (Bachis et al., 2006).
Figure 1. Differential intracellular localization of gp120 and Tat.
Cortical neurons were prepared from E18 rat embryos as previously described (Avdoshina et al., 2010). Neurons were exposed to (A) gp120IIIB (5 nM) and (B) Tat (100 nM) for different time points. Neurons were then fixed and stained with anti-gp120 and anti-Tat antibodies (red), and co-stained with class III β-tubulin (green) and DAPI (blue). The figure shows representative images obtained 6 hr after gp120 or Tat exposure using a confocal microscope. Neurons were optically sliced and a Z-stack was created using the FluoView software. Orthogonal projections were created to show perinuclear and intranuclear localization of gp120 and Tat immunoreactivity, respectively. Bar = 10 μm.
The entry of gp120 is a CXCR4-dependent mechanism (Bachis et al., 2003; Bachis et al., 2006). CXCR4 undergoes spontaneous and ligand-mediated endocytosis (Orsini et al., 1999; Signoret et al., 2000). Endocytosis is a fundamental cellular function involved in nutrient uptake, pathogen removal, and transport of signaling molecules from an extracellular environment to the cytoplasm (Ungewickell and Hinrichsen, 2007). The endocytosed material is first moved into an early endosome, from which it may either be returned to the plasma membrane via a recycling endosome, or transferred into a late endosome. The late endosome may move its contents into the trans-Golgi network, or into a lysosome, in which case the contents will be targeted for degradation (Maxfield and McGraw, 2004). However, there is evidence that abnormal ceramide metabolism in relation to aberrant lysosomal function can cause neurodegenerative diseases (Ditaranto-Desimone et al., 2003). Thus, once gp120 accumulates inside lysosomes, it may increase the production of toxic cytoplasmic inclusions such as ceramide (Haughey et al., 2004; Jana and Pahan, 2004). Data showing that HAD patients have high levels of brain ceramide (Haughey et al., 2004) support this neurotoxic mechanism of gp120.
Ceramide may not be the only cellular mechanism of neurotoxicity involving lysosomes. In fact, homeostasis in neurons involves clearance of proteins by autophagy, an evolutionary conserved pathway that involves sequestration of cytoplasmic material into the lysosomes (Kundu and Thompson, 2008). Autophagic vacuoles have been shown to accumulate in affected neurons in subjects with Alzheimer’s and Parkinson’s diseases (Wei et al., 2007; Pickford et al., 2008). The induction of autophagy is also associated with axonal degeneration in Purkinje cells (Wang et al., 2006) and it is also seen in HAD (Gelman et al., 2005). When lysosomes cannot recycle/degrade efficiently, an abnormal collection of undigested material within neurons occurs, leading to cell death (Zhang et al., 2001). Gp120 may alter the regulated retrograde flux that balances axonal transport and autophagy and cause apoptosis. Indeed, the death receptor signaling molecule TRAIL, which is up-regulated by HIV (Ryan et al., 2004), regulates both apoptosis and autophagy. p53, which is also activated by gp120 (Garden et al., 2004), is another activator of autophagy (Wang et al., 2009). Finally the ubiquitin proteosome system, which is altered in HAD subjects (Nguyen et al., 2010) and is crucial for protein degradation is also implicated in autophagy (Kim et al., 2008). Thus, gp120 endocytosis may impair various cellular functions crucial for neuronal homeostasis. These considerations suggest that if gp120 accumulates inside lysosomes or other organelles it promotes neuronal apoptosis by preventing physiological autophagy.
Gp120 has also been shown to cause axonal degeneration (Melli et al., 2006) and dendritic injury (Everall et al., 2002; Iskander et al., 2004), two key pathological events that may account for the synaptodendritic atrophy observed in HAD (Masliah et al., 1997). Gp120-mediated neuronal degeneration can be prevented by nocodazole or colchicine (Bachis et al., 2006), two inhibitors of fast axonal transport (James et al., 1970; Samson et al., 1979), indicating that trafficking of gp120 along the axon is crucial for its neurotoxicity. The gp120-mediated axonal degeneration has four key features: 1: It takes place both in the CNS as well as in the peripheral nervous system (Bachis et al., 2006; Melli et al., 2006); 2: It occurs only when gp120 is applied directly to axons (Melli et al., 2006); 3: It is CXCR4 dependent (Bachis et al., 2006; Melli et al., 2006) and 4: It is caspase-3 dependent. Therefore, it is different from that observed during CNS development, which is caspase independent (Raff et al., 2002). In contrast, gp120-mediated degeneration of dendrites occurs without a significant change in apoptosis (Iskander et al., 2004). Interestingly, gp120 is seen around synaptic vesicles (Bachis et al., 2006) and associates with dendrites (Fig. 2A) and axonal microtubules (Fig. 2B). Such cellular mechanism may have a profound significance for gp120 neurotoxicity if we consider that microtubules are essential for intracellular transport of pro-survival proteins in both axons and dendrites. In addition, axonal transport in neurons necessitates the most rapid and lengthy mitochondrial movements and is required to maintain local ATP levels at the synapse. Microtubule/cytoskeleton-dependent movement of mitochondria has been shown to influence a variety of cellular behaviors including proliferation, differentiation and apoptotic events (Boldogh and Pon, 2007; Chen and Chan, 2009). Therefore, gp120 may be neurotoxic by reducing movements along microtubules or by impairing the function of motor proteins such as dyneins and kinesins, that, when impaired, promote a “dying-back” pattern of degeneration. This phenomenon may explain the synaptic simplification/atrophy seen in HAD subjects as well as the pathophysiology of degenerating sensory fibers and prominent loss of unmyelinated fibers that are seen in AIDS patients (Estanislao et al., 2004).
Figure 2. gp120 associates with microtubules.

Cerebellar granule neurons were prepared from P8 rat pups as previously described (Bachis et al., 2003). Neurons were exposed to (A) gp120IIIB (5 nM) or (B) colloidal gold (25 nm mean particle size) gp120 for 30 min. In A, neurons were fixed and stained with a gp120 antibody (green) and microtubule associated protein-2 antibody (red) to label dendrites. Cells were then counterstained with DAPI (blue) and analyzed with a confocal microscope. Bar=10 μm. In B, neurons were processed for electron microscopy analysis of gold gp120 as previously described (Bachis et al., 2006). The picture shows several axons as determined by the presence of synaptic vesicle (SV) and the absence of polyribosomes. Red arrow indicates gp120 associated with microtubules (MT). M=mitochondria. Scale bar: 200 nm. (Modified from Bachis et al., 2006).
Gp120 reduces neurotrophic support
Axonal transport is absolutely necessary for delivering or transporting proteins, including neurotrophic factors, to and from the nerve terminals. Neurons rely on axonal transport of neurotrophic factors for their survival. For instance, the neurotrophin brain derived neurotrophic factor (BDNF), one of the most abundant neurotrophins in the adult brain, is produced in cortical neurons and anterogradely delivered to striatal neurons (Altar et al., 1997), where it is particularly important for their survival and for the activity of the cortico-striatal synapses (Zuccato and Cattaneo, 2007). Conversely, loss of BDNF has been suggested to be a risk factor in chronic diseases of the basal ganglia such as Parkinson’s (Nagatsu et al., 2000) and Huntington’s diseases (Xie et al., 2010). BDNF is also abundant in the hippocampus where it is important for maintaining dendritic morphology and synaptic function (Horch and Katz, 2002), the survival of neurons and their connections (Mattson, 1997; Xu et al., 2000), and long-term potentiation (Figurov et al., 1996; Zakharenko et al., 2003). On the contrary, loss of BDNF has been associated with Alzheimer’s disease and impaired cognition (Phillips et al., 1991). Indeed, there is evidence that reducing the production of BDNF in the hippocampus decreases hippocampal neuronal survival (Erickson et al., 2011).
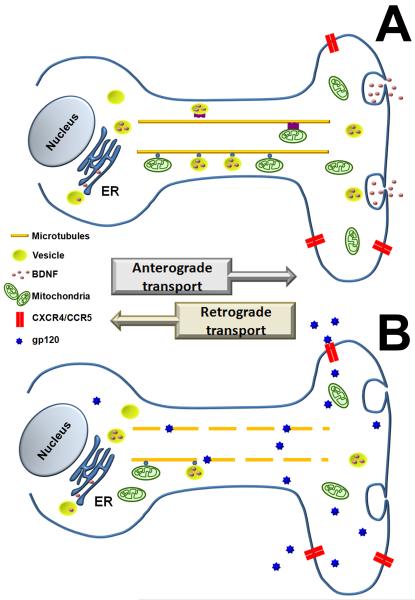
Given the tight relation between BDNF and neuronal survival, one may suggest that gp120 neurotoxicity includes a reduction of BDNF and other neurotrophic factors at the synapses. In vivo studies have shown that gp120 decreases the levels of BDNF in cortico-striatal terminals without affecting BDNF in the cell bodies (Nosheny et al., 2004), suggesting that gp120 modifies the anterograde transport of BDNF. Most likely this phenomenon is related to the ability of gp120 to alter microtubules/axonal transport. These data provide a scenario by which HIV shedding of gp120 promotes neuronal apoptosis by decreasing the microtubule-directed trafficking of vesicles containing BDNF (Fig. 3). A reduced availability of BDNF in the axonal terminal will culminate in a decreased release of BDNF which will affect profoundly neuronal homeostasis and the ability of neurons to counteract the inflammatory responses and production of cytokines that are caused by HIV infection (Kraft-Terry et al., 2010).
Figure 3. Hypothetical model of HIV neurotoxicity.
(A) BDNF processed in the ER apparatus is packed and stored in vesicles. Vesicles are anterogradely transported via microtubules to the axonal terminal. From here, BDNF is released in an activity dependent manner. (B) Extracellular gp120 binds to CXCR4/CCR5 chemokine receptors and it is internalized. Endocytosed gp120 associates with microtubules and disrupts the microtubular transport of BDNF containing vesicles resulting in a decrease in BDNF content in the synapse.
Conclusions
The studies described here and elsewhere show how HIV promotes neuronal cell death by indirect mechanisms involving viral proteins. Neurotoxic actions of viral proteins include an increase in the production of pro-inflammatory cytokines and a reduction of anti-apoptotic neurotrophic factors, such as BDNF and glial-cell derived neurotrophic factor (Nosheny et al., 2006) . Reduction of BDNF culminates in an up-regulation of CXCR4 and CCR5 (Nosheny et al., 2004; Ahmed et al., 2008; Avdoshina et al., 2011), two HIV co-receptors that also promote neuronal apoptosis. In addition, BDNF promotes adult neurogenesis (Li et al., 2008) that is believed to be essential for specific cognitive functions that decline in HIV positive subjects (Venkatesan et al., 2007). Moreover, BDNF exhibits protective activity against the neurotoxic effect of gp120 both in vitro (Bachis et al., 2003) and in vivo (Nosheny et al., 2007). Thus, BDNF could be a valid adjunctive therapy to reduce pathological signs of HAD that are due to loss of synapses. Most importantly, BDNF could be used in MND subjects who exhibit the clinical hallmarks of HAD (Pumpradit et al., 2010), albeit with less severe signs and symptoms (Cysique and Brew, 2009), to prevent the onset of frank dementia. An adjunct therapy is particularly important because of viral mutation and HAART resistance, failure of drugs to access viral sanctuaries in the brain, and toxicities of HAART.
Acknowledgements
This work was supported by National Institute of Health grants DA026174 and NS040670.
References
- Acquas E, Bachis A, Nosheny RL, Cernak I, Mocchetti I. Human immunodeficiency virus type 1 protein gp120 causes neuronal cell death in the rat brain by activating caspases. Neurotox Res. 2004;5:605–615. doi: 10.1007/BF03033180. [DOI] [PubMed] [Google Scholar]
- Ahmed F, Tessarollo L, Thiele C, Mocchetti I. Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain. Brain Res. 2008;1227:1–11. doi: 10.1016/j.brainres.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O’Connor MJ, Doms RW, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58:1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Becker J, Campbell L, Parsadanian M, Mhyre T, Tessarollo L, Mocchetti I. Neurotrophins modulate the expression of chemokine receptors in the brain. J NeuroVirol. 2011;17:58–62. doi: 10.1007/s13365-010-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. The chemokine receptor CXCR4 and not the N-methyl-D-aspartate receptor mediates gp120 neurotoxicity in cerebellar granule cells. J Neurosci Res. 2004;75:75–82. doi: 10.1002/jnr.10826. [DOI] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Ann NY Acad Sci. 2005;1053:247–257. doi: 10.1196/annals.1344.022. [DOI] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010;32:570–578. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of Human Immunodeficiency Virus Type 1 envelope glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Aloe L, Berliocchi L, Costa N, Finazzi-Agro A, Nistico G. Intracerebral injection of human immunodeficiency virus type 1 coat protein gp120 differentially affects the expression of nerve growth factor and nitric oxide synthase in the hippocampus of rat. Proc Natl Acad Sci U S A. 1996;93:928–933. doi: 10.1073/pnas.93.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: The role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind RA, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–344. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- Bodner A, Toth PT, Miller RJ. Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol. 2004;188:246–253. doi: 10.1016/j.expneurol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Boldogh IR, Pon LA. Mitochondria on the move. Trends in Cell Biology. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol. 2011;12:189–197. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Darbinian N, Darbinyan A, Czernik M, Peruzzi F, Khalili K, Reiss K, Gordon J, Amini S. HIV-1 Tat inhibits NGF-induced Egr-1 transcriptional activity and consequent p35 expression in neural cells. J Cell Physiol. 2008;216:128–134. doi: 10.1002/jcp.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Ditaranto-Desimone K, Saito M, Tekirian TL, Saito M, Berg M, Dubowchik G, Soreghan B, Thomas S, Marks N, Yang AJ. Neuronal endosomal/lysosomal membrane destabilization activates caspases and induces abnormal accumulation of the lipid secondary messenger ceramide. Brain Res Bull. 2003;59:523–531. doi: 10.1016/s0361-9230(02)00948-6. [DOI] [PubMed] [Google Scholar]
- Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, Simmonds P. HIV-1 tropism and co-receptor use. Nature. 1997;385:495–496. doi: 10.1038/385495a0. [DOI] [PubMed] [Google Scholar]
- Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2011;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estanislao L, Carter K, McArthur J, Olney R, Simpson D. A randomized controlled trial of 5% lidocaine gel for HIV-associated distal symmetric polyneuropathy. J Acquir Immune Defic Syndr. 2004;37:1584–1586. doi: 10.1097/00126334-200412150-00010. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MVL, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Nat Acad Sci USA. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Everall IP, Hudson L, al-Sarraj S, Honavar M, Lantos P, Kerwin R. Decreased expression of AMPA receptor messenger RNA and protein in AIDS: a model for HIV-associated neurotoxicity. Nat Med. 1995;1:1174–1178. doi: 10.1038/nm1195-1174. [DOI] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fox L, Alford M, Achim C, Mallory M, Masliah E. Neurodegeneration of somatostatin immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1997;56:360–368. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Fu W-Y, Chen Y, Sahin M, Zhao X-S, Shi L, Bikoff JB, Lai K-O, Yung W-H, Fu AKY, Greenberg ME, Ip NY. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. Faseb J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Holzer CEI, Fabian RH, Schuenke KW, Keherly MJ, Richey FJ, Lahart CJ. Potential role for white matter lysosome expansion in HIV-associated dementia. JAIDS. 2005;39:422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman HE. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol. 1998;72:3340–3350. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Holden CP, Haughey NJ, Nath A, Geiger JD. Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience. 1999;91:1369–1378. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1:7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol (Berl) 2000;99:376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth BU, Dikic I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. [DOI] [PubMed] [Google Scholar]
- James HJ, Sharer LR, Zhang Q, Wang HG, Epstein LG, Reed JC, Gelbard HA. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25:380–386. doi: 10.1046/j.1365-2990.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- James KAC, Bray JJ, Morgan IG, Austin L. The effect of colchicine on the transport of axonal protein in the chicken. Biochem J. 1970;117:767–771. doi: 10.1042/bj1170767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Human Immunodeficiency Virus Type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, Jhamandas J, Power C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–3711. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci U S A. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–1646. [PubMed] [Google Scholar]
- Koot M, van ’t Wout AlB, Kootstra NA, de Goede REY, Tersmette M, Schuitemaker H. Relation between Changes in Cellular Load, Evolution of Viral Phenotype, and the Clonal Composition of Virus Populations in the Course of Human Immunodeficiency Virus Type 1 Infection. J Infect Dis. 1996;173:349–354. doi: 10.1093/infdis/173.2.349. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Parkinsonism in HIV dementia. J Neural Transm. 2002;109:767–775. doi: 10.1007/s007020200063. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon C-H, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuroprotective signal transduction: relevance to stroke. Neurosci Biobehav Rev. 1997;21:193–206. doi: 10.1016/s0149-7634(96)00010-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner JP, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani D, Mazzoni M, Zauli G, Mischiati C, Gibellini D, Giacca M, Capitani S. HIV-1 Tat induces tyrosine phosphorylation of p125FAK and its association with phosphoinositide 3-kinase in PC12 cells. AIDS. 1998;12:1275–1284. doi: 10.1097/00002030-199811000-00008. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000;60:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Naska S, Park KJ, Hannigan GE, Dedhar S, Miller FD, Kaplan DR. An essential role for the integrin-linked kinase-glycogen synthase kinase-3 beta pathway during dendrite initiation and growth. J Neurosci. 2006;26:13344–13356. doi: 10.1523/JNEUROSCI.4462-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186:S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Berger J. HIV Dementia. Curr Treat Options Neurol. 2004;6:139–151. doi: 10.1007/s11940-004-0023-6. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–194. [PubMed] [Google Scholar]
- Nguyen TP, Soukup VM, Gelman BB. Persistent hijacking of brain proteasomes in HIV-associated dementia. Am J Pathol. 2010;176:893–902. doi: 10.2353/ajpath.2010.090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Aden SA, De Bernardi MA, Mocchetti I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J Neurobiol. 2006;66:1311–1321. doi: 10.1002/neu.20288. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Amhed F, Yakovlev AG, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Parent J-L, Mundell SJ, Benovic JL. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identificaton of residues in the C-terminas tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000;74:9717–9726. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Kerza-Kwiatecki AP, Gendelman HE, McCarthy M, Nath A, Podack ER, Shapshak P, Wiley CA. Review: neuronal injury in HIV infection. J Neurovirol. 1999;5:327–341. doi: 10.3109/13550289909029474. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polianova MT, Ruscetti FW, Pert CB, Ruff MR. Chemokine receptor-5 (CCR5) is a receptor for the HIV entry inhibitor peptide T (DAPTA) Antiviral Res. 2005;67:83–92. doi: 10.1016/j.antiviral.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Rogers DT, Mulholland PJ, Littleton JM, Wilkins LH, Jr., Self RL, Nath A. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res. 2002;954:300–307. doi: 10.1016/s0006-8993(02)03360-7. [DOI] [PubMed] [Google Scholar]
- Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197(Suppl 3):S294–306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Pumpradit W, Ananworanich J, Lolak S, Shikuma C, Paul R, Siangphoe U, Chaoniti N, Kaew-On P, Paris R, Ruxrungtham K, Valcour V. Neurocognitive impairment and psychiatric comorbidity in well-controlled human immunodeficiency virus-infected Thais from the 2NN Cohort Study. J Neurovirol. 2010;16:76–82. doi: 10.3109/13550280903493914. [DOI] [PubMed] [Google Scholar]
- Radja F, Kay DG, Albrecht S, Jolicoeur P. Oligodendrocyte-specific expression of human immunodeficiency virus type 1 Nef in transgenic mice leads to vacuolar myelopathy and alters oligodendrocyte phenotype in vitro. J Virol. 2003;77:11745–11753. doi: 10.1128/JVI.77.21.11745-11753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal Self-Destruction and Neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathol (Berl) 1991;82:39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Ryan LA, Peng H, Erichsen DA, Huang Y, Persidsky Y, Zhou Y, Gendelman HE, Zheng J. TNF-related apoptosis-inducing ligand mediates human neuronal apoptosis: links to HIV-1-associated dementia. J Neuroimmunol. 2004;148:127–139. doi: 10.1016/j.jneuroim.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Samson F, Donoso JA, Heller-Bettinger I, Watson D, Himes RH. Nocodazole action on tubulin assembly, axonal ultrastructure and fast axoplasmic transport. J Pharmacol Exp Ther. 1979;208:411–417. [PubMed] [Google Scholar]
- Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, El-Hage N, Campbell ME, Lutz SE, Knapp PE, Nath A, Hauser KF. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–790. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: Differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Wang CX, Song DK, Wang P, Shuaib A, Hao C. Interferon gamma Induces Neurite Outgrowth by Up-regulation of p35 Neuron-specific Cyclin-dependent Kinase 5 Activator via Activation of ERK1/2 Pathway. J Biol Chem. 2005;280:12896–12901. doi: 10.1074/jbc.M412139200. [DOI] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol. 2003;9:399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- Stevenson M, Gendelman HE. Cellular and viral determinants that regulate HIV-1 infection in macrophages. J Leukoc Biol. 1994;56:278–288. doi: 10.1002/jlb.56.3.278. [DOI] [PubMed] [Google Scholar]
- Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, Buch S. Neuronal Apoptosis Is Mediated by CXCL10 Overexpression in Simian Human Immunodeficiency Virus Encephalitis. The American Journal of Pathology. 2004;164:1557–1566. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem. 2003;84:169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994a;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Meyers K, Atwood W, Conant K, Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994b;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1034. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J, Brailoiu GC, Unterwald EM. Localization of CXCR4 in the forebrain of the adult rat. Brain Res. 2010;1315:53–62. doi: 10.1016/j.brainres.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: The Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Ming Gl, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64:2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, Power C. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology. 2010;75:1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-J, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dong XX, Cao Y, Liang ZQ, Han R, Wu JC, Gu ZL, Qin ZH. p53 induction contributes to excitotoxic neuronal death in rat striatum through apoptotic and autophagic mechanisms. Eur J Neurosci. 2009;30:2258–2270. doi: 10.1111/j.1460-9568.2009.07025.x. [DOI] [PubMed] [Google Scholar]
- Wei J, Fujita M, Nakai M, Waragai M, Watabe K, Akatsu H, Rockenstein E, Masliah E, Hashimoto M. Enhanced lysosomal pathology caused by beta-synuclein mutants linked to dementia with Lewy bodies. J Biol Chem. 2007;282:28904–28914. doi: 10.1074/jbc.M703711200. [DOI] [PubMed] [Google Scholar]
- Xie Y, Hayden MR, Xu B. BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci. 2010;30:14708–14718. doi: 10.1523/JNEUROSCI.1637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, Reichardt LF. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000;26:233–245. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Butler JD, Levin SW, Wisniewski KE, Brooks SS, Mukherjee AB. Lysosomal ceroid depletion by drugs: Therapeutic implications for a hereditary neurodegenerative disease of childhood. Nat Med. 2001;7:478. doi: 10.1038/86554. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zhou M, Deng L, Lacoste V, Park HU, Pumfery A, Kashanchi F, Brady JN, Kumar A. Coordination of transcription factor phosphorylation and histone methylation by the P TEFb kinase during human immunodeficiency virus type 1 transcription. J Virol. 2004;78:13522–13533. doi: 10.1128/JVI.78.24.13522-13533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]