Abstract
Background:
Malaria infestation during pregnancy is mostly asymptomatic and untreated especially in unbooked pregnancies. It presents with almost all the fetal complications of overt malaria in pregnancy. The aim of this study was to determine the effect of asymptomatic malaria parasitaemia on the neonates of unbooked parturients delivered at term at the Federal Teaching Hospital, Abakaliki.
Materials and Methods:
This study was conducted in the labour ward complex of the Federal Teaching Hospital, Abakaliki from March to May 2012. Unbooked pregnant women who fulfilled the inclusion criteria and gave consent were consecutively recruited. Cord blood and placenta tissue were collected for haemoglobin concentration determination and histology, respectively. Birth weights were determined with an electronic weighing machine. Statistical Analysis was done with 2008 Epi Info™ software and level of significant was set at P-value <0.05.
Results:
A total of 250 unbooked parturients were recruited, of which 194 (77.6%) had asymptomatic malaria parasitaemia while 227 (90.8%) had placental parasitisation. The prevalence of low birth weight in the study was 16.4%. There was significant relationship between asymptomatic malaria parasitemia and birth weight (X2 = 43.70, P-value < 0.001). There were no low-birth-weight deliveries among paturients without placental parasitemia. No neonate, however, had anaemia in the study.
Conclusion:
Asymptomatic malaria parasitemia and placental parasitisation by malaria parasites contribute to the outcome of the foetal birth weight. Asymptomatic malaria parasitaemia and placental parasitaemia did not result in a corresponding foetal anaemia on babies delivered.
Keywords: Asymptomatic malaria parasitaemia, labour, neonatal outcome, Nigeria, pregnant women
INTRODUCTION
Malaria in pregnancy is an important cause of anaemia, miscarriages, intrauterine growth restriction, low birth weight, still birth and other pregnancy-related complications.1 Several studies have reported that 125 million women worldwide and 25-50 million African women who get pregnant annually are at risk of malaria.2,3,4,5 Ninety percent of global malaria burden occur in Africa south of Sahara where malaria in pregnancy has been most evaluated.6 Seventy percent of pregnant women in Nigeria suffer malaria with maternal and foetal complications.1 Most pregnant women with malaria infestation are asymptomatic thus are undetected and untreated.7
Foetal complications of malaria in pregnancy include early pregnancy loss, intrauterine growth restriction, vertical transmission of malaria, intrauterine death, low birth weight and congenital malaria. Most of the foetal complications are attributed to placental malaria and reports have shown that the human placenta is an ideal site for the accumulation of Plasmodium falciparum and as a consequence, serious health problems arise for the mother and her baby.8 Studies have shown that placental sequestration of Plasmodium falciparum result in accumulation of parasitised erythrocytes in the intervillous space, infiltration by inflammatory cells and release of pro-inflammatory mediators with malaria pigment and perivillous fibrinoid deposites, proliferation of cytotrophoblastic cells and thickening of basement membrane which impair materno- foetal exchange, often resulting in adverse pregnancy outcome.9,10,11 There is a postulation that the biological basis for susceptibility to malaria in pregnancy is due to accumulation of erythrocytes infected by Plasmodium falciparum in the placenta through adhesion to molecules such as chondroitin sulphate A.12 Studies have also shown that placental malaria induce pathological lesions that lead to intrauterine growth restriction and low birth weight and poses a great challenge to control as it occurs in asymptomatic malaria parasitaemia.13,14,15,16,17,18
This study was aimed at determining the prevalence of placental malaria and neonatal outcomes among unbooked pregnant women presenting in labour in the Federal Teaching Hospital, Abakaliki, Southeastern Nigeria. Those detected with the problem were given appropriate treatment in the Teaching Hospital.
MATERIALS AND METHODS
This was a prospective cross-sectional study conducted in the labour ward complex of the Federal Teaching Hospital, Ebonyi state, Southeastern Nigeria, over a period of 3 months from March to May, 2012. This is the major tertiary hospital located in the state capital Abakaliki. About 75% of the population are rural dwellers with subsistence farming as the major occupation.19 Malaria transmission is endemic in the state.
The study population included all the unbooked pregnant women presenting in labour at term within the period of study. The minimum sample size of 206 parturients was calculated using the formula proposed by Daniel, Lwanga and Lameshow.20,21,22 The exclusion criteria were, those who refused consent, preterm delivery, multiple gestation, HIV in pregnancy, antepartum haemorrhage and symptomatic parturients. A structured data sheet was administered to the women by the authors after explaining to them the purpose of the study and informed consent obtained. The information obtained were of age, parity, gestational age and social class. Blood sample was collected from the antecubital vein under aseptic procedure. A thick and thin blood films were prepared immediately on two glass slides labeled for each parturient. At delivery, cord blood was collected and preserved in a labeled EDTA bottle for foetal haemoglobin estimation. A large placental biopsy was collected and fixed in 10% neutral-buffered formalin for histopathological studies.
The blood films were allowed to air dry and the thin films fixed with absolute methanol for 1 minute, then both were stained with 5% Giemsa stain for 20 minutes, rinsed with water, air dried and microscopic examination done under oil immersion at 100× magnification. The fixed placental tissue biopsies were processed in the histopathology unit, embedded in paraffin wax and sectioned unto slides by standard technique. The sections were stained with haematoxylin-eosin stain for detection of infection. The foetal haemoglobin estimation was done using the hemocue system (hemocue AB, Angel-holm Sweden) which consist of a pre-caliberated, portable, battery or main operated photometer. No dilution was required as blood was run by capillary action directly into a curvette containing sodium nitrite and sodium azide that convert the haemoglobin to azide methaemoglobin. The absorbance was then measured at a wavelength of 565 and 880Nm. This is reliable as high level of bilirubin, lipids or white blood cells do not affect measurements.23 Anaemia was determined using the WHO standard of haemoglobin concentration less than 11.0g/dl. The neonates were weighed with an electronic weighing machine and the birth weight recorded. The coded data was fed into the computer using 2008 Epi Info™ statistical software (version 3.5.1, Centers for Disease Control, Atlanta, GA, USA) and analysis done. A P-value < 0.05 was considered significant. The Research and Ethics Committee of the Federal Teaching Hospital approved the study protocol.
RESULTS
Two hundred and fifty women were screened for asymptomatic malaria parasitaemia and placental parasitisation during the study period. Out of this number, (194/250) 77.6% had peripheral parasitaemia while (227/250) 90.8% had placental parasitisation. Two hundred and fifty babies were delivered out of which 141/250 (56.4%) were male neonates while 109/250 (43.6%) were female neonates. The mean birth weight in the study was 2.9 ± 0.6 kg and ranged from 1.4 to 4.5 kg. The prevalence of low birth weight in the study was 41/250 (16.4%) neonates. One hundred and twenty-six (50.4%) neonates weighed ≥3.0 kg while 83/250 (33.2%) neonates weighed between 2.5 and 2.9 kg. A total of 39/41 (95.1%) of the low-birth-weight neonates were deliveries of parasitaemic mothers. The 2/41 (4.9%) low-birth-weight neonates delivered to parturients without peripheral parasitaemia, however, had placental parasitisation. All the neonates that weighed less than 1.5 kg were deliveries of parasitaemic mothers. This was statistically significant (X2 = 43.70, P-value <0.001) [Table 1].
Table 1.
Birth weight and asymptomatic malaria parasitaemia
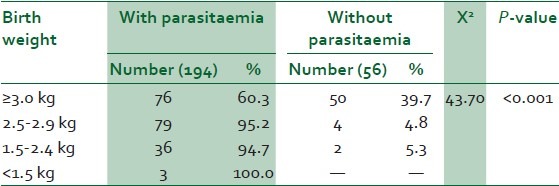
Sixty-six of the neonates that weighed 3.0 kg and above were males while 60 were females. Only three neonates weighed less than 1.5 kg, two males and one female. There was no statistically significant difference in birth weight between male and female neonates (X2 = 3.19, P-value = 0.3631) [Figure 1].
Figure 1.
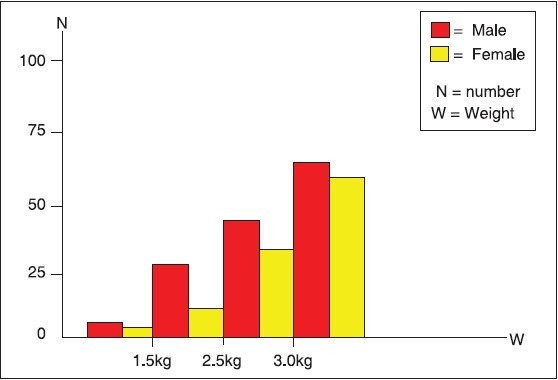
Foetal birth weight and sex distribution
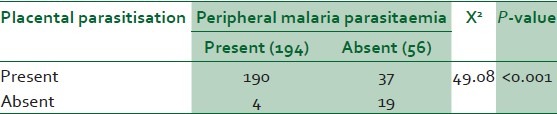
Table 2 showed birth weight distribution and placental parasitisation. All the low-birth-weight babies were deliveries of the parturients with placental parasitisation. No neonate among those without placental malaria weighed less than 3.0 kg. In the study, 190 parturients with placental parasitisation had peripheral parasitaemia while 37 did not have. Only four parturients had peripheral parasitaemia without placental parasitisation [Table 3]. The difference was statistically significant (X2 = 49.08, P-value < 0.001).
Table 2.
Birth weight and placental malaria parasitisation
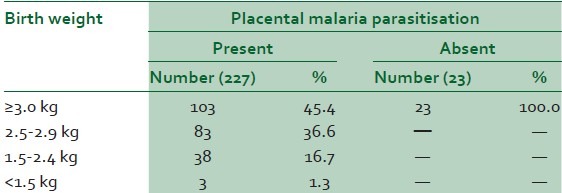
Table 3.
Placental parasitisation and peripheral parasitaemia
Among the parturients with peripheral parasitaemia, 21.1% had birth weight <2.5 kg while 18.0% of those with placental parasitisation had birth weight less than 2.5 kg. There was no neonate with haemoglobin concentration less than 11.0 g/dl. Logistic regression showed negative correlation between malaria parasitaemia with foetal haemoglobin concentration. The only parasite specie identified during the study period was Plasmodium falciparum.
DISCUSSION
There was high prevalence of asymptomatic malaria parasitaemia and placental parasitisation in this study, 77.6% and 90.8%, respectively. This shows the superiority of placental histology over blood film in the diagnosis of asymptomatic malaria parasitaemia in pregnancy. This compares with the report by Rogerson et al,18 of 91% for placental histology, 63% for placental blood film and 47% for peripheral blood film. This was however slightly lower than the 97.4% reported by Achang - Kimbi JK et al.24 There was no neonatal anaemia in the study but logistic regression showed negative correlation between neonatal haemoglobin concentration and asymptomatic malaria parasitaemia as shown by negative coefficient of-0.904 and correlation coefficient (r^2) of 0.04. The prevalence of low birth weight in the study was 21.1% for peripheral parasitaemia and 18.0% for placental parasitisation. This compares with the report of 19.0% in previous studies in sub-Saharan Africa.25 Low birth weight following asymptomatic malaria parasitaemia is an important contributor to neonatal and infant morbidity and mortality in malaria endemic areas like ours. This may be due to placental malaria and associated host response-induced changes in placental structure and function affecting pregnancy growth hormone and predisposing the offspring to hypertension and vascular dysfunction leading to foetal growth restriction, significantly lower birth weight and ponderal index.26,27,28 No neonate, however, died or had features of malaria during the course of the study and none had any problem at 6-weeks postnatal clinic. There was no statistically significant difference in birth weight between male and female neonates in the study suggesting that foetal sex may not be a factor in the neonatal outcome of asymptomatic malaria parasitaemia in pregnancy.
CONCLUSIONS
There was high prevalence of placental malaria parasitisation in the study group. Placental histology is a more sensitive method of diagnosing malaria in pregnancy at delivery when compared with peripheral blood film for malaria parasitaemia. Asymptomatic malaria parasitaemia and placental parasitaemia did not result in a corresponding foetal anaemia on the babies delivered. Plasmodium falciparium was the only type of Plasmodium species identified in the blood film.
RECOMMENDATIONS
There is need to scale up preventive measures (ITN, IPT and effective treatment) against malaria in pregnancy due to the observed foetal impact of asymptomatic malaria parasitaemia. Vector control measures should also be put in place to reduce the level of malaria transmission in our environment. Adequate counselling and advocacy is required to encourage early antenatal booking and proper assessment of the services available during the antenatal care.
ACKNOWLEDGMENT
We sincerely appreciate the management and laboratory staff of the Federal Teaching Hospital Abakaliki, Ebonyi State for their technical support in carrying out the laboratory investigations in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Federal Ministry of Health. National malaria control programme in Nigeria Abuja: Roll Back Malaria Annual Report. 2005:8–9. [Google Scholar]
- 2.World Health Organisation. Malaria: High risk groups/pregnancy. [Last accessed on Aug]. Available from: http://www.who.int/malaria/areas/high_risk_groups/en/
- 3.World Health Organisation. Roll Back Malaria: Malaria in pregnancy; RBM infosheet. [Last accessed on 2011 Sept21]. Available from: http://www.rbm.who.int/cmc_upload/0/000/015/369/RBMInfosheet_4.htm .
- 4.Desai M, terKuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. The epidermology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 5.Dellicour S, Tatem AJ, Guerra CA, Snow RW, terKuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: A demographic study. PLoS Med. 2010;7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organisation. Lives at Risk: Malaria in pregnancy. 2003; information sheet Published 2003. [Last accessed on 2011 Mar 30]. Available from: http://www.who.int/features/2003/04b/en/
- 7.Okpere EE. Malaria in pregnancy. In: Okpere EE, editor. Clinical Obstetrics. Revised ed. Benin: UNIBEN Press; 2004. pp. 56–61. [Google Scholar]
- 8.Uneke CJ. Impact of placental plasmodium falciparum malaria on pregnancy and perinatal outcome in Sub-Saharan Africa: 1: Introduction to placental malaria. Yale J Biol Med. 2007;80:39–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria inpregnancy: Pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–17. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 10.Opare-Addo HS, Odoi AT. Malaria in pregnancy. In: Kwawukume EY, Emuveyan EE, editors. Comprehensive Obstetrics in the Tropics. Accra: Asante and Hittscher Printing Press Limited; 2002. pp. 250–60. [Google Scholar]
- 11.Kagoma SM, Titus KK, Godfrey M. Asymptomatic parasitemia and placental malaria infection among pregnant women in Kigoma urban district, western Tanzania. East Afr J Public Health. 2006;3:14–8. [Google Scholar]
- 12.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–5. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 13.Shulman CE, Dorman EK. Importance and prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg. 2003;97:30–5. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 14.Onyenekwe CC, Meludu SC, Dioka CE, Salimonu LS. Prevalence of asymptomatic malaria parasitaemia amongst pregnant women. Indian J Malariol. 2002;39:60–5. [PubMed] [Google Scholar]
- 15.Kassan SN, Nesbitt S, Hunt LP, Oster N, Soothill P, Sergi C. Pregnancy outcomes in women with or without placental malaria infection. Int J Gynaecol Obstet. 2006;93:225–32. doi: 10.1016/j.ijgo.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–9. [PubMed] [Google Scholar]
- 17.Matteelli A, Donato F, Shein A, Muchi JA, Abass AK, Mariani M, et al. Malaria infection and birth weight in urban Zanzibar, Tanzania. AnnTrop Med Parasitol. 1996;90:125–34. doi: 10.1080/00034983.1996.11813036. [DOI] [PubMed] [Google Scholar]
- 18.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodiun falciparum malaria at delivery: Comparison of blood film preparation methods and of blood films and histology. J ClinMicrobiol. 2003;41:1370–4. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umeora OU, Egwuatu VE. The role of unorthodox and traditional birth care in maternal mortality. Trop Doct. 2010;40:13–7. doi: 10.1258/td.2009.080207. [DOI] [PubMed] [Google Scholar]
- 20.Daniel WW. Biostatistics; A foundation for analysis in the health sciences. 7th ed. New York: John Wiley and sons; 1999. p. 9. [Google Scholar]
- 21.Lwanga SK, Lameshow S. Sample size determination in health studies: A practical manual. Geneva: World Health Organization; 1991. p. 25. [Google Scholar]
- 22.Uneke CJ, Sunday-Adeoye I, Iyare FE, Ugwuja EI, Duhlinska DD. Impact of maternal Plasmodium falciparum malaria and haematological parameters on pregnancy and its outcome in Southeastern Nigeria. J Vector Borne Dis. 2007;44:285–90. [PubMed] [Google Scholar]
- 23.Dacie, Lewis . In: Practical Hematology. 9th ed. Lewis SO, Bain BJ, Dates I, editors. London: Churchill Livingstone; 2001. p. 23. [Google Scholar]
- 24.Anchang-Kimbi JK, Achidi EA, Nkegoum B, Sverremark-Ekström E, Troye-Blomberg M. Diagnostic comparison of malaria infection in peripheral blood, placental blood and placental biopsies in Cameroonian parturient women. Malar J. 2009;8:126. doi: 10.1186/1475-2875-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev. 2004;17:760–9. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adebami OJ, Owa JA, Oyedeji GA, Oyelami OA, Omoniyi-Esan GO. Associationbetween placental and cord blood malaria infection and fetal malnutrition in an area of malaria holoendemicity. Am J Trop Med Hyg. 2007;77:209–13. [PubMed] [Google Scholar]
- 27.Akum AE, Kuoh AJ, Minang JT, Achimbom MJ, Troye-Blomberg M. The effect of maternal, umbilical cord and placental malaria parasitaemia on the birth weight of newborns from South-western Cameroon. ActaPaediatr. 2005;94:917–23. doi: 10.1111/j.1651-2227.2005.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 28.Brabin BJ, Warsame M, Uddenfeldt-Wort U, Dellicour S, Hill J, Gies S, et al. Monitoring and evaluation of malaria in pregnancy-developing a rational basis for control. Malar J. 2008;7:S56. doi: 10.1186/1475-2875-7-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]


