Abstract
Congenital bony syngnathia, a rare but severe human birth defect, is characterized by bony fusion of the mandible to the maxilla. However, the genetic mechanisms underlying this birth defect are poorly understood, largely due to limitation of available animal models. Here we present evidence that transgenic expression of Bmp4 in neural crest cells causes a series of craniofacial malformations in mice, including a bony fusion between the maxilla and hypoplastic mandible, resembling the bony syngnathia syndrome in humans. In addition, the anterior portion of the palatal shelves emerged from the mandibular arch instead of the maxilla in the mutants. Gene expression assays showed an altered expression of several facial patterning genes, including Hand2, Dlx2, Msx1, Barx1, Foxc2 and Fgf8, in the maxillary and mandibular processes of the mutants, indicating mis-patterned cranial neural crest (CNC) derived cells in the facial region. However, despite of formation of cleft palate and ectopic cartilage, forced expression of a constitutively active form of BMP receptor-Ia (caBmprIa) in CNC lineage did not produce the syngnathia phenotype, suggesting a non-cell autonomous effect of the augmented BMP4 signaling. Our studies demonstrate that aberrant BMP4-mediated signaling in CNC cells leads to mis-patterned facial skeleton and congenital bony syngnathia, and suggest an implication of mutations in BMP signaling pathway in human bony syngnathia.
Keywords: Bmp signaling, cranial neural crest, syngnathia, cleft palate, craniofacial patterning
Introduction
The neural crest cells (NCCs) comprise a transient and multipotent cell population unique to vertebrates. In the developing embryo, NCCs give rise to a variety of cell types essential for normal embryogenesis, including neurons, muscle cells, adipocytes, melanocytes, fibroblasts, chondrocytes, osteoblasts and osteocytes (Hall, 1999; Le Douarin and Kalcheim, 1999; Thorogood, 1993). During craniofacial morphogenesis, cranial neural crest (CNC) cells give rise to craniofacial skeleton including maxillary and mandibular bones (Chai et al., 2000; Jiang et al., 2002). CNC cells follow distinct pathways and migrate towards their ventrol-lateral destination, contributing to a series of segments, the branchial arches 1–6 (BA). BA1 is further divided into the maxillary processes and the mandibular processes. The maxillary processes, in coordination with medial and lateral nasal processes, develops into the upper jaw, and the mandibular processes give rise to the lower jaw (Lee et al., 2004). Consequently, the maxillary and mandiblular processes differentiate into characteristic tissues, such as the secondary palate (from the maxillary process) or the dentary bone (from the mandibular process), respectively. Disruption of CNC development often causes craniofacial malformations, the most common birth defects in humans. Understanding the regulatory mechanisms of CNC development is thus crucial for prognosis and development of novel therapeutic strategies against these craniofacial anomalies.
Syngnathia, a birth defect, is defined as abnormal fusion of upper and lower jaws by either fibrous or bony tissues. Clinically, bony syngnathia (fusion of the jaws by bone) is extremely rare, and is often associated with anomalies of other CNC derivatives, such as cleft palate, aglossia, and hypoplasia of the mandible (Knoll et al., 2000), suggesting a role of CNC cells in this birth defect. However, the etiologies of congenital bony syngnathia in human remain largely unknown because of lack of evidence of any family tendency, drug or toxin exposure, or consanguinity for this condition (Dawson et al., 1997; Knoll et al., 2000; Shah and Desai, 2013). While an involvement of vitamin A in such condition was proposed, as evidenced by the induction of bony syngnathia with clefting of the secondary palate in rats administrated with a large dose of vitamin A (Nanda, 1970), very few animal models with genetic alteration for such defect have been described. A recent study reveals that deficiency of Foxc1 and Fgf8 causes bony syngnathia phenotype in mouse models (Inman et al., 2013). Interestingly, vitamin A, Foxc1, and Fgf8 have been shown to interact with BMP signaling (Baleato et al., 2005; Inman et al., 2008; St Amand.et al, 2000; Sun et al., 2013), suggesting a possible role for altered BMP signaling activity in this birth defect.
Bone morphogenetic proteins (BMPs) play pivotal roles in development and disease (Bandyopadhyay et al., 2013). Activation of BMP signaling is engaged by binding of BMP ligands to the type I (BMPRIa, BMPRIb, or ActRIa) and type II (BMPRII) transmembrane receptor complex, leading to phosphorylation of cytoplasmic adaptors Smad1/5/8. Phosphorylated Smad1/5/8 subsequently bind to Smad4 and translocate into the nucleus, where the Smad complex regulates target gene transcription and controls cell fate and behavior. In addition to this Smad-dependent (canonical) pathway, ligand-bound receptor complex also activates the mitogen-activated protein kinse (MAPK) pathways, known as non-canonical pathway. During development and homeostasis, BMP signaling is tightly regulated by various mediators at multiple levels, to maintain its activity at a proper level (Balemans and Van Hul, 2002; Botchkarev and Sharov, 2004; Yanagita, 2009). At the extracellular level, Noggin, Chordin, Twsg1, Follistatin, Cerberus, Ectodin, and Gremlin act BMP antagonists to modulate BMP ligand-receptor binding. At the cytoplasmic level, Smurf mediates the level of Smad1/5/8 and their availability, and Smad6/7 interfere their phosphorylation. In the nucleus, Ski, SNIP1 and other transcriptional co-factors interact with Smad complexes and operate their binding to target gene promoter (Bandyopadhyay et al., 2013). Dysregulation of BMP signaling usually leads to abnormal development and diseases.
The essential and conserved roles for BMP signaling in craniofacial development have been demonstrated across the species in vertebrates (Nie et al., 2006; Parada and Chai, 2012). In birds, Bmp4 is an essential regulator to control the beak morphology of Darwin’s finch (Abzhanov et al., 2004; Wu et al., 2004). In fish, Bmp4 was shown to control craniofacial skeleton formation and patterning (Albertson et al., 2005). In humans, mutations in BMP4 or its downstream target gene MSX1 are implicated in cleft lip/palate formation (Suzuki et al., 2009; van den Boogaard et al., 2000). On the other hand, single-nucleotide polymorphism of NOGGIN has been linked to mandibular hypoplasia (Gutiérrez et al., 2010). In mice, gene dosage of BMP signaling pathway components has been shown to correlate with the size and shape of mandible (Boell et al., 2013). Tissue specific deletion of Bmp4 by Nestin-Cre in craniofacial epithelium and mesenchyme causes cleft lip and inactivation of BmprIa using either Nestin-Cre or Wnt1Cre results in the formation of both cleft lip and cleft palate (Li et al., 2011; Liu et al., 2005). On the other hand, mice lacking BMP antagonists, such as Noggin, Chordin, or Twsg1, exhibit a spectrum of craniofacial abnormalities, including cleft palate, hypoplastic/absent mandible, and facial truncation (He et al., 2010; Stottmann et al., 2001). These studies indicate that a finely tuned BMP signaling level is required for normal craniofacial formation. In addition to BMP signaling, recent advances in craniofacial studies and availability of genetically modified mouse models have revealed an increasingly complicated regulatory network in the facial skeleton patterning and the involvement of additional signaling pathways (Chai and Maxson, 2006).
In this study, we investigated the effect of gain-of-function of BMP4-mediated signaling in CNC lineage in a transgenic mouse model. We found that mice with targeted Bmp4 expression in CNC cells exhibit bony fusion between the maxillary and mandibular skeletons, as well as cleft palate and hypoplastic mandible, resembling congenital bony syngnathia in humans.
Materials and Methods
Animals
Conditional Bmp4 transgenic animal (pMes-Bmp4) was generated by pronuclear injection of the pMes-IRES-Egfp vector containing the full-length cDNA of mouse Bmp4, as described before (He et al., 2010; Xiong et al., 2009). The pMes-Bmp4 transgenic vector was constructed by inserting the Bmp4 sequence into the site downstream of the β-actin promoter and a Loxp flanked STOP cassette and the upstream of the Ires-Egfp sequences. Transgenic founders and transgenic mice were identified by a PCR based genotyping. The generation and genotyping of pMes-caBmprIa, Wnt1Cre, and Osr2Cre mice have been described before (Danielian et al., 1998; He et al., 2010; Lan et al., 2007). pMes-Bmp4, pMes-caBmprIa, and Wnt1Cre were maintained on the CD1 outbred genetic background, and Osr2Cre was maintained in the C57BL/6J background. The animal studies described in this paper were approved by the Tulane University Institutional Animal Care and Use Committee and by the Animal Use Committee of Fujian Normal University.
Histology, immunostaining, and in situ hybridization
For histological analysis, staged embryos were collected from pregnant mice and fixed in 4% paraformaldehyde (PFA) in ice-cold PBS for overnight. The yolk sac was used for genotyping. The fixed embryos were then dehydrated with gradient ethanol washes and embedded in paraffin. Coronal section were cut at 10 µm thickness and subjected to Hematoxylene and Eosin (H&E) or Azon red/Aniline blue staining (Presnell and Schreibman, 1997), or to immunostaining using anti-Ki67 antibody (Spring Bioscience) following the Manufacturer’s instruction, or to section in situ hybridization as described previously (St. Amand et al., 2000). For whole-mount in situ hybridization, staged mouse embryos were fixed in PFA and bleached in 6% H2O2 before being dehydrated in methanol. Whole-mount in situ hybridization was performed as described previously (Zhang et al., 1999). All in situ hybridization assays were performed three times independently.
Alkaline phosphotase staining, Alcian blue staining, and Tunel assay
Paraffin-embedded and sectioned samples were rehydrated and subjected to BM Purple AP Substrate staining. Alcian blue staining were carried out as described previously (Li et al., 2004), and Tunel assay was conducted as reported previously (Alappat et al., 2005).
Skeletal preparation
Pups were collected at postnatal day 0 (P0). Bone and cartilage were stained with Alizarin red and Alcian blue as described previously (Zhang et al., 2000).
Results
Forced expression of Bmp4 in CNC lineage causes congenital bony syngnathia and hypoplastic craniofacial skeletons in mice
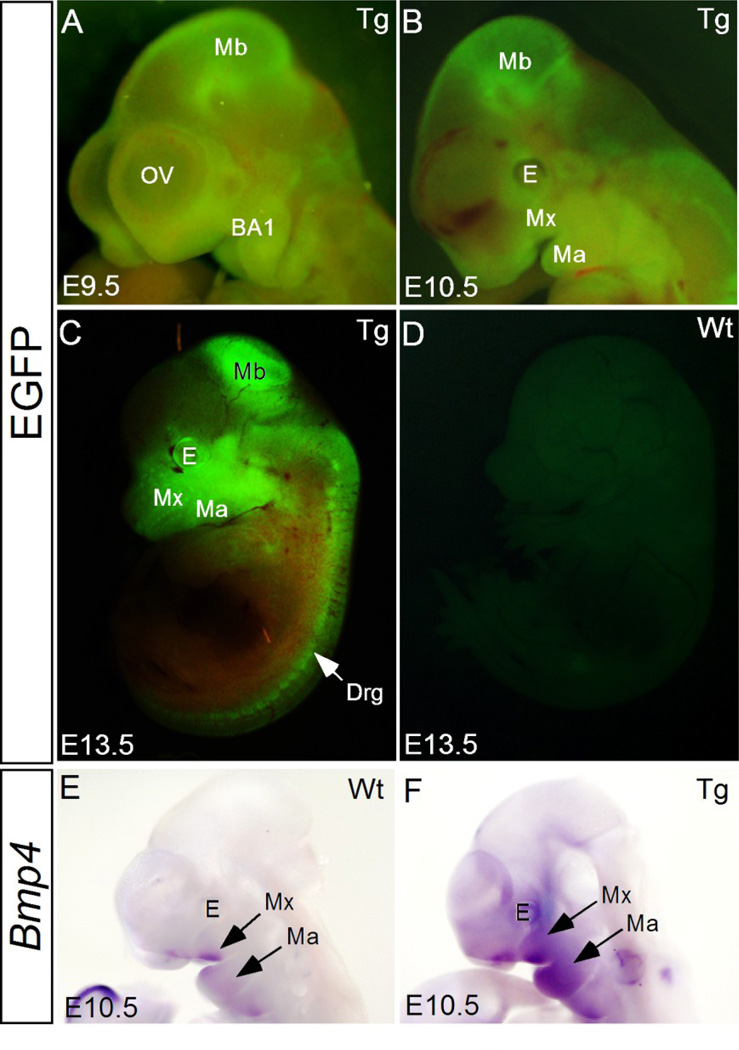
We have shown previously that elevated BMP signaling, either by inactivating Noggin or by forced expression of a constitutively active form of BMPRIa (BmprIa) in CNC lineage or in epithelium, disrupts normal development of the palate and tooth (He et al., 2010; Li et al., 2013). To further investigate the potential role of BMP signaling in craniofacial morphogenesis, we generated pMes-Bmp4 conditional transgenic mice. Out of total eleven founders that have been generated, five transgenic lines were established with germline transmission. When crossed to Wnt1Cre mice, lines 1, 2, and 4 produced double transgenic embryos (Wnt1Cre;pMes-Bmp4) with reproducible phenotype. The double transgenic embryos from line 3 or 5 did not show a distinguishable phenotype. Line 1 was thus used to generate Wnt1Cre;pMes-Bmp4 mice in this study. Since the transgenic allele harbors an Ires-Egfp cassette downstream of the Bmp4 transgene, removal of the STOP cassette by Cre recombinase activates the expression of Bmp4 and Egfp simultaneously, allowing tracing of neural crest cell migration. We found that at embryonic day 9.5 (E9.5), Bmp4-expressing CNC cells have already populated in the neural crest derived tissues that have been well characterized in other studies, such as the craniofacial mesenchyme including the maxillary and mandibular processes, periocular mesenchyme, and midbrain (Fig. 1A), suggesting that Bmp4-overexpression did not halt migration of CNC cells to their targeted areas. Strong Egfp expression remained in these organs/tissues at E10.5 and E13.5 (Fig. 1B and C). In situ hybridization assay further confirmed ectopic Bmp4 expression in the CNC-derived tissues, particularly in the facial primordia including the maxillary and mandibular prominences in Wnt1Cre;pMes-Bmp4 embryos at E10.5 (Fig. 1E and F). The slight variation of the Bmp4 expression and the EGFP expression pattern in the transgenic mice could arise from the low sensitivity of in situ hybridization and the penetration of in situ probe.
Figure 1. Wnt1Cre directed Bmp4 expression in the neural crest cells.
(A–D) Migration of transgene-expressing CNC cells, evidenced by Egfp expression, to their targeted destinations at E9.5 (A), E10.5 (B), and E13.5 (C), as compared to the lack of Egfp expression in E13.5 wild type control (D). (E, F) At E10.5, Bmp4 expression is restricted to the distal end of the frontonasal process, maxilla, mandible, and the second pharyngeal arch in wild type control (E), but was extensively expressed throughout the maxilla and mandible, and other neural crest derivatives in Wnt1Cre;pMes-Bmp4 embryo (F). E, eye; BA1, the first branchial arch; Ma, mandible; Mb, midbrain; Mx, maxilla; OV, optical vesicle; drg, dorsal root ganglion.
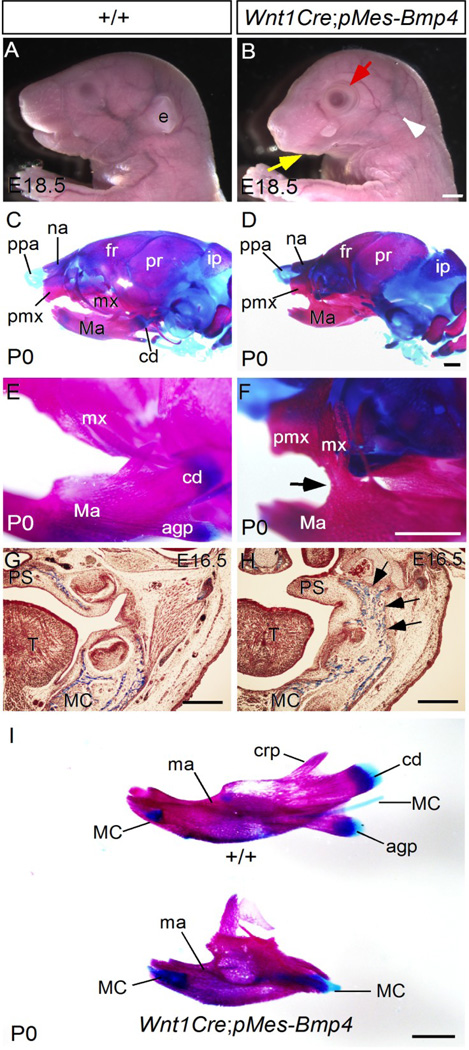
Gross morphological examination of Wnt1Cre;pMes-Bmp4 mice at E18.5 identified severe craniofacial developmental defects including a shortened snout, open eyelid, missing external ear, and an agnathia-like phenotype, as compared to controls (Fig. 2A and B). Skeleton preparations further revealed defects in a number of CNC derived skeletal elements, including the hypoplastic nasal bone, frontal bone, maxilla, and mandible, as well as the absence of the condyle, coronoid process, and angular process, as compared to controls (Fig. 2C–F and I). Strikingly, bony fusion of the jaws was also observed (Fig. 2F; 4/4 skeleton preparations), resembling the congenital bony syngnathia in humans. The bony structures connecting the maxilla and the mandible appeared to be derived from the posterior portion of the maxilla (Fig. 2F). This bony fusion of the jaws could be identified as early as E16.5 (Fig. 2G and H), and was present in all samples examined (n > 20). TMJ agenesis is a common malformation observed in patients afflicted with syngnathia, and this phenotype at the jaw joints is also found in Wnt1Cre;pMes-Bmp4 mice (data not shown).
Figure 2. Craniofacial defects in Wnt1Cre;pMes-Bmp4 mice.
(A, B) Whole mount view of control (A) and Wnt1Cre;pMes-Bmp4 head (B) at E18.5. The mutant embryo shows microagnathia (yellow arrow), open eye lid (red arrow) and absence of eternal ear (blank arrowhead). (C–F) Skeletal preparations of wild type control (C, E) and Wnt1Cre-pMes-Bmp4 mice at P0 show hypoplastic frontal bone, mandible, maxilla, and bony fusion (arrow in F) between the maxilla and mandible in Wnt1Cre;pMes-Bmp4 mice. (G, H) Coronal sections of E16.5 (G) control and Wnt1Cre;pMes-Bmp4 head reveal bony structures (arrows in H) between the mandible and maxilla of Wnt1Cre;pMes-Bmp4 mice. (I) Skeletal preparations of P0 control and Wnt1Cre;pMes-Bmp4 mandible show hypoplastic mandible, and absence of the angular process, condyle, and coronoid process. Agp, angular process; cd, condyle; crp, coronoid process; ee, external ear; fr, frontal bone; ip; interparietal bone; ma, mandible; MC, Meckel’s cartilage; mx, maxilla; na, nasal bone; pmx, premaxilla; ppa, prominentia pars anterior; and pr, parietal bone. Scale bar = 500 µm.
Wnt1Cre;pMes-Bmp4 mice exhibit mandible agenesis accompanying by abnormal osteoblast differentiation and ectopic apoptosis
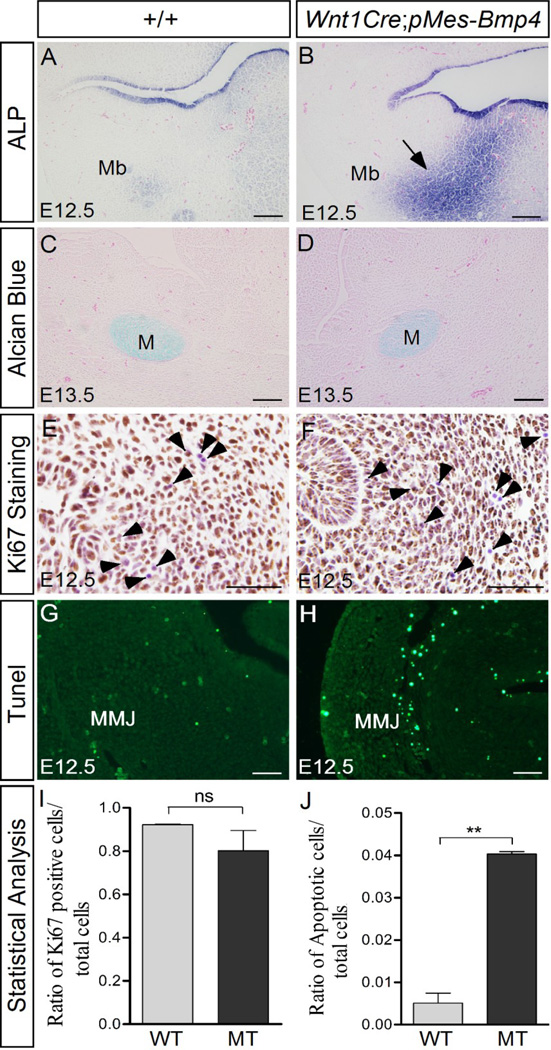
To understand the cellular mechanisms underlying the bony syngnathia, alkaline phosphatase (ALP) activity was assayed in the mandible and maxillo-mandibular junction. At E12.5, the wild type controls exhibit only background level staining in the mandible mesenchyme (Fig. 3A). Remarkably, strong ALP staining was detected in the mutant mandible (Fig. 3B). This strong ALP staining was also seen in the mutant mandible at E13.5 (data not shown). On the other hand, Alcian blue staining results did not reveal distinguishable phenotype in the mutant mandible at both E12.5 and E13.5 (Fig. 3C, D; and data not shown). These results indicate that Bmp4 expression in the CNC lineage enhances the osteoblast differentiation in the mutants, but does not facilitate cartilage formation. Cell proliferation and apoptosis levels were further evaluated in the control and mutant embryos. Immunostaining result with anti-Ki67 antibody revealed a slight decrease of cell proliferation rate in the mutant maxillo-mandibular junction at E12.5 (Fig. 3E, F and I). At the same time, Tunel assay demonstrated abundant ectopic cell apoptosis in the mutant maxillo-mandibular junction as compared to wild type controls (Fig. 3G, H and J). These observations indicate that over-dosed BMP4 leads to premature and enhanced ossification, which could contribute to bony syngnathia. The elevated cell apoptosis in the transgenic maxillo-mandibular junction appears to be a major cellular mechanism underlying the agnathia-like phenotype in the transgenic animals.
Figure 3. Wnt1Cre-driven Bmp4 expression causes abnormal osteoblast differentiation and apoptosis in the mandible.
(A, B) Alkaline phosphatase (ALP) staining reveals moderate osteoblast differentiation in the control mandible at E12.5 (A), but strong ALP activity in the mutant mandible mesenchyme (arrow in B). (C, D) Alcian blue staining results show comparable chondrocyte formation in the control and mutant mandible. (E, F, I) Immunostaining with anti-Ki67 antibody reveals a slight decrease of cell proliferation level in the mutant mandible. Arrowheads point to Ki67-negative cells that were counterstained by Hematoxylene (purple). (G, H, and I) Tunel assay shows abundant ectopic cell apoptosis in the mutant maxillo-mandibular junction. M, Meckel’s cartilage; Mb, mandible; MMJ, maxillo-mandibular junction. Scale bar = 100-µm in (A–D), and = 50-µm in (E–H).
Expression of Bmp4 in CNC lineage disrupts patterning of the secondary palate and results in cleft palate formation
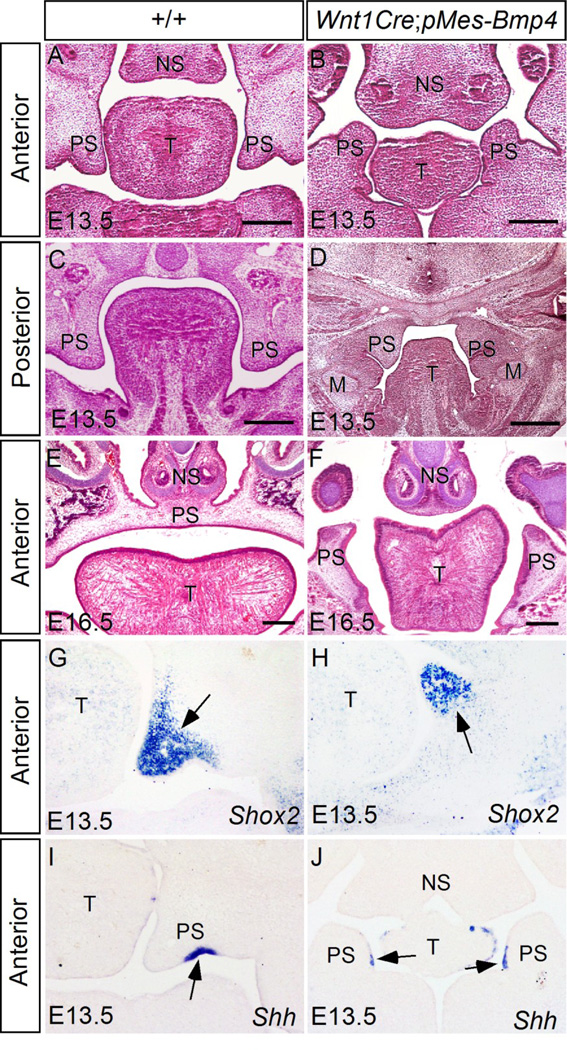
Since congenital bony syngnathia is often accompanied with cleft palate and other craniofacial abnormalities, we conducted histological analysis on palatogenesis of Wnt1Cre;pMes-Bmp4 embryos. At E13.5, in wild type embryos, the maxilla-derived secondary palatal shelves are well formed aside the tongue laterally, with their anterior tips pointing ventrally toward the mandible along the anterior-posterior axis (Fig. 4A and C). In contrast, at this stage in transgenic animals, the anterior portion of the palatal shelves (anterior to the first molar) did not grow out from the maxilla, instead, a pair of palatal shelf-like structures originating from the mandible was identified, which adopted a reversed direction with their tip pointing dorsally toward the nasal septum (Fig. 4B). At the posterior level, the mutant palate shelves appeared smaller and fused with the mandible abnormally (Fig. 4D). The palate phenotype was consistently observed in mutant embryos at E16.5 when control embryos developed an intact palatal shelf separating the primitive oronasal cavity into two individual spaces, the mutant palatal shelf-like structures remained separated and pointing dorsally along the tongue at the anterior level (Fig. 4E and F). To determine if the protrusions from the anterior mandible in mutant embryos are indeed palatal tissue, we examined the expression of palatal tissue markers by in situ hybridization. Shox2 is expressed restrictedly in the anterior palatal mesenchyme from the onset of palatogenesis in wild type embryo (Yu et al., 2005; Fig. 4G). Similarly, Shox2 expression was detected in the protrusions from the transgenic mandible (Fig. 4H). During normal palatogenesis, Shh is expressed in the palate epithelium at the future oral side (ventral side) (Fig. 4I). In mutant embryo, Shh mRNA was detected in the medial side epithelium of the mandibular protrusions, an orientation identical to the control (Fig. 4J). These results confirm the palatal tissue origin of the mandibular protrusions, suggesting that Bmp4 expression in CNC cells may have altered the destination of a subgroup of CNC cells that supposed to migrate to the maxillary region, but does not affect their developmental fate.
Figure 4. Wnt1Cre;pMes-Bmp4 mice exhibit reversed growth of palatal shelves from the mandible and cleft palate defect.
(A–F) Histology analyses of wild type control (A, C, E) and Wnt1Cre;pMe-sBmp4 (B, D, F) embryos at E13.5 (A–D) and E16.5 (E, F) reveal reversed growth of the palatal shelves from the mandible at the anterior portion (B) and abnormal fusion of palatal shelves with the mandible at the posterior portion (D), as well as cleft palate defect (F) in Wnt1Cre;pMes-Bmp4 mice. (G–J) In situ hybridization shows expression of Shox2 (G, H) and Shh (I, J) in the anterior palatal shelves of E13.5 control (G, I) and Wnt1Cre;pMes-Bmp4 (H, J) embryos, confirming the aberrant orientation of the anterior palatal shelves in the Wnt1Cre;pMes-Bmp4 mice. Arrows in (G–J) point to the gene expression domains. M, Meckel’s cartilage; T, tongue; NS, nasal septum, PS, palatal shelf. Scale bar = 100 µm.
Mis-patterned maxillary and mandibular processes in Wnt1Cre;pMes-Bmp4 embryos
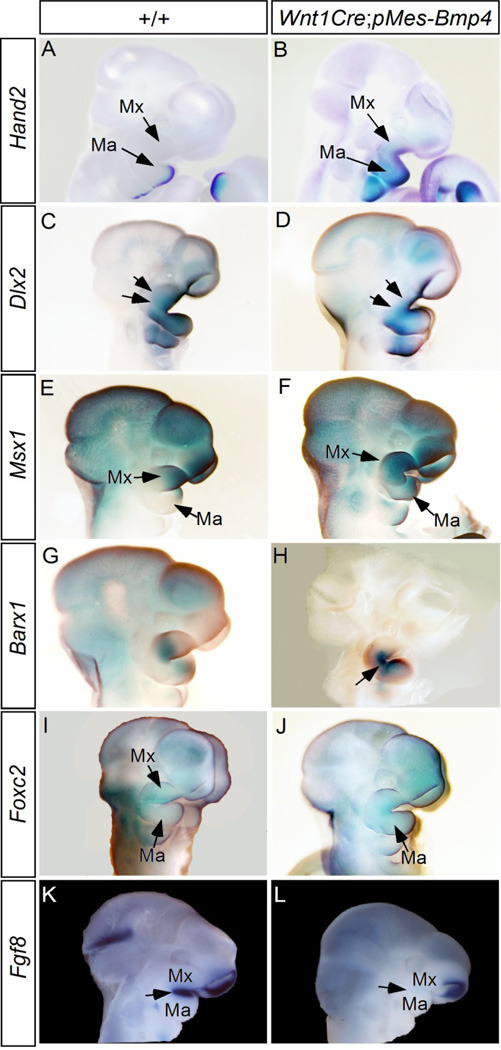
The conversion of the posterior maxilla to the fusion bony structures and the reversed growth of the anterior palatal shelves from the mandible suggest mis-patterned CNC-derived maxillary and mandibular processes in Wnt1Cre;pMes-Bmp4 mice. To test this hypothesis, we conducted whole-mount in situ hybridization to assay the expression patterns of a number of maxillary and mandibular landmark genes in E10.5 embryos. Hand2, a downstream target of Bmp4, is expressed in the mandibular mesenchyme specifically at the distal aspect in wild type embryo (Howard et al., 2000; Xiong et al., 2009) (Fig. 5A). However, in the mutants, Hand2 expression domain expanded to the entire mandible and was ectopically activated in the maxillary prominence (Fig. 5B). Dlx genes play crucial roles in patterning the first branchial arch (Depew et al., 2005). Dlx2 is essential for normal maxillary development, and Dlx2 mRNA is expressed in the maxillary and mandibular ectoderm in wild type embryo (Depew et al., 2005; Qiu et al., 1995) (Fig. 5C). Our results show that expression of Bmp4 in CNC cells attenuated Dlx2 expression in the maxilla but did not affect its expression in the mandible (Fig. 5D). Previous studies have established Msx1 as a Bmp4 target gene and its essential role in normal palate development and tooth morphogenesis (Chen et al., 1996; Satokata and Maas, 1994; Zhang et al., 2002; Zhang et al., 2009). In controls, Msx1 was expressed in the distal portion of the maxilla and mandible (Fig. 5E), but in the mutants, Msx1 expression domain expanded to the entire maxilla and mandible (Fig. 5F). Barx1 is normally expressed in the proximal mesenchyme of the mandible and maxilla but is not in the maxillo-mandibular junction (MacKenzie et al., 2009; Tissier-Seta et al., 1995) (Fig. 5G). In the mutant, ectopic Barx1 expression was found in the maxillo-mandibular junction (Fig. 5H). Foxc2 was expressed in the mesenchyme of the maxilla of control embryo (Fig. 5I), but its expression domain extended to in the mandibular process of transgenic embryo (Fig. 5J). In the “Hinge and Caps” model for the jaw organization and patterning, it was proposed that the Fgf8-expressing oral ectoderm exerts its positional information to pattern and maintain gene expression in the jaw-forming CNCs (Depew and Compagnucci, 2008). Since Fgf8 deficiency causes syngnathia phenotype, and BMP signaling functions as an antagonist of Fgf8 during craniofacial development, we also examined Fgf8 expression (Inman et al., 2008; St Amand et al, 2000). At E10.5, Fgf8 is expressed in the ectoderm covering maxillary and mandibular processes in controls (Fig. 5K), but in the mutant, Fgf8 expression in these domains was barely detected (Fig. 5L). Thus the loss of Fgf8 expression in the oral ectoderm could also be responsible to the altered expression patterns of those genes reported above. On the other hand, the expression patterns of Pitx1, Axin2, Dlx1, Dlx5, and Dlx6 in the mutant embryos remained comparable to that in the controls (Fig. S1). These observations indicate altered patterning of the maxillary and mandibular processes in Wnt1Cre;pMes-Bmp4 mice, and that the dysregulated expression of these genes may underlie the facial defects observed.
Figure 5. Altered expression of maxillo-mandibular landscape genes in E10.5 Wnt1Cre;pMes-Bmp4 embryos.
(A, B) Hand2 is expressed in the distal region of the control mandible and absent from the maxilla (A), but its expression domain expands to the maxilla, the secondary pharyngeal arch, and the proximal region of the mandible in Wnt1Cre;pMes-Bmp4 embryo (B). (C, D) Dlx2 expression is detected in the maxilla (arrows), mandible, and second pharyngeal arch in control embryo (C), but is specifically attenuated in the maxilla (arrows) and the distal region of the mandible and the second pharyngeal arch (D). (E, F) Msx1 expression is restricted to the distal region of the frontonasal process, maxilla, and mandible in the wild type embryos (E), but is expanded to the proximal domain of the maxilla and mandible, as well as the secondary pharyngeal arch in Wnt1Cre;pMes-Bmp4 embryo (F). (G, H) Barx1 is expressed in the proximal region of the maxilla and mid-portion of the mandible in control (G), but its expression is activated in the proximal region of the mandible and in the maxillo-mandibular junction (arrow) of mutant (H). (I, J) Foxc2 is expressed in the maxilla but not in the mandible of control embryo (I), but is ectopically activated in the mandible of Wnt1Cre;pMes-Bmp4 embryo (J). (K, L) Fgf8 expression (arrow) is detected in the epithelium of the maxilla and mandible in control embryo (K), But in the mutant, Fgf8 expression (arrow) in these tissues is barely detected. Note retained Fgf8 expression in the nasal placode in the mutant. Ma, mandible; Mx, maxilla.
Activation of BmprIa-mediated signaling in CNC lineage causes severe but distinct craniofacial phenotype
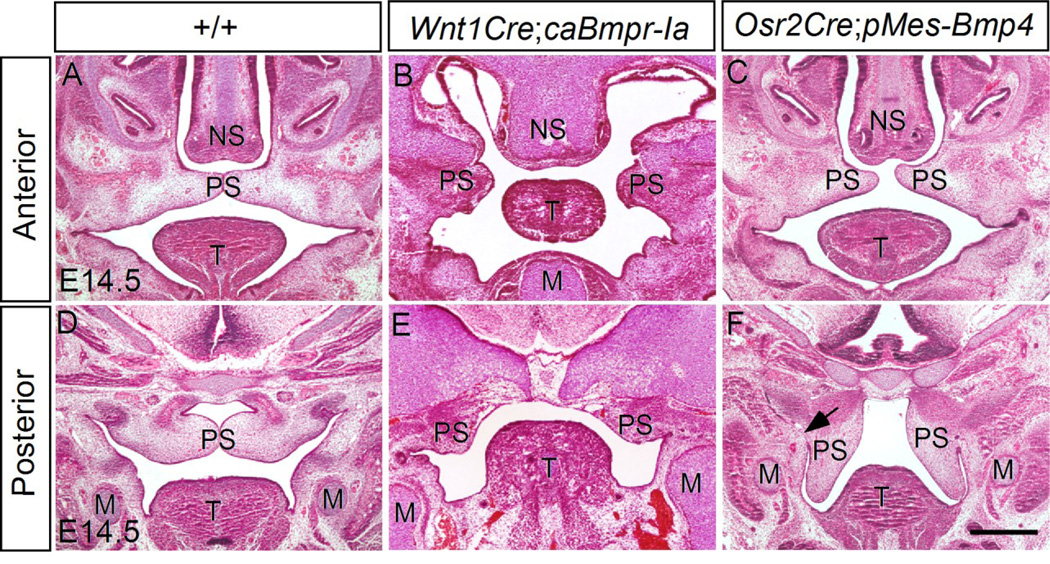
BMP signaling is primarily transduced through the receptor complex of BMPR-II and one of the three type I BMP receptors (BMPR-Ia, BMPR-Ib, ActRIa). Among these type I receptors, BmprIa has been shown to play an essential role in craniofacial development (Li et al., 2011; Li et al., 2013; Liu et al., 2005). Since Wnt1Cre-driven expression of a constitutively active form of BMPR-IB (caBmprIb) did not produce any visible phenotype in mice (Li et al., 2011), we reevaluated craniofacial phenotype of mice expressing caBmprIa in CNC lineage (Wnt1Cre;pMes-caBmprIa) mice and confirmed a lack of syngnathia-like phenotype in these mice (data not shown). Histological analysis also confirmed the origin of palatal shelves, although dramatically deformed, from the maxillary region in Wnt1Cre;pMes-caBmprIa mice (Fig. 6B, E). To investigate if the “inverted” palate phenotype in Wnt1Cre;pMes-Bmp4 mice is an intrinsic defect of the palatal shelves or a consequence of mis-patterned palatal progenitor cells, ectopic Bmp4 expression was induced specifically in the palatal mesenchyme by compounding the pMes-Bmp4 transgenic allele to the Osr2Cre allele that is expressed specifically in the palatal mesenchyme at the onset of palatogenesis (Lan et al., 2007). Despite of malformed palatal shelves in Osr2Cre;pMes-Bmp4 embryos, their origin from the maxillary processes remained unaltered (Fig. 6C, F). However, abnormal fusion of the posterior palatal shelves with the mandible was identified in Osr2Cre;pMes-Bmp4 mice (Fig. 6F), an identical phenotype that was observed in mice carrying mutations in either Hand2 or Noggin, or ectopic expression of BmprIa in the palatal epithelium (He et al., 2010; Xiong et al., 2009). Taking together, these results suggest that the augmented Bmp4 expression in CNC cells appears to effect in a non-cell autonomous manner to alter the destination of a sub-population of CNC cells that are already committed to the fate of the secondary palatal shelves, although we could not rule out the possibility that the different phenotypes reflect the variant levels of BMP signaling activity between Wnt1Cre-pMesBmp4 and Wnt1Cre;pMes-caBmprIa mice.
Figure 6. Elevated BMP signaling leads to cleft palate and abnormal fusion of palatal shelves with the mandible.
(A, D) The control secondary palatal shelves has completed elevation and initiated fusion to form an intact palatal shelf at both anterior (A) posterior level (D). (B, E) Wnt1Cre;pMes-caBmprIa embryo shows cleft palate at both the anterior (B) and posterior level (E). The palatal shelves are severely hypoplastic as compared to the control. (C, F) Osr2Cre;pMe-sBmp4 embryo exhibits a cleft palate phenotype. At the anterior level, the palatal shelves elevated but failed to contact (C); at the posterior level, the palatal shelves remains at the vertical orientation, showing abnormal fusion with the mandible (F). M, Meckel’s cartilage; T, tongue; NS, nasal septum; PS, palatal shelf. Scale bar = 500 µm.
Discussion
Syngnathia is rare but severe craniofacial malformation in human births, and the underlying mechanisms remain largely unknown. In the present study, we show that Wnt1Cre driven Bmp4 expression in NCCs causes bony fusion between the maxilla and mandible, as well as cleft palate and hypoplastic maxillary and mandibular processes, resembling the congenital bony syngnathia syndrome in humans. We further dissected the etiologies underlying this syngnathia model by examining the cellular mechanisms, as well as expression of facial landmark genes and BMP signaling targets. Our results suggest that the augmented BMP4-mediated signaling may act in a non-cell autonomous manner to mis-guide the migration of a subgroup of CNC cells to the facial destination, accompanied by altered expression of several facial patterning genes. Our studies present a unique animal model for human bony syngnathia and provide potential mechanisms underlying its formation.
Syngnathia and abnormal BMP signaling activity
Syngnathia presents abnormal maxillo-mandibular adhesion in the form of soft tissue (synachiae) or bony tissue (synostosis) or a combination of both (Knoll et al., 2000). While the etiologies of this birth defect remain to be elucidated, our previous and present studies have implicated dysregulated BMP signaling pathway in such birth defect. We have shown previously that an abnormal fusion of the posterior palatal shelves with the mandible, a synachiae-like phenotype, occurs in genetically-engineered mouse models harboring aberrant BMP signaling or its downstream target gene (He et al., 2010; Xiong et al., 2009). This phenotype is recapitulated in Osr2Cre;pMes-Bmp4 embryos. On the other hand, augmentation of BMP4-mediated signaling in CNC cells leads to a much severe phenotype, synostosis or bony syngnathia. Besides synostosis, Wnt1Cre;pMes-Bmp4 mice also exhibit a series of craniofacial defects, including cleft palate and hypoplastic maxilla and mandible, which are also observed in patients carrying syngnathia (Broome et al., 2013; Knoll et al., 2000; Parkins and Boamah, 2009). These studies demonstrate that dysregulated BMP signaling or its downstream targets could lead to both synachiae and synostosis, making components of BMP signaling pathway candidate genes for such human congenital disease. A recent study showed that deficiency of Foxc1 or Fgf8 also causes bony syngnathia in mice (Inman et al., 2013). Foxc1 acts to reduce the phosphorylation level of Smad1/5/8, and repress BMP-dependent gene expression in the osteogenic precursor cells (Sun et al., 2013). Specifically in the jaw, Foxc1 deficiency leads to decreased Fgf8 expression in the oral ectoderm of the first pharyngeal arch. Mice carrying compound mutations in both Foxc1 and Fgf8, or in Fgf8 alone exhibit bony syngnathia phenotype, indicating a genetic interaction between these two genes in jaw development. In current study, we found a decreased Fgf8 expression in the oral ectoderm of Wnt1Cre;pMes-Bmp4 mice, suggesting an integration of BMP signaling into the Foxc1-Fgf8 pathway in regulating jaw development. In addition, the altered Fgf8 expression in the Wnt1Cre;pMes-Bmp4 oral ectoderm also suggests a non-cell autonomous effect of Bmp4 expression in syngnathia formation, since ectopic Bmp4 expression is driven by Wnt1Cre in neural crest derived mesenchymal cells.
BMP signaling and NCC development
NCC development involves several distinct steps, including induction, migration, and differentiation. Extensive studies have pinpointed to the essential role of BMP signaling in NCC induction across the species as well as its involvement in the regulation of migration and differentiation (Stuhlmiller and García-Castro, 2012). Despite of the expression of BmprIa in the premigratory NCCs, tissue specific inactivation of BmprIa in premigratory NCCs does not affect NCC induction and migration (Panchision et al., 2001; Morikawa et al., 2009). However, in Bmp2-null mice, NCCs form but fail to migrate (Zhang et al., 1996; Kanzler et al., 2000; Correia et al., 2007). Lineage tracing studies have revealed the contribution of CNC cells to facial skeletons, with CNC cells derived from the forebrain and rostral midbran colonizing the frontonasal region, and the caudal midbrain-derived CNC cells populating the maxillary component of the BA1 and giving rise to the upper jaw and palatal mesenchyme, while the hindbrain-derived CNC cells producing the lower jaw, hyoid bone, and adjacent regions of the neck (reviewed in Trainor, 2010). The segregation of distinct CNC cell populations and the precise and region-specific migratory pathways are established and tightly regulated by the axial registration (such as Hox gene expression) of CNC cells and their associated mesodermal and ectodermal cells during induction and migration, as well as environmental cues (Noden, 1991; Trainor and Tam, 1995; Trainor, 2010). However, it has not yet been reported that if BMP signaling is involved in the regulation of guiding the migration of CNC cells to their final destinations. Since the majority of CNC cells are unipotent and multipotential cells with fairly restricted developmental potential (Baroffio et al., 1991), our results suggest that Wnt1Cre directed Bmp4 expression may alter the normal patterning of maxilla and mandible, and very likely direct a subgroup of palatal progenitor cells to the mandibular process. Further lineage tracing studies are required to test this hypothesis. BMP4 protein is diffusible and its function is mediated in a cell autonomous or non-cell autonomous fashion, depending on the expression of its receptors. However, forced expression of caBmprIa by the Wnt1Cre allele in CNC cells, despite of producing craniofacial abnormalities, was not able to recapitulate the craniofacial phenotype in Wnt1Cre;pMes-Bmp4 mice, suggesting that the non-cell autonomous manner of overexpressed Bmp4 may be responsible primarily for the production of the observed phenotypes. It is possible that BMP receptors are expressed in the ectoderm, mesoderm, and endoderm tissues with which the CNC cells interact, and signaling of BMP4 to these tissues alters their axis registration, leading to mis-patterned facial identity. This hypothesis is further supported by our data showing decreased Fgf8 expression in Wnt1Cre;pMes-Bmp4 mice. However, since BmprIa is also expressed in NCCs (Panchision et al., 2001), a synergistic effect of both non-cell autonomous and cell autonomous actions cannot be ruled out. Additionally, such differential craniofacial phenotypes observed in Wnt1Cre;pMes-caBmprIa and Wnt1Cre;pMes-Bmp4 could also reflect the differential activity levels of BMP signaling in these mutants.
BMP signaling and Maxillo-mandibular patterning and development
Although the essential role for BMP signaling in bone formation has been well appreciated, the formation of hypoplastic mandible in Wnt1Cre;pMes-Bmp4 and the agnathia phenotype in Noggin+/−/Chordin−/− and Twsg1−/− mice (Stottmann et al., 2001) indicate that overdosed BMP activities are detrimental to facial skeletal formation. It has been demonstrated that overdosed BMP signaling results in dramatic apoptosis in NCC cells and their derivatives (Stottmann et al., 2001; Anderson et al., 2006; MacKenzie et al., 2009). Since the amount of CNC cells populating their final destinations is crucial for normal facial skeletal development (Trainor, 2010), it is very likely that the reduced CNC-derived cell number, apparently attributed to elevated cell apoptosis, represents the primary causative of mandibular hypoplasia in Wnt1Cre;pMes-Bmp4 mice.
Previous studies have established an essential role for Endothelin-1 (Edn1) signaling in maxillo-mandibular patterning and development, as Endothelin-A receptor (Ednra) deficiency transforms the mandible into maxillary skeleton and ectopic activation of Edn1 signaling reverses the cell fate of the maxillary skeleton into mandible in mice (Ruest et al., 2004; Sato et al., 2008). Accompanied with these cell fate transformations is the down-regulation of Dlx5, Dlx6, and Hand2 in the mandibular arch of Ednra deficient mice and ectopic activation of these genes in the maxillary process of mice expressing ectopic Edn1 signaling (Ruest et al., 2004; Sato et al., 2008), suggesting an important role of the Edn1/Ednra-Dlx5/6-Hand2 signaling pathway in maxillo-mandibular patterning. We have shown previously that BMP signaling positively regulates Hand2 expression in the developing palatal shelves (Xiong et al., 2009). Indeed, in the present study, we show that Hand2 is also ectopically activated in the maxillary region from where the palatal shelf derives in Wnt1Cre;pMes-Bmp4 mice. Despite that mis-expression of Hand2 in the Ednra domain could also induce the transformation of maxillary structures to mandible (Sato et al., 2008), however, similar phenotype was not observed in Wn1Cre;pMes-Bmp4 mice. Such a difference could be attributed to the different level of ectopic Hand2 in the maxillary region or the simultaneously dys-regulated expression of other transcription factors including Dlx2, Msx1, Barx1, and Foxc2 in Wnt1Cre;pMes-Bmp4 mice. While both BMP and Edn1 signaling pathways act upstream of Hand2, BMP signaling appears to regulate Hand2 expression independently from the Edn1 signaling in CNC cells, because the Edn1 signaling is known to regulate Hand2 expression through the mediation of Dlx5/6 whose expression was found unaltered in Wnt1Cre;pMes-Bmp4 embryos (Fig. S1). Indeed it was demonstrated that these two signaling pathways exert different functions in craniofacial morphogenesis (Zuniga et al., 2011). Nevertheless, our studies further emphasize the central importance of a tightly balanced BMP signaling in maxillo-mandibular patterning and development.
Supplementary Material
Research Highlights.
Transgenic Bmp4 expression in neural crest leads to severe craniofacial defects.
Mice carrying transgenic Bmp4 expression resemble bony syngnathia in humans.
Elevated BMP4 signaling mis-guides migration of a subgroup of palatal progenitors.
Mutations in BMP signaling pathway may be implicated in human bony syngnathia.
Acknowledgements
This work was supported by the National Institutes of Health grants (R01 DE14044, DE17792) to Y.C., and by “973” Project (2010CB944800) from the Ministry of Science and Technology of China and by grants (81100730, 30771132) from the National Natural Science Foundation of China to X.H. and Y.Z. L.Y. was supported by a scholarship from the China Scholarship Council (No. 201208440191).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen Y. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev. Biol. 2005;277:102–113. doi: 10.1016/j.ydbio.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Albertson RC, Streelman JT, Kocher TD, Yelick PC. Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proc. Natl. Acad. Sci. USA. 2005;102:16287–16292. doi: 10.1073/pnas.0506649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Stottmann RW, Choi M, Klingensmith J. Endogenous bone morphogenetic protein antagonists regulate mammalian neural crest generation and survival. Dev. Dyn. 2006;235:2507–2520. doi: 10.1002/dvdy.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular Regulation of BMP Signaling in Vertebrates: A Cocktail of Modulators. Dev. Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Baleato RM, Aitken RJ, Roman SD. Vatamin A regulation of BMP4 expression in the male germ line. Dev. Biol. 2005;286:78–90. doi: 10.1016/j.ydbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Yadav PS, Prashar P. BMP signaling in development and diseases: a pharmacological perspective. Biochem. Pharmacol. 2013;85:857–864. doi: 10.1016/j.bcp.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Baroffio A, Dupin E, Le Douarin NM. Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development. 1991;112:301–305. doi: 10.1242/dev.112.1.301. [DOI] [PubMed] [Google Scholar]
- Boell L, Pallares LF, Brodski C, Chen Y, Christian JL, Kousa YA, Kuss P, Nelsen S, Novikov O, Schutte BC, Wang Y, Tautz D. Exploring the effects of gene dosage on mandible shape in mice as a model for studying the genetic basis of natural variation. Dev. Genes Evol. 2013;223:279–287. doi: 10.1007/s00427-013-0443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–526. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- Broome M, Vial Y, Jacquemont S, Sergi C, Kamnasaran D, Giannoni E. Complete Maxillo-Mandibular Syngnathia in a Newborn with Multiple Congenital Malformations. Pediatr Neonatol. 2013 doi: 10.1016/j.pedneo.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev. Dyn. 2007;236:2493–2501. doi: 10.1002/dvdy.21256. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Dawson KH, Gruss JS, Myall RW. Congenital bone syngnathia: a proposed classification. Celft Palate Craniofac. J. 1997;34:141–146. doi: 10.1597/1545-1569_1997_034_0141_cbsapc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Compagnucci C. Tweaking the hinge and caps: Testing a model of the organization of jaws. J. Exp. Zool. (Mol. Dev. Evol.) 2008;310B:315–335. doi: 10.1002/jez.b.21205. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J. Anat. 2005;207:501–561. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez SJ, Gómez M, Rey JA, Ochoa M, Gutiérrez SM, Prieto JC. Polymorphisms of the noggin gene and mandibular micrognathia: a first approximation. Acta Odontol. Latinoamericana. 2010;23:13–19. [PubMed] [Google Scholar]
- Hall BK. The neural crest in development and evolution. Springer Verlag; 1999. [Google Scholar]
- He F, Xiong W, Wang Y, Matsui M, Yu X, Chai Y, Klingensmith J, Chen Y. Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev. Biol. 2010;347:109–121. doi: 10.1016/j.ydbio.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MJ, Stanke M, Schneider C, Wu X, Rohrer H. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development. 2000;127:4073–4081. doi: 10.1242/dev.127.18.4073. [DOI] [PubMed] [Google Scholar]
- Inman KE, Purcell P, Kume T, Trainor PA. Interaction between Foxc1 and Fgf8 during mammalian jaw patterning and in the pathogenesis of syngnathia. PLoS Genet. 2013;9:e1003949. doi: 10.1371/journal.pgen.1003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- Knoll B, Karas D, Persing JA, Shin J. Complete congenital bony syngnathia in a case of oromandibular limb hypogenesis syndrome. J.Craniofac. Surg. 2000;11:398–404. doi: 10.1097/00001665-200011040-00023. [DOI] [PubMed] [Google Scholar]
- Lan Y, Wang Q, Ovitt CE, Jiang R. A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis. 2007;45:618–624. doi: 10.1002/dvg.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin ML, Kalcheim C. The Neural Crest. London: Cambridge university press; 1999. [Google Scholar]
- Lee SH, Bedard O, Buchtova M, Fu K, Richman JM. A new origin for the maxillary jaw. Dev. Biol. 2004;276:207–224. doi: 10.1016/j.ydbio.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Li L, Lin M, Wang Y, Cserjesi P, Chen Z, Chen Y. BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development. Dev. Biol. 2011;349:451–461. doi: 10.1016/j.ydbio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Y, Lin M, Yuan G, Yang G, Zheng Y, Chen Y. Augmented BMPRIA-Mediated BMP Signaling in Cranial Neural Crest Lineage Leads to Cleft Palate Formation and Delayed Tooth Differentiation. PLoS ONE. 2013;8:e66107. doi: 10.1371/journal.pone.0066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang W, Yuan Z, Yang Y, Jia H, Cai W. A rapid and reliable double staining technique for mouse embryonic bone and cartilage. J. Chin. Med. Univ. 2004;23(2):189–190. [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- MacKenzie B, Wolff R, Lowe N, Billington CJ, Jr, Peterson A, Schmidt B, Graf D, Mina M, Gopalakrishnan R, Petryk A. Twisted gastrulation limits apoptosis in the distal region of the mandibular arch in mice. Dev. Biol. 2009;328:13–23. doi: 10.1016/j.ydbio.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and – independent pathways. Development. 2009;136:3575–3584. doi: 10.1242/dev.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda R. Maxillomandibular ankylosis and cleft palate in rat embryos. J. Dent. Res. 1970;49:1086–1090. doi: 10.1177/00220345700490051501. [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Internat. J. Dev. Biol. 2006;50:511–521. doi: 10.1387/ijdb.052101xn. [DOI] [PubMed] [Google Scholar]
- Noden DM. Cell movement and control of patterned tissue assembly during craniofacial development. J. Craniofac. Genet. Dev. Biol. 1991;11:192–213. [PubMed] [Google Scholar]
- Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–2110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C, Chai Y. Roles of BMP signaling pathway in lip and palate development. Front. Oral Biol. 2012;16:60–70. doi: 10.1159/000337617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins GE, Boamah MO. Congenital maxillomandibular syngnathia: case report. J.Cranio-Maxillo-Facial Surg. 2009;37:276–278. doi: 10.1016/j.jcms.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Presnell JK, Schreibman MP. Humason’s Animal Tissue Techniques. Fifth edition. Baltimore, MD: The Johns Hopkins University Press; 1997. [Google Scholar]
- Qiu M, Bulfone A, Martinez S, Meneses JJ, Shimamura K, Pedersen RA, Rubenstein JL. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9:2523–2538. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- Ruest LB, Xiang X, Lim KC, Levi G, Clouthier DE. Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development. 2004;131:4413–4423. doi: 10.1242/dev.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JR, Desai SN. Congenital unilateral complex bony syngnathia with non-formation of TM joint and zygomatic arch disruption, presenting at 11 years of age. J. Cranio. Max. Dis. 2013;2:59–63. [Google Scholar]
- Sato T, Kurihara Y, Asai R, Kawamura Y, Tonami K, Uchijima Y, Heude E, Ekker M, Levi G, Kurihara H. An endothelin-1 switch specifies maxillomandibular identity. Proc. Natl. Acad. Sci. USA. 2008;105:18806–18811. doi: 10.1073/pnas.0807345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- St. Amand TR, Zhang Y, Semina EV, Zhao X, Hu YP, Nguyen L, Murray JC, Chen Y. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev. Biol. 2000;217:323–332. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev. Biol. 2001;240:457–473. doi: 10.1006/dbio.2001.0479. [DOI] [PubMed] [Google Scholar]
- Stuhlmiller TJ, García-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cell. Mol. Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ishii M, Ting M-C, Maxson R. Foxc1 controls the growth of the murine frontal bone rudiment by direct regulation of a Bmp response threshold of Msx2. Development. 2013;140:1034–1044. doi: 10.1242/dev.085225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Marazita ML, Cooper ME, Miwa N, Hing A, Jugessur A, Natsume N, Shimozato K, Ohbayashi N, Suzuki Y, Niimi T, Minami K, Yamamoto M, Altannamar TJ, Erkhembaatar T, Furukawa H, Daack-Hirsch S, L'Heureux J, Brandon CA, Weinberg SM, Neiswanger K, Deleyiannis FW, de Salamanca JE, Vieira AR, Lidral AC, Martin JF, Murray JC. Mutations in BMP4 are associated with subepithelial, microform, and overt cleft lip. Am. J. Hum. Genet. 2009;84:406–411. doi: 10.1016/j.ajhg.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood P. Differentiation and morphogenesis of cranial skeletal tissues. In: Hanken J, Hall BK, editors. The skull. Vol. 1. Chicago: University of Chicago Press; 1993. pp. 112–152. [Google Scholar]
- Tissier-Seta JP, Mucchielli ML, Mark M, Mattei MG, Goridis C, Brunet JF. Barx1, a new mouse homeodomain transcription factor expressed in cranio-facial ectomesenchyme and the stomach. Mechanisms of Development. 1995;51:3–15. doi: 10.1016/0925-4773(94)00343-l. [DOI] [PubMed] [Google Scholar]
- Trainor PA. Craniofacial birth defects: The role of neural crest cells in the etiology and pathhogenesis of Treacher Collins syndrome and the potential for prevention. Am. J. Med. Genet. A. 2010;152(A):2984–2994. doi: 10.1002/ajmg.a.33454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA, Tam PPL. Cranial paraxial mesoderm and neural crest of the mouse embryo: co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development. 1995;121:2569–2582. doi: 10.1242/dev.121.8.2569. [DOI] [PubMed] [Google Scholar]
- van den Boogaard MJ, Dorland M, Beemer FA, van Amstel HK. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat. Genet. 2000;24:342–343. doi: 10.1038/74155. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Scienc. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, He F, Morikawa Y, Yu X, Zhang Z, Lan Y, Jiang R, Cserjesi P, Chen Y. Hand2 is required in the epithelium for palatogenesis in mice. Dev. Biol. 2009;330:131–141. doi: 10.1016/j.ydbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M. BMP modulators regulate the function of BMP during body patterning and disease progression. Biofactors. 2009;35:113–119. doi: 10.1002/biof.15. [DOI] [PubMed] [Google Scholar]
- Yu L, Gu S, Alappat S, Song Y, Yan M, Zhang X, Zhang G, Jiang Y, Zhang Z, Zhang Y, Chen Y. Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development. 2005;132:4397–4406. doi: 10.1242/dev.02013. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are non-viable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao X, Hu Y, Amand TS, Zhang M, Ramamurthy R, Qiu M, Chen Y. Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev. Dyn. 1999;215:45–53. doi: 10.1002/(SICI)1097-0177(199905)215:1<45::AID-DVDY5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lan Y, Chai Y, Jiang R. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science. 2009;323:1232–1234. doi: 10.1126/science.1167418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yu X, Zhang Y, Geronimo B, Lovelie A, Fromm SH, Chen YP. Targeted Misexpression of Constitutively Active BMP Receptor-IB Causes Bifurcation, Duplication, and Posterior Transformation of Digit in Mouse Limb. Dev. Biol. 2000;220:154–167. doi: 10.1006/dbio.2000.9637. [DOI] [PubMed] [Google Scholar]
- Zuniga E, Rippen M, Alexander C, Schilling TF, Crump JG. Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development. 2011;138:5147–5156. doi: 10.1242/dev.067785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.