Abstract
Ixodes scapularis is a medically important tick species that transmits causative agents of important human tick-borne diseases including borreliosis, anaplasmosis and babesiosis. An understanding of how this tick feeds is needed prior to the development of novel methods to protect the human population against tick-borne disease infections. This study characterizes a blood meal-induced I. scapularis (Ixsc) tick saliva serine protease inhibitor (serpin (S)), in-house referred to as IxscS-1E1. The hypothesis that ticks use serpins to evade the host's defense response to tick feeding is based on the assumption that tick serpins inhibit functions of protease mediators of the host's anti-tick defense response. Thus, it is significant that consistent with hallmark characteristics of inhibitory serpins, Pichia pastoris-expressed recombinant IxscS-1E1 (rIxscS-1E1) can trap thrombin and trypsin in SDS- and heat-stable complexes, and reduce the activity of the two proteases in a dose-responsive manner. Additionally, rIxscS-1E1 also inhibited, but did not apparently form detectable complexes with, cathepsin G and factor Xa. Our data also show that rIxscS-1E1 may not inhibit chymotrypsin, kallikrein, chymase, plasmin, elastase and papain even at a much higher rIxscS-1E1 concentration. Native IxscS-1E1 potentially plays a role(s) in facilitating I. scapularis tick evasion of the host's hemostatic defense as revealed by the ability of rIxscS-1E1 to inhibit adenosine diphosphate (ADP)- and thrombin-activated platelet aggregation, and delay activated partial prothrombin time (APTT) and thrombin time (TT) plasma clotting in a dose-responsive manner. We conclude that native IxscS-1E1 is part of the tick saliva protein complex that mediates its anti-hemostatic, and potentially inflammatory, functions by inhibiting the actions of thrombin, trypsin and other yet unknown trypsin-like proteases at the tick-host interface.
Keywords: Ixodes scapularis, Tick-feeding physiology, Serine protease inhibitors, Tick anti-blood clotting function, Tick anti-platelet aggregation function
1. Introduction
Ixodes spp. ticks transmit a large number of human tick-borne disease (TBD) agents including Borrelia burgdoferri, Anaplasma phagocytophilum, Babesia microti, Powassan virus, tick-borne encephalitis, Omsk hemorrhagic fever and Louping hill (Ebel and Kramer, 2004; Yano et al., 2005; Michalski et al., 2006; Růžek et al., 2010; Burri et al., 2011; Anderson and Armstrong, 2012; Lommano et al., 2012; Subramanian et al., 2012; Vannier et al., 2012). The impact of Ixodes spp. on public health was the underlying rationale for sequencing of the Ixodes scapularis genome (Hill and Wikel, 2005; Pagel et al., 2007). The availability of the I. scapularis genome sequence data provides opportunities for in-depth studies on the molecular basis of tick feeding physiology. We are interested in understanding the role(s) of serine protease inhibitors (serpin) in I. scapularis tick feeding physiology. These data may lead to a better understanding of how I. scapularis and other ticks acquire, maintain and transmit tick-borne agents.
The tick feeding style of lacerating host tissue and feeding on blood that bleeds into the wounded site is expected to trigger serine protease mediated tissue repair pathways such as inflammation, complement activation and blood clotting, all of which are controlled by serpins. Ticks accomplish feeding by secreting a myriad of proteins into the feeding site to evade the host's anti-tick defense. Given that the host's anti-tick defense is serpin-regulated serine protease pathways (Gettins 2002; Huntington 2006; Huntington and Church, 2007; Raul et al., 2007; Kaiserman et al., 2010; Silverman et al., 2010; Whisstock et al., 2010), it was hypothesized that ticks could utilize this family of proteins to evade the host's anti-tick defense (Mulenga et al., 2001). Serpin-encoding cDNAs have been cloned for multiple tick species (Leboulle et al., 2002a Nene et al., 2002, 2004; Mulenga et al., 2003, 2007, 2009; Sugino et al., 2003; Imamura et al, 2005; Ribeiro et al., 2006, 2011; Chmelar et al., 2011; Sonenshine et al., 2011; Karim et al., 2012; Rodriguez-Vale et al., 2012; Yu et al., 2013). Currently several unpublished serpin sequences from Rhipicephalus puchelus are available in GenBank (Accession numbers JAA54306.1-JAA54316.1, JAA54167.1, JAA62387.1, JAA63258.1, JAA63611.1, JAA53966.1) .
The hypothesis that ticks utilize serpins to evade the host's anti-tick defense (Mulenga et al., 2001) is premised on the assumption that ticks inject inhibitory serpins into the host during feeding, and that these serpins inhibit mammalian protease mediators of the host's anti-tick defense. Tick serpins effective against blood clotting, complement activation, inflammation and platelet aggregation functions have been identified in Ixodes ricinus (Leboulle et al., 2002a; Prevot et al., 2006, 2009; Kovářová, et al., 2010; Chmellar et al., 2011), Rhipicephalus haemaphysaloides (Yu et al., 2013), and Amblyomma americanum (Mulenga et al., 2013a). In other studies the effect of recombinant serpins as tick vaccines was demonstrated when ticks that fed on animals immunized with recombinant tick serpins displayed reduced feeding efficiency (Imamura et al., 2005, 2006, 2008; Prevot et al., 2007).
The objective of this study was to functionally characterize tick saliva serine protease inhibitor (IxscS-1E1) in the context of I. scapularis tick feeding physiology. The gene encoding for IxscS-1E1 likely occurs in a cluster with 10 other serpins in the I. scapularis genome, and potentially exhibits as an extracellular protein (Mulenga et al., 2009). Temporal and spatial transcription analysis revealed that IxscS-1E1 was blood meal responsive and displayed a dichotomous mRNA expression pattern in the tick salivary gland (SG) and midgut (MG) (Mulenga et al., 2009). In the SG, IxscS-1E1 mRNA was induced and differentially up-regulated in response to feeding activity from the 24 h feeding time point. In contrast, in the MG, IxscS-1E1 mRNA was expressed in unfed ticks and was down-regulated in response to tick feeding activity (Mulenga et al., 2009). We conclude that native IxscS-1E1 is part of the tick saliva protein complex that mediates its anti-hemostatic, and potentially inflammatory, functions by inhibiting the actions of thrombin, trypsin and other yet unknown trypsin-like proteases at the tick-host interface.
2. Materials and methods
2.1. Chemicals and proteases
In this study, the proteases, bovine trypsin, chymotrypsin and thrombin, porcine elastase and kallikrein, human chymase and plasmin (plasma), and human neutrophil cathepsin G, were purchased from Sigma (St. Louis, Missouri, USA). The other proteases, papain (papaya) and human plasma factor Xa were purchased from Spectrum (Gardena, California, USA) and Fisher Scientific (Middletown, Virginia, USA), respectively. P-nitroanilide (pNA)-labeled chromogenic peptide substrates for trypsin (Arg-pNA), chymotrypsin (Ala-Ala-Val-Ala-pNA), papain (Glu-Phe-Leu-pNA), plasmin and factor Xa (Gly- Arg-pNA), elastase (Pro-Val- pNA), chymase and cathepsin G (Ala-Ala-Pro-Phe- pNA), thrombin and kallikrein (Pro-Phe-Arg-pNA) were purchased from Sigma. Reagents for in vitro plasma clotting time assays, prothrombin time (PT), activated partial prothrombin time (APTT) and thrombin time (TT) as well as accompanying normal reference human plasma was purchased from Pacific Hemostasis through Fisher Scientific (Middletown, Virginia, USA). Adenosine diphosphate (ADP) was obtained from Chrono-Log Coorporation (Harvetown, Pennsylvania, USA). Cattle whole blood was collected at the Texas A & M University meat processing plant, USA.
2.2. Cloning and sequencing analyses
The IxscS-1E1 open reading frame (ORF) was assembled from expressed sequence tags (ESTs; EW88858) and genome sequence fragments (ISW023621) that were downloaded from GenBank and VectorBase. For cloning, PCR primers (For: ATGAAAGTTCTCGTGACGTTCCTG; Rev: TCAAAGGTGGTTCACTTGCCCTG) were designed from the assembled ORF sequences. The ORF was amplified from 120 h partially fed I. scapularis tick salivary gland cDNA template. Synthesis of cDNA used here was previously described by Ibelli et al. (2013). The PCR product was cloned into a pGEMT (Promega, Fitchburg, Wisconsin, USA) TA cloning vector and sequenced using the BigDye Sequencing Master Mix (Life Technologies, Grand Island, New York, USA) with T7 or SP6 promoter primers using routine procedures. Sequence analysis was done using MacVector software (Acceryls, San Diego, CA, USA).
2.3. Expression and affinity purification of recombinant IxscS-1E1 (rIxscS-1E1)
The Pichia pastoris and pPICZ plasmid system was used to express rIxscS-1E1 according to the manufacturer's instructions (Life Technologies, Carlsbad, California, USA). SignalP (http://www.cbs.dtu.dk) analysis of the translated IxscS-1E1 ORF revealed a 16 amino acid signal peptide (SP) (Mulenga et al., 2009). To express mature rIxscS-1E1, a forward primer excluding the SP coding domain with the EcoRI site (GAATTCTGGGGAAGAGGACAAACTCACAG) and reverse primer with the NotI site (GCGGCCGCAAGGTGGTTCACTTGCCCTG) were synthesized. The recombinant pPICZ A - IxscS-1E1 plasmid was used to transform X-33 P. pastoris competent cells as previously described (Mulenga et al., 2013). Positive transformants, selected for methanol utilizati were cultured at 28 C to A600 of 1 before inducing rIxscS-1E1 expression. Daily addition of methanol to a 5% final concentration induced rIxscS-1E1 protein expression.
The X-33 P. pastoris and pPICZα plasmid system secretes a recombinant protein fused with a C-terminus six-histidine tag into the media that is used for detection of expression and affinity purification of the recombinant protein. Pilot assays confirmed that rIxscS-1E1 reached high expression levels at day 5. To purify from large-scale cultures, rIxscS-1E1 was precipitated from a 5 day 1 L-spent media by ammonium sulfate ((NH4)2+SO4) saturation (525 g/L) overnight at 4 C with stirring. Precipitated rIxscS-1E1 was processed for affinity purification under native conditions using the NiCl2+ charged HiTrapTM Chelating HP column (GE Healthcare Bio- Sciences Corp, Piscataway, NJ, USA) as previously described (Mulenga et al., 2013a, b). Affinity purified rIxscS-1E1 was dialyzed against 0.1 M HEPES buffer containing 150 mM NaCl (pH 7.4) or normal saline (0.9% NaCl in sterile distilled water). Purified rIxcsS-1E1 was concentrated by centrifugation using Jumbosep centrifugal spin filter devices with a 30 kDa size cut-off point (Pall Life Sciences, Port Washington, New York, USA). Protein quantification was done using the Bradford assay according to instructions of the manufacturer (Thermo Scientific, Barrington, Ilinois, USA).
2.4. Deglycosylation
To determine whether rIxscS-1E1 was glycosylated, affinity-purified rIxscS-1E1 was treated with deglycosylation enzyme mix according to the manufacturer's instructions (New England Biolabs, Ipswich, Massachusetts, USA). Briefly, 1 μg of enzyme mix in appropriate reaction buffer was incubated at 37 C with 10 μg of rIxscS-1E1 for 30 min. Subsequently non-treated rIxscS-1E1 and the treated sample were subjected to SDS-PAGE with silver staining. The deglycosylation enzyme mix used in the study was validated to remove both N- and O-linked sugars.
2.5. Production of antibodies to replete-fed I. scapularis tick saliva proteins
Antibodies to replete-fed (allowed to feed to completion) I. scapularis tick saliva proteins were produced as previously published (Chalaire et al., 2011; Mulenga et al., 2013a). Briefly New Zealand White rabbits were repeatedly (four times) infested with 30 (15 on each ear) female I. scapularis ticks to provoke tick resistance. Ticks were fed on top of the rabbit ear to prevent them from getting into the ear canal. Ticks were restricted on top of the rabbit ear by placing them into a 5.08 cm orthopedic stockinette (containment cell) that was attached on top of the rabbit ear using Kamar adhesive (Kamar Inc., Steamboat Springs, Colorado, USA). Two weeks after the final infestation, rabbits were exsanguinated under humane conditions as provided in the animal use protocol approved by the Institutional Animal Care and Use (Committee Texas A & M University, USA). Care and maintenance of animals was provided by licensed veterinarians and/or approved staff of the Comparative Medicine Program at Texas A & M University (USA) in accordance with USA federal government requirements. Rabbit blood was kept at 4°C overnight to allow for maximum separation of serum. Collected immune serum was stored at −80 C in 1 ml aliquots.
2.6. Validation of IxscS-1E1 immunogenicity and secretion into the host during tick feeding
To validate whether native IxscS-1E1 was immunogenic and secreted into the vertebrate host during tick feeding, we subjected rIxscS-1E1 to western blotting analysis using antibodies to replete-fed adult I. scapularis tick saliva proteins. Preliminary analysis revealed that rIxscS-1E1 was N-glycosylated. Thus, to investigate whether antibodies to IxscS-1E1 were directed to glycans, and/or protein backbone, deglycosylated and non-treated rIxscS-1E1 were subjected to western blotting analysis. Detection of the positive signal was done using DAB (3, 3'-diaminobenzidine) (Thermo Scientific Pierce, Rockford, Illinois, USA), the chromogenic substrate for horseradish peroxidase (HRP).
2.7. Inhibitor function profiling of rIxscS-1E1
The inhibitor function of rIxscS-1E1 was investigated using 10 proteases including trypsin, chymotrypsin, elastase, chymase, kallikrein, plasmin, factor Xa, thrombin, papain and cathepsin G. Assays were done using the progress curve method under continuous conditions (Schechter and Plotnick, 2004; Askew et al., 2007; Huang et al., 2008) and adopted by us (Mulenga et al., 2013b) with slight modifications. All assays were done in duplicate in 100 mM HEPES buffer (pH 7.4) containing 150 mM NaCl, pH 7.4. Enzyme and inhibitor combinations provided here were determined in several rounds of optimization trials with the optimized levels repeated twice. Thrombin (0.54 μM) was assayed against 0.031, 0.122, 0.489 and 1.96 μM rIxscS-1E1. Cathepsin G (0.267 μM), factor Xa (0.0217 μM), and trypsin (6.68 μM) were assayed against 0.154, 0.618, 2.47 and 9.89 μM rIxscS-1E1. In preliminary assays with high rIxscS-1E1 amounts, no inhibitory activity was observed against papain, chymotrypsin, plasmin, chymase, kallikrein or elastase and thus, these proteases were removed from the dose-responsive analysis. rIxscS-1E1 and protease were routinely co-incubated at 37 C for 10 min and then peptide substrates were added to the reaction mix. Optimized peptide substrate amounts added to the reaction mix were 50 μM for trypsin and thrombin, 100 μM for cathepsin G and factor Xa. Peptide substrate hydrolysis was progressively monitored at A450nm every 20 s for over 30 min using the VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, USA) set to 37 C.
Substrate hydrolysis data was acquired using SoftMax Pro software (Molecular Devices) set to the default basic kinetics protocol. This analysis estimated endpoint maximum enzyme velocity (Vmax) as a proxy for residual enzyme activity. To calculate the percentage of enzymatic inhibition levels, Vmax estimates in the presence of different rIxscS-1E1 concentrations were expressed as a percentage of Vmax in the absence (0 μM) of rIxscS-1E1. This was then subtracted from the assumed 100% enzyme activity in the 0 μM rIxscS-1E1 reaction. Statistical analysis was done as described in Section 2.12.
2.8. Formation of SDS- and heat-stable complexes between rIxscS-1E1 and the target protease
The mechanism of inhibition by serpins is to trap the target protease into a SDS- and heat-stable complex (Gettins, 2002). To determine whether this was consistent for rIxscS-1E1, aliquots of rIxscS-1E1 and proteases reactions (see Section 2.7) were subjected to routine western blotting analysis with antibodies to the target protease or by silver staining. In this analysis, we detected complexes between rIxscS-1E1 and thrombin or trypsin. To investigate whether complex formation was time-dependent, 3.96 μM rIxscS-1E1 and 0.81 μM thrombin or 7.03 μM rIxscS-1E1 and 0.625 μM trypsin were incubated at 37 C and/or room temperature. These reactions were then sampled at 0 (immediately after mixing), 5, 30, 60 min and overnight. Aliquots were immediately mixed with loading buffer and then subjected to western blotting analyses using protease-specific antibodies. The antibodies to trypsin, thrombin, cathepsin G and factor Xa were purchased from Rockland (Gilbertsville, Pennsylvania, USA), Fitzgerald (Acton, Massachusetts, USA), Enzo Life Sciences (Farmingdale, New York, USA), and New England Biolabs, respectively. Positive signals were detected by chemiluminescence.
2.9. Effect of rIxscS-1E1 on platelet aggregation
The effect of rIxscS-1E1 against platelet aggregation function was investigated using the whole blood platelet aggregation assay as described (Mulenga et al., 2013a, b). Citrated cattle blood (500 μL) was diluted with an equal volume of normal saline and then pre-incubated for 10 min at 37 C with various amounts of rIxscS-1E1. Addition of 20 μM ADP or 0.5U/ L of thrombin triggered platelet aggregation. Platelet aggregation as a function of increased electrical resistance (ohms, Ω) was monitored and recorded using the whole blood platelet aggregometer (Chrono-Log Coorporation). Platelet aggregation assays were done in duplicate and statistical analyses were performed as described in Section 2.12.
2.10. Effect of rIxscS-1E1 on in vitro blood clotting assays
The effects of rIxscS-1E1 on the extrinsic, intrinsic and common blood activation pathways were, respectively, assayed using modified PT, APTT and TT according to the manufacturer's instructions (Pacific Hemostasis, Waltham, Massachusetts, USA) with modifications. The modifications were that we used, respectively, 25 μL and 30 μL of plasma instead of the recommended 100 μL in the PT and APPT assays. In the TT assay, 25 μL of the TT reagent was used instead of the recommended 100 μL. The rationale for modification was to attempt to have excess rIxscS-1E1 in the reaction. We believe that this removed the possibility of high amounts of target clotting factors in plasma masking the effect of rIxscS-1E1. All assays were done in duplicate and statistical analyses were performed as described in Section 2.12.
In the PT assay various amounts (0, 0.52, 1.02, 2.03 and 4.09 μM) of rIxscS-1E1 were pre-incubated for 10 min at 37 C with 25 μL of reference human plasma diluted up to 100 μL with 100 mM HEPES buffer, pH 7.4. Adding 200 μL of pre-warmed thromboplastin reagent triggered plasma clotting. Plasma clotting time was immediately monitored and determined using the KC1 DELTA coagulometer (Trinity Biotech, Parsippany, New Jersey, USA).
In the APTT assay, 100 μL of the APTT reagent and 30 μL of citrated reference human plasma were pre- (0, 0.26, 0.52, 1.02 and 2.04 μM) of rIxscS-1E1 with the reaction volume adjusted to 200 μL with 100 mM HEPES buffer, pH 7.4. Addition of 100 μL of 25 mM CaCl pre-warmed to 37 C triggered plasma clotting time was immediately monitored and determined as described above.
In the TT assay, various amounts (0, 0.52, 1.02, 2.03 and 4.09 μM) of rIxscS-1E1 were pre-incubated for 10 min at 37 C with 25 μL of the TT reagent (which contains thrombin and calcium) with the reaction volume adjusted to 100 μL with 100 mM HEPES buffer, pH 7.4. Addition of 200 μL of pre-warmed plasma triggered conversion of fibrinogen to fibrin followed by clot formation. Clotting time was determined as described for the PT assay.
2.11. Effect of rIxscS-1E1 on complement activation
The effect of rIxscS-1E1 on complement activation was investigated using the MicroVue CH50 ELISA kit according to the manufacturer's instructions (Quidel, San Diego, California, USA) modified as described (Mulenga et al., 2013a, b). This kit reports the amount of terminal complement complexes (TCC) that are formed when the complement system is activated via the classical pathway (Kojouharova et al., 2010). Briefly, in the first step 14 μL of reference human serum supplied with the kit were pre-incubated at 37 C for 15 min without or with 1.33 and 4.67 μM of rIxscS-1E1. In the second step, 86 μL of the complement activator solution was added to the reaction mix and incubation continued for 1 h at 37 C. In the third step, a 200 μL aliquot of 1:200 dilution of the reaction mix was applied onto ELISA plates that were pre-coated with a monoclonal antibody to human TCC. In the fourth step, HRP-conjugated antibody to human TCC was added to plates and incubated for 1 h at room temperature. In the fifth step, a chromogenic HRP substrate was added to wells to detect bound TCC. Color intensity as proxy for bound TCC was quantified at A450nm using the DUB 640 spectrophotometer. Assays were done in duplicate and statistical significance done as described in Section 2.12.
2.12. Statistical analysis
The one-way ANOVA and Tukey Honestly Significant Difference (HSD) calculator in PRISM software (GraphPad Software, Inc. La Jolla, California, USA) were used to determine whether effects of different rIxscS-1E1 concentrations on protease activity, blood clotting, complement activation and platelet aggregation assays were statistically significant. Additionally the unpaired student's t-test calculator in PRISM was used to determine whether the dose-responsive effects of rIxscS-1E1 on platelet aggregation and complement activation assays were statistically significant Observed differences were graphed as mean S.E.M. in PRISM. Differences were considered significant if P < 0.05.
3. Results
3.1. ORF cloning, expression, affinity purification and western blotting analyses of rIxscS-1E1
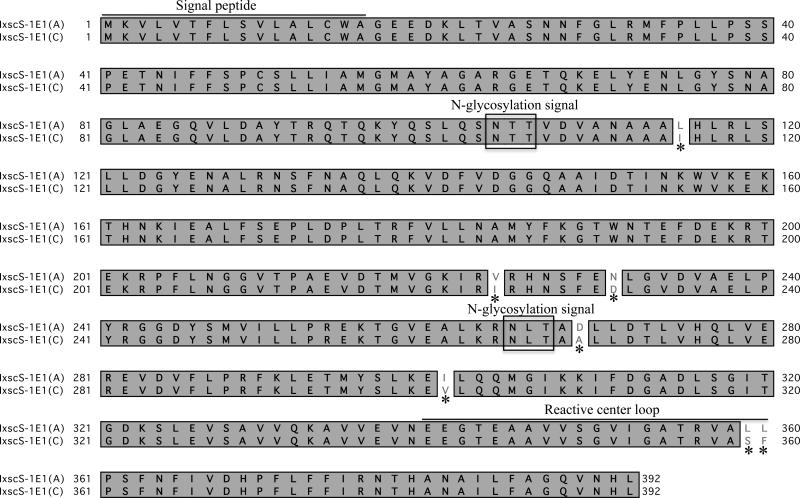
IxscS-1E1 ORF, (GenBank accession number KF990169) was cloned using PCR primers that were designed from source sequences ISW023621 and EW88858 (Mulenga et al., 2009). The assembled and the cloned IxscS-1E1 ORFs were 98% identical at the amino acid level (not shown). Pairwise sequence alignment analysis shown in Fig. 1 revealed a seven amino acid difference between the cloned (IxscS-1E1 (C)) and assembled (IxscS-1E1 (A) ORFs. It is important to note that two of the seven amino acid differences between the cloned and assembled ORFs are in the reactive center loop (RCL), which is important for serpin substrate specificity (Gettins, 2002). Comparative sequence analysis between IxscS-1E1 (A) and other tick serpins has been previously published (Mulenga et al., 2009).
Fig 1.
Cloning of the open reading frame (ORF) for Ixodes scapularis serpin (IxscS-1E1). ORF PCR primers were designed from the sequence of the ORF assembled from two sequence fragments (GenBank Accession numbers ISCW023621 and EW88858). Translated amino acid sequences of the cloned (IxscS-1E1(C)) and assembled (IxscS-1E1 (A)) ORFs were aligned using MacVector software. The signal peptide, putative N-glycosylation sites and the reactive center loop are noted. Asterisks (*) denote amino acid differences between the ORFs. Identical amino acid residues are boxed and shaded gray.
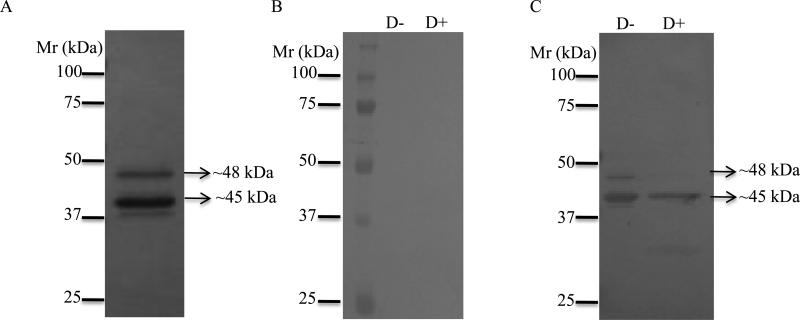
The cloned IxscS-1E1 ORF was successfully expressed in P. pastoris and affinity purified under native conditions. Affinity purification of rIxscS-1E1 was verified by silver staining (Fig. 2A). Fig. 2B shows that rIxscS-1E1 did not bind pre-immune serum, but it bound antibodies to replete-fed I. scapularis tick saliva proteins (Fig. 2C) indicating that native IxscS-1E1 was immunogenic and that it was injected into the host during tick feeding. It is apparent in Fig. 2A that two major rIxscS-1E1 forms were expressed, the long form migrating at ~48 kDa, and the short form migrating at ~45 kDa. Treatment of rIxscS-1E1 with the deglycosylation enzyme mix eliminated the upper band (Fig. 2C lane 2), indicating that it is the glycosylated form. Mature IxscS-1E1 is 377 amino acid residues long with ~41.7 kDa calculated molecular weight. If secreted into media as occurred with rIxscS-1E1, the pPICZα plasmid adds ~3.5 kDa protein fusion to the recombinant protein. In this case the expected molecular mass for rIxscS-1E1 is ~45 kDa, which appears to be consistent with the observed band in Fig. 2C lane 2. It is also important to note that a 1:100 antibody dilution was used in experiments summarized in Fig. 2C. While the rIxscS-1E1 protein band was detectable at lower antibody concentrations (1:500 and 1:1000), the protein band intensity was much weaker (data not shown).
Fig. 2.
Expression, deglycosylation and western blotting analyses of recombinant Ixodes scapularis serpin (rIxscS-1E1). Affinity purified rIxscS-1E1 was subjected to SDS-PAGE with silver staining (A). Untreated (D−) and treated with deglycosylation enzyme mix (D+) rIxscS-1E1 was subjected to routine western blotting analysis using (B) 1:100 dilution pre-immune sera, or (C) immune sera to replete-fed I. scapularis tick saliva proteins.
3.2. rIxscS-1E1 is an inhibitor of thrombin and trypsin
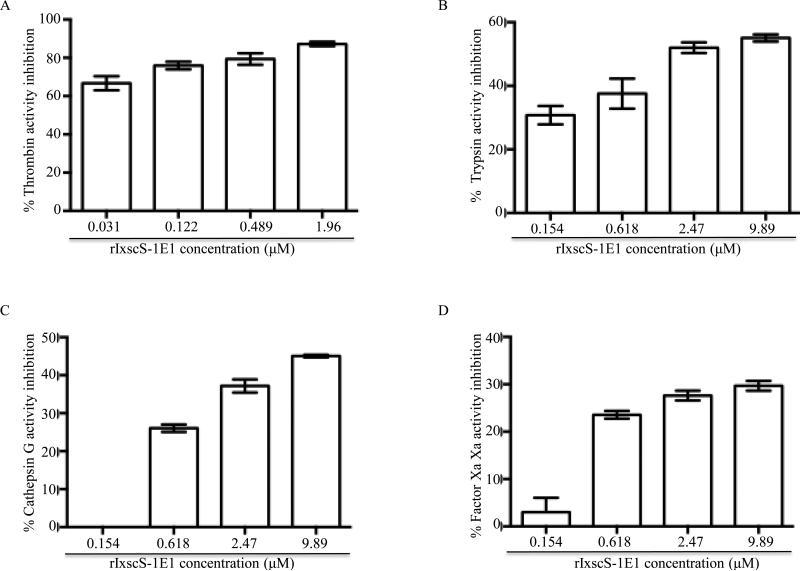
Fig. 3 shows that the amounts of rIxscS-1E1 indicated reduced enzymeic activity of thrombin (Fig. 3A), trypsin (Fig. 3B), cathepsin G (Fig. 3C), and factor Xa (Fig. 3D) in a dose-responsive manner. Activity of 0.54 μM thrombin was reduced by ~ 70 1.43, ~77 1.01, ~79 2.50 and 87 1.15% (Fig. 3A), 6.68 μM trypsin by ~44 1.12, ~48 06.1, ~53 1.17 and ~55 0.70% (Fig. 3B), 0.267 μM Cathepsin G by 0, ~26 0.98, ~37 2.28 and 45 0.33% (Fig. 3C), and 0.0217 μM factor Xa by 0, ~24 0.98, ~28 1.03 and ~30 1.02% (Fig. 3D). One-way ANOVA revealed that differences among treatments in Figs. 3A, B, C and D were significant with F (3,4) = 8.52, P = 0.033; F (3,4) = 28.12, P = 0.0038; F (3,4) = 244.62, P 0.0001; and F (3,4) = 17.97, P = 0.0083, respectively. Subsequently Tukey HSD analysis shows that mean inhibition (MI) of thrombin activity at 0.031 μM rIxscS-1E1 was significantly lower than 0.489 μM (P 0.05) and 1.96 μM (P 0.001), but not 0.122 μM. Similarly MI of thrombin activity at 0.122 μM rIxscS-1E1 was significantly lower than 1.96 μM rIxscS-1E1 (P 0.05), butnot 0.489 μM. Additionally, differences in MI between 0.489 and 1.96 μM rIxscS-1E1 are not statistically significant, although apparent. In Fig. 3B, 0.154 μM rIxscS-1E1 MI of trypsin activity was significantly lower than 2.47 μM (P 0.05) and 9.89 μM (P 0.01) but not 0.618 μM. Similarly MI of trypsin activity at 0.618 μM rIxscS-1E1 was significantly lower than 2.47 μM (P 0.05) and 9.89 μM (P 0.01), but not between 2.47 μM and 9.89 μM rIxscS-1E1. In Fig. 3C, 0.267 μM rIxscS-1E1 reduction of MI of cathepsin G activity was significantly lower than 2.47 and 9.89 μM rIxscS-1E1 with P 0.01, but not 0.618 μM. Similarly 0. 618 μM inhibition of cathepsin G was significantly lower than 9.89 μM rIxscS-1E1 with P 0.05. In Fig. 3D, although differences in the MI of factor Xa activity are apparent, the dose-responsive effect was not statistically significant.
Fig. 3.
Inhibitor function profiling of recombinant Ixodes scapularis serpin (rIxscS-1E1). Various amounts of affinity purified rIxscS-1E1 (indicated) were pre-incubated with (A) 0.54 μM thrombin, (B) 6.68 μM trypsin, (C) 0.267 μ,M cathepsin G, (D) 0.0217 μM factor Xafor 10min at 37°C. Following incubation, peptide substrates were added and hydrolysis continuously monitored every 20 s up to 30min at A405nm using the VersaMax microplate reader. Substrate hydrolysis data was acquired using the SoftMax Pro software set to the default kinetics parameters to estimate end point maximum enzyme velocity (Vmax) or residual enzyme activity (REA). Vmax or REA at different rIxscS-1E1 concentrations were expressed as percentages of Vmax in the absence of rIxscS-1E1. Subtracting the percentage of REA from the assumed 100% enzyme activity in the absence of rIxscS-1E1 calculated the percentage of enzyme inhibition levels summarized as the mean ± S.E.M. of two assays.
3.3. rIxscS-1E1 traps thrombin and trypsin is SDS- and heat-stable complexes
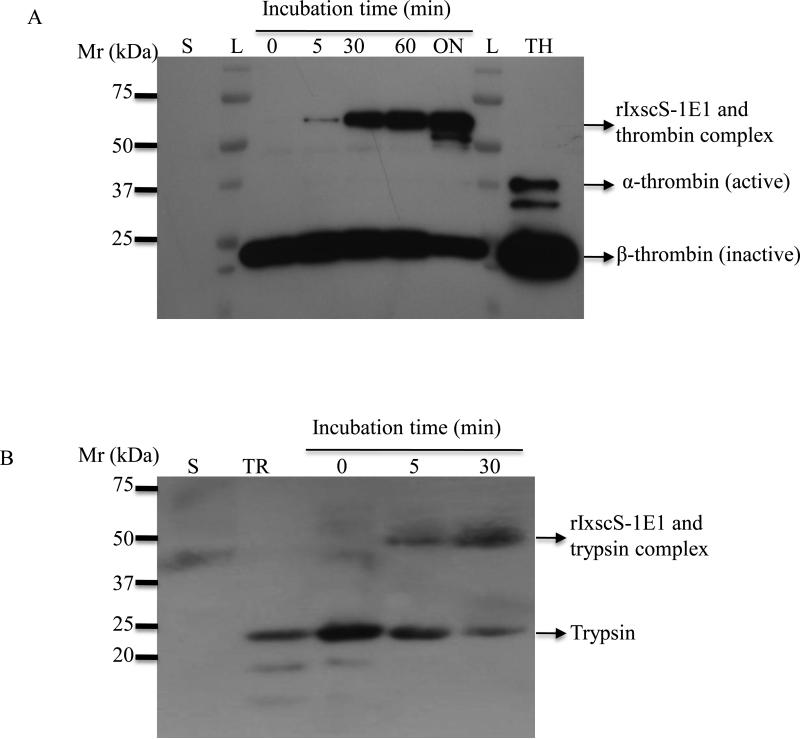
Fig. 4 show that rIxscS-1E1 trapped thrombin and trypsin in SDS- and heat-stable complexes. When aliquots of reactions in Fig. 3 were subjected to western blotting analysis, rIxscS-1E1 complexes with trypsin and thrombin were observed at the highest rIxscS-1E1 concentration (not shown). To investigate whether complex formation between rIxscS-1E1 and thrombin (Fig. 4A) or trypsin (Fig. 4B) was time dependent, a single reaction was sampled at different time points and subjected to western blotting analysis. Based on complex band intensity, the amount of complex formed between rIxscS-1E1 and thrombin (Fig. 4A) or trypsin (Fig. 4B) increased with time. It should be noted that the trypsin reaction was not sampled at 60 min and overnight time points. The observed ~50/52 and 70 kDa complexes of rIxscS-1E1 with trypsin and thrombin are within the expected molecular size range. The mechanism of inhibitory serpin function involves the protease cleaving and releasing the C-terminal end of the serpin through the P1 site (Gettins, 2002). Subsequently the cleaved serpin and the protease form a stable complex. Removal of the C-terminal end from translated rIxscS-1E1 leaves ~37.5 kDa protein backbone. Thus, the observed ~50/52 (Fig. 4B) and 70 kDa (Figs. 4A) are consistent with the expected molecular size of the complex between the cleaved 37.5 kDa rIxscS-1E1 and the 23.3 kDa trypsin or ~35 kDa bovine thrombin. It is important to note that the antibody to thrombin used in Fig. 4 was raised against the prothrombin molecule, and hence the appearance of other prothrombin components in Fig. 4A. Following activation, prothrombin separates into two major subunits, α-thrombin (active subunit, ~35/37 kDa) and β-thrombin (inactive subunit, ~22 kDa) as indicated in Fig. 4A. Complexes between rIxscS-1E1 and cathepsin G or factor Xa were not detected (data not shown).
Fig 4.
Formation of SDS- and heat-stable complexes between recombinant Ixodes scapularis serpin (rIxscS-1E1) and target protease. A single reaction of rIxscS-1E1 and (A) thrombin or (B) trypsin was incubated at room temperature and sampled at 0, 5, 30, 60 min and overnight (ON). Overnight and the 60 min aliquots were not run for trypsin (B). Aliquots were subjected to western blotting analysis using antibodies to thrombin and trypsin as described in Section 2.8. Complexes between rIxcsS-1E1 and thrombin (A) or trypsin (B) are indicated by arrows. L, molecular size ladder; TH, thrombin; S, rIxscS-1E1.
3.4. rIxscS-1E1 inhibits platelet aggregation
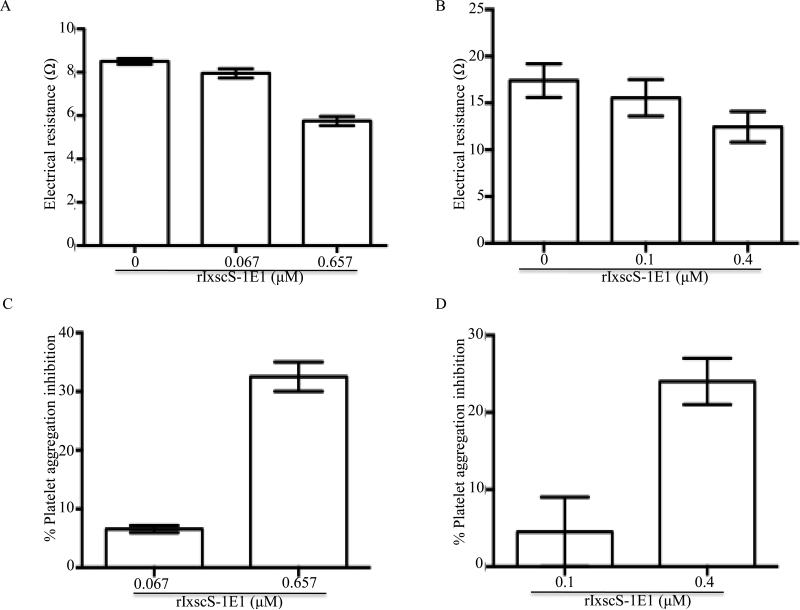
When subjected to the classical complement activation pathway assay, rIxscS-1E1 did not show any effect (data not shown). The whole blood approach was successfully used to measure the effects of various amounts of rIxscS-1E1 on ADP- (Fig. 5A) and thrombin-induced (Fig. 5B) platelet aggregation. In this assay the level of electrical resistance in ohms (Ω) is correlated with the level of platelet aggregation. In Fig. 5A, electrical resistance of ADP-induced platelet aggregation was reduced from 8.5 0.1 Ω in the absence (0 μM) of rIxscS-1E1 to 7.95 0.15 Ω and 5.75 0.15 Ω when 0.067 μM and 0.659 μM rIxscS-1E1 were pre-incubated with whole blood, respectively. Similarly, in Fig. 5B, electrical resistance of thrombin-induced platelet aggregation was reduced from 16.5 2.95 Ω in the absence (0 μM) of rIxscS-1E1 to 15.55 1.95 Ω and 12.45 1.65 Ω when 0.1 μM and 0.4 μM rIxscS-1E1 was pre-incubated with whole blood, respectively. One-way ANOVA demonstrated statistical significant differences between different treatments in Fig. 5A (F (2, 3) = 115.5, P = 0.0015) but not in Fig. 5B. When subjected to Tukey HSD analysis, differences in electrical resistance between 0 μM and 0.659 μM (P = 0.0015) and 0.067 μM and 0.65 μM (P = 0.0029) rIxscS-1E1 treatments were statistically significant, but not between 0 μM and 0.067 μM. To determine the perecentage of platelet aggregation inhibition levels, observed Ω at different rIxscS-1E1 concentrations were expressed as a percentage of electrical resistance at 0 μM rIxscS-1E1 (Figs. 5C, D). In Fig. 5C, 0.06 μM IsxcS-1E1 reduction of ADP-induced platelet aggregation by 6.5 0.66% was statistically significantrly lower than 32.36 0.1% at 0.659 μM (P = 0.0021) as revealed by unpaired t-test. In Fig. 5D, the difference in the percentage of reduction of thrombin-induced platelet aggregation between 0.1 μM (4.5 4.5%) and 0.4 μM (23 4%) rIxscS-1E1 treatments, while apparent, is not statistically significant, as revealed by an unpaired t-test.
Fig. 5.
The effect of recombinant Ixodes scapularis serpin (rIxscS-1E1) on platelet aggregation function. Various amounts, of rIxscS-1E1 (as indicated in A-D) were co-incubated with cattle whole blood for 10 min at 37°C as described in Section 2.10. Addition of 20 μM adenosine diphosphate (AD ) or 0.5U/μL of thrombin triggered platelet aggregation. Platelet aggregation was monitored over 8 min using the whole blood platelet aggregometer as described in Section 2.10. (A B) The observed electrical resistance as an index for platelet aggregation. (C, D) The percentage of inhibition of ADP- and thrombin-triggered platelet aggregation, respectively. Experiments were duplicated.
3.5. rIxscS-1E1 delays plasma clotting in a dose-responsive manner
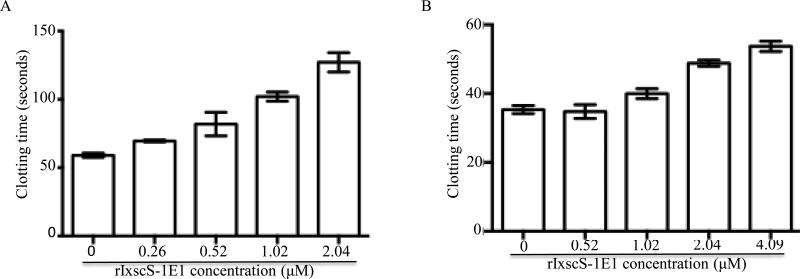
The effect of rIxscS-1E1 on plasma clotting time (PCT) was investigated using in vitro blood clotting assays as summarized in Fig. 6. In the prothrombin time (PT) assay to measure effects on the extrinsic blood clotting activation pathway various amounts of rIxscS-1E1 did not have any effect on PCT (not shown). In the activated partial prothrombin time (APTT) (Fig. 6A) and thrombin time (TT) (Fig. 6B) assays to measure the effect of rIxscS- 1E1 on the intrinsic and common blood clotting activation pathways, PCT was delayed in a statistically significant dose-responsive manner as revealed by one-way ANOVA with F (4, 5) = 26.48, P = 0.0015 and F (4, 5) = 48.67, P = 0.0003, respectively. In Fig. 6A, Tukey HSD analysis revealed that PCT at 0.26 μM (69.6 0.99 s) and 0.52 μM (81.95 12.23 s) rIxscS-1E1 while apparently dalayed by 10.5 2.3 s and 22.85 7.5 s, respectively, were not statistically significant different from PCT at 0 μM (59.1 2.263s), while PCT at 1.02 μM (102 4.74s, P = 0.0115) and 2.04 M (120.1 9.97s, P = 0.0014) rIxscS-1E1, respectively, were significantly delayed by 42.95 4.95 and 68.05 8.65 s. Similarly in Fig. 6B, 0 μM PCT (35.35 1.63 s) and 0.52 μM (34.8 2.83 s) are not different, 1.02 μM PCT (39 1.34 s) was apparently delayed by 4.1 0.2 s but not statistically significant, while PCT at 2.03 μM (50.3 0.85 s, P = 0.0019) and 4.09 μM s, (53.2 1.41 s, P = 0.008) were significantly delayed by 14.95 1.75 and 17.85 2.15 s, respectively.
Fig. 6.
The effect of recombinant Ixodes scapularis serpin (rIxscS-1E1) on plasma clotting time in the activated partial thromboplastin time (APTT, A), and thrombin time (TT, B) assays. Indicated amounts of rIxscS-1E1 were pre-incubated with (A) citrated human reference plasma or (B) the TT reagent at 37°C for 10 min. Following activation of plasma clotting, the time to clot formation was determined using the KC1 DELTA coagulometer as described in Section 2.10.
4. Discussion
Except for a limited number of serpins from I. ricinus (Leboulle et al., 2002a; Prevot et al., 2002, 2006; Kovářová et al., 2010; Chmelar et al., 2011), R. haemaphysaloides (Yu et al., 2013), and A. americanum (Mulenga et al., 2013) that have been functionally characterized, the majority of tick serpins in databases remain uncharacterized. IxscS-1E1 characterized in this study occurs in a cluster with 10 other I. scapularis serpins (Mulenga et al., 2009). This study is part of a series which functionally characterizes this cluster of serpins in the context of I. scapularis tick feeding physiology. To understand the role(s) of serpins, expression of recombinant serpins with appropriate post-translational modifications will be important. Thus, it was encouraging to note that rIxscS-1E1 had post-translational N-glycosylation modification consistent with sequence analysis that predicted two putative N-glycosylation sites in IxscS-1E1 (Mulenga et al., 2009). This observation gave us confidence in the potential for data in this study to reflect events in vivo. It may be possible that P. pastori- expressed recombinant proteins may be hyperglycosylated. Thus, there is a potential that levels of glycosylation between native IxscS-1E1 and rIxcsS-1E1 may be different. In identifying and characterizing tick saliva proteins that regulate tick feeding, it is important to verify secretion of candidate proteins into the host during tick feeding. Additionally, from the perspective of our long-term interest in identifying target antigens for tick vaccine development, it is important that candidate tick saliva proteins are verified as immunogenic. Thus, the observation that rIxscS-1E1 specifically reacted with antibodies to replete-fed I. scapularis tick saliva proteins was significant as it demonstrated that the native IxscS-1E1 was immunogenic and that it was apparently secreted into the host during tick feeding. We would like to note here that we observed a strong rIxscS-1E1 antibody-binding signal at a much higher antibody concentration (1:100). This could be explained by the possibility that native IxscS-1E1 is not a predominant tick saliva protein or that it is not highly immunogenic.
In the literature, the designation of a tick protein being injected into the host during tick feeding has been based on a candidate gene being expressed in the tick salivary gland and possessing the signal peptide (Valenzuela et al., 2002; Ribeiro et al., 2006). Data emerging in our laboratory (unpublished data) suggest that this is not always the case. There is also evidence of tick salivary gland expressed proteins such as the tick histamine release factor, which does not have the classical signal peptide, but is apparently secreted into the host during tick feeding (Mulenga et al., 2003, 2005). On this basis, we are of the opinion that expression in the salivary gland and possession of the signal peptide alone is not sufficient evidence to designate a protein as being injected into the host during tick feeding.
The hypothesis that ticks utilize serpins to facilitate the tick's evasion of the host's defense response to tick feeding is premised on the assumption that candidate tick serpins are inhibitors of mammalian protease mediators of the host's defense response to tick feeding (Mulenga et al., 2001). Thus, an important goal in this study was to determine the functionality of rIxscS-1E1. In mammals, serpins generally fall into two categories: i) those with inhibitor functions against serine and cysteine proteases, and ii) those without inhibitor functions but which are involved in other important pathways (Gettins, 2002; Huntington, 2006; Silverman et al., 2010; Whisstock et al., 2010). Data in this study demonstrated that rIxscS-1E1 inhibited activities of thrombin and trypsin, and possibly cathepsin G and factor Xa. The inhibitory mechanism of serpins is to trap and destroy their target protease in a stable complex (Gettins, 2002; Huntington, 2006). Kinetically the consequence of serpins trapping the protease is reducing maximum enzyme velocity of the target protease (residual enzyme activity) in a dose-responsive manner as was observed with rIxscS-1E1 in this study. Another interesting observation in this study was the formation of SDS- and heat-stable complexes between rIxscS-1E1 and thrombin or trypsin. This was significant in that it is consistent with the hallmark feature of a typical inhibitory serpin mode of action (Huntington, 2006; Wang et al., 2013). The failure to detect complexes between rIxscS-1E1 and cathepsin G and factor Xa could be explained by the possibility that the optimum molar ratio at which complexes could be detected was not attained in this study. It is also possible that rIxscS-1E1 did not form complexes with the two proteases because it is a poor inhibitor of these proteases.
Several lines of evidence have demonstrated that both thrombin and trypsin play important roles in mediating the host's anti-tick defense pathways, inflammation, platelet aggregation function, wound healing and blood clotting. From this perspective, the observation that rIxscS-1E1 inhibited thrombin and trypsin is significant. In addition to its central role in the blood clotting activation cascade, thrombin function is also important in inflammation, platelet function, cell proliferation and angiogenesis (Huntington and Baglin, 2003; Siller-Matula et al., 2011; Calucci and Semeraro, 2012; Chambers and Scotton, 2012; Hunt et al., 2012; Rothmeier and Ruff, 2012; Heemskerk et al., 2013), all of which represent the host's first line of defense to tick feeding activity. Similarly, trypsin in serum and skin plays important roles in inflammation through its activation of protease activated G-coupled receptor 1 (Koshikawa et al., 1998; Satoshi et al., 2001; Meyer-Hoffer et al., 2004; Hunt et al., 2012; Rothmeier and Ruff 2012), and wound healing through cell proliferation functions (White et al., 2013). It is also noteworthy that rIxscS-1E1, which has a basic residue (R) at the P1 site, inhibited trypsin and trypsin-like serine proteases, thrombin, cathepsin G and factor Xa. The data provided here are supported by site-directed mutagenesis studies that showed that replacing the P1 site with a basic amino acid residue converted Manduca sexta (Li et al., 1999) and I. ricinus (Leboulle et al., 2002a, b; Prevot et al., 2006) to efficient inhibitors of trypsin-like proteases. Of significance to our long-term research interests, human serpins, α1-antichymotrypsin, α2-antiplasmin, antithrombin III, protein C inhibitor and C1 inhibitor that regulate pathways representing the host's first line of defense tick feeding, inflammation, blood clotting and complement activation have basic amino acid residues at their P1 sites (Gettins, 2002). Studies reviewed here and data from this study strongly suggest a high possibility for native IxscS-1E1 to be among tick saliva proteins that facilitate tick and host interactions.
The observation that rIxscS-1E1 inhibited thrombin, an important protease of the blood clotting pathway, and our data that native IxscS-1E1 is injected into the host during I. scapularis tick feeding, prompted us to gauge insight into probable role(s) of this protein at the tick-feeding site. The observation that rIxscS-1E1 prevented platelet aggregation and delayed plasma clotting in a dose-responsive manner suggested that native IxscS-1E1 may be part of tick saliva proteins that mediate tick evasion of the host's hemostatic defense mechanism. It is noteworthy that at the highest concentration of rIxscS-1E1, we could only observe 23 4% and 32.36 0.1% inhibition of thrombin- and ADP-activated platelet aggregation. This may be explained by the fact that several pathways, collagen, ADP, and thromboxane contribute to platelet aggregation (Brass, 2003; Offermanns, 2006; Davi and Patrono, 2007; Varga-Szabo et al., 2008; Stalker et al., 2012). Thus, there is a possibility that levels of inhibition observed here reflected contributions of thrombin and ADP to the final platelet aggregation levels.
Blood clotting can be activated via three pathways, extrinsic and intrinsic pathways, which culminate into the common pathway when the fibrin clot is formed. In vitro assays, PT, APTT, and the TT time are routinely used to probe the effects of tick saliva proteins on the extrinsic, intrinsic and common blood clotting activation pathways, respectively (Okhura et al., 2011; Oliveira et al., 2012; Mulenga et al., 2013a, b). Data from this study indicate that rIxscS-1E1 affected the intrinsic and common pathways, but not the extrinsic pathway. Physiologically, the blood clotting system is activated via the extrinsic pathway when injury to blood vessels exposes the tissue factor, which initiates a cascade of actions that culminates in activating prothrombin to thrombin (Mercer and Chambers, 2013). Activated thrombin catalyzes the conversion of fibrinogen to fibrin to initiate clot formation at the common pathway level (Mercer and Chambers, 2013). An intense amplification of plasma clotting occurs at the intrinsic pathway level in which thrombin catalyzes activation of key blood clotting factors XI, IX, VII and X (Mercer and Chambers, 2013). Given that thrombin is the principal protease of both the intrinsic and common blood clotting activation pathways (Mercer and Chambers, 2013), it is fitting that an anti-thrombin such as rIxscS-1E1 delayed plasma-clotting time in APTT and TT assays in a statistically significant dose-responsive manner. Comparable to the mammalian blood clotting system, principal components of the arthropod haemolymph clotting system include serine proteases and serpins (Theopold et al., 2002; Iwanaga and Lee, 2005; Dushay, 2009). Thus, there is a chance that native IxscS-1E1 is part of the tick haemolymph clotting regulatory mechanism and that the observed anti-plasma clotting effects are artifact. However, the apparent secretion of native IxscS-1E1 into the host during tick feeding increases confidence that native IxscS-1E1 protein acts at the tick-host interface.
In conclusion, data from this study add to an emerging list of tick serpins that inhibit proteases which mediate the host defense to tick feeding (Leboulle et al., 2002a; Prevot et al., 2002; 2006; Kovářová et al., 2010; Chmelar et al., 2011; Mulenga et al., 2013; Yu et al., 2013). It is also important to note here that there is a-two amino acid deference in the RCL between the cloned (characterized in this study) and assembled ORFs. Whether or not the two amino acid differences in the RCL denotes allelic difference remains to be confirmed. The next experiments will be to determine whether or not blocking function of IxscS-1E1 through immunization affects I. scapularis tick feeding.
Highlights.
Ixodes scapularis (Ixsc) serpin (S) 1E1 is injected into the host during tick feeding
Recombinant (r) IxscS-1E1 is an inhibitor of thrombin and trypsin
rIxscS-1E1 inhibited platelet aggregation function in a dose-responsive manner
rIxscS-1E1 delayed prevented fibrin clot formation in a dose-responsive manner
Acknowledgements
This research was supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID), USA, grants (AI093858, AI074789, AI074789-01A1S1) to AM. MS is a College Station high school science teacher who was supported by NIH-American Recovery and Reinvestment Act summer employment supplement grant (AI074789-01A1S1) to AM. AMGI was an international visiting student supported by a Conselho Nacional de Desenvolvimento Científico e Tecnológico scholarship (201690/2010-1) from the Brazilian government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JF, Armstrong PM. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am. J. Trop. Med. Hyg. 2012;87:754–759. doi: 10.4269/ajtmh.2012.12-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew DJ, Askew YS, Kato Y, Luke CJ, Pak SC, Brömme D, Silverman GA. The amplified mouse squamous cell carcinoma antigen gene locus contains a serpin (Serpinb3b) that inhibits both papain-like cysteine and trypsin-like serine proteinases. Genomics. 2004;84:166–175. doi: 10.1016/j.ygeno.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, Kaslow RA. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Berger BW, Clemmensen OJ, Ackerman AB. Lyme disease is a spirochetosis. Am. J. Dermatopathol. 1983;5:115–124. [PubMed] [Google Scholar]
- Brass LF. Thrombin and platelet activation. Chest. 2003;124:18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, A.G., Hayes SF, Péter O, Aeschlimann A. Erythema chronicum migransa tickborne spirochetosis. Acta Trop. 1983;40:79–83. [PubMed] [Google Scholar]
- Burri C, Dupasquier C, Bastic V, Gern L. Pathogens of emerging tick-borne diseases, Anaplasma phagocytophilum, Rickettsia spp., and Babesia spp., in Ixodes ticks collected from rodents at four sites in Switzerland (Canton of Bern) Vector Borne Zoonotic Dis. 2011;11:939–944. doi: 10.1089/vbz.2010.0215. [DOI] [PubMed] [Google Scholar]
- Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A. Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 is secreted into tick saliva during tick feeding. J. Exp. Biol. 2011;214:665–673. doi: 10.1242/jeb.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RC, Scotton CJ. Coagulation cascade proteinases in lung injury and fibrosis. Proc. Am. Thorac. Soc. 2012;9:96–101. doi: 10.1513/pats.201201-006AW. [DOI] [PubMed] [Google Scholar]
- Chmelar J, Oliveira CJ, Rezacova P, Francischetti IM, Kovarova Z, Pejler G, Kopacek P, Ribeiro JM, Mares M, Kopecky J, Kotsyfakis M. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood. 2011;117:736–744. doi: 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci M, Semeraro N. Thrombin activatable fibrinolysis inhibitor: at the nexus of fibrinolysis and inflammation. Thromb. Res. 2012;129:314–319. doi: 10.1016/j.thromres.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- de Castro JJ. Sustainable tick and tick-borne diseases control in livestock improvement in developing countries. Vet. Parasitol. 1997;7:77–97. doi: 10.1016/s0304-4017(97)00033-2. [DOI] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard J-F, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialists. Nucl. Acids Res. 2008;36:W465–469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay MS. Insect hemolymph clotting. Cell Mol. Life Sci. 2009;66:2643–2650. doi: 10.1007/s00018-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am. J. Trop. Med. Hyg. 2004;71:268–271. [PubMed] [Google Scholar]
- Flicek BF. Rickettsial and other tick-borne infections. Crit. Care Nurs. Clin. North Am. 2007;19:27–38. doi: 10.1016/j.ccell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gettins PGW. Serpin structure, mechanism, and function. Chem. Rev. 2002;102:4751–4803. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- Gratz N, editor. Tick-borne disease of the USA and Canada. Cambridge University Press; Cambridge: 2006. [Google Scholar]
- Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J. Thromb. Haemost. 2013;11:2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- Hill CA, Wikel SK. The Ixodes scapularis Genome Project: an opportunity for advancing tick research. Trends Parasitol. 2005;21:151–153. doi: 10.1016/j.pt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Hunt JM, Tuder R. Alpha 1 anti-trypsin: one protein, many functions. Curr. Mol. Med. 2012;12:827–835. doi: 10.2174/156652412801318755. [DOI] [PubMed] [Google Scholar]
- Huntington JA. Shape-shifting serpins – advantages of a mobile mechanism. Trends Biochem. Sci. 2006;31:427–435. doi: 10.1016/j.tibs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Huntington JA, Baglin TP. Targeting thrombin--rational drug design from natural mechanisms. Trends Pharmacol. Sci. 2003;24:589–595. doi: 10.1016/j.tips.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Imamura S, da Silva Vaz Junior I, Sugino M, Ohashi K, Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine. 2005;23:1301–1311. doi: 10.1016/j.vaccine.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Imamura S, Konnai S, Vaz Ida S, Yamada S, Nakajima C, Ito Y, Tajima T, Yasuda J, Simuunza M, Onuma M, Ohashi K. Effects of anti-tick cocktail vaccine against Rhipicephalus appendiculatus. Jpn. J. Vet. Res. 2008;56:85–98. [PubMed] [Google Scholar]
- Imamura S, Namangala B, Tajima T, Tembo ME, Yasuda J, Ohashi K, Onuma M. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine. 2006;24:2230–2237. doi: 10.1016/j.vaccine.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Iwanaga S, Lee BL. Recent advances in the innate immunity of invertebrate animals. J Biochem. Mol. Biol. 2005;38:128–150. doi: 10.5483/bmbrep.2005.38.2.128. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kaiserman D, Bird PI. Control of granzymes by serpins. Cell Death Differ. 2010;17:586–595. doi: 10.1038/cdd.2009.169. [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One. 2011;6:e28525. doi: 10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojouharova M, Reid K, Gadjeva M. New insights into the molecular mechanisms of classical complement activation. Mol. Immunol. 2010;47:2154–2160. doi: 10.1016/j.molimm.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Hasegawa S, Nagashima Y, Mitsuhashi K, Tsubota Y, Miyata S, Miyagi Y, Yasumitsu H, Miyazaki K. Expression of trypsin by epithelial cells of various tissues, leukocytes, and neurons in human and mouse. Am J Pathol. 1998;153:937–944. doi: 10.1016/S0002-9440(10)65635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovářová Z, Chmelař J, Sanda M, Brynda J, Mareš M, Rezáčová P. Crystallization and diffraction analysis of the serpin IRS-2 from the hard tick Ixodes ricinus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010;66:1453–1457. doi: 10.1107/S1744309110032343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboulle G, Rochez C, Louahed J, Ruti B, Brossard M, Bollen A, Godfroid E. Isolation of Ixodes Ricinus salivary gland mRNA encoding factors induced during blood feeding. Am. J. Trop. Med. Hyg. 2002a;66:225–33. doi: 10.4269/ajtmh.2002.66.225. [DOI] [PubMed] [Google Scholar]
- Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, Godfroid E. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J. Biol. Chem. 2002b;277:10083–10089. doi: 10.1074/jbc.M111391200. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Z, Canagarajah B, Jiang H, Kanost M, Goldsmith EJ. The structure of active serpin 1K from Manduca sexta. Structure. 1999;7:103–109. doi: 10.1016/s0969-2126(99)80013-6. [DOI] [PubMed] [Google Scholar]
- Lommano E, Burri C, Maeder G, Guerne M, Bastic V, Patalas E, Gern L. Prevalence and genotyping of tick-borne encephalitis virus in questing Ixodes ricinus ticks in a new endemic area in western Switzerland. J. Med. Entomol. 2012;49:156–164. doi: 10.1603/me11044. [DOI] [PubMed] [Google Scholar]
- Mercer PF, Chambers RC. Coagulation and coagulation signaling in fibrosis. Biochim. Biophys. Acta. 2013;1832:1018–1027. doi: 10.1016/j.bbadis.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Meyer-Hoffert U, Rogalski C, Seifert S, Schmeling G, Wingertszahn J, Proksch E, Wiedow O. Trypsin induces epidermal proliferation and inflammation in murine skin. Exp Dermatol. 2004;13:234–241. doi: 10.1111/j.0906-6705.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- Michalski M, Rosenfield C, Erickson M, Selle R, Bates K, Essar D, Massung R. Anaplasma phagocytophilum in central and western Wisconsin: a molecular survey. Parasitol. Res. 2006;99:694–699. doi: 10.1007/s00436-006-0217-9. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Kim TK, Ibelli AM. Deorphanization and target validation of cross-tick species conserved novel Amblyomma americanum tick saliva protein. Int. J. Parasitol. 2013;43:439–451. doi: 10.1016/j.ijpara.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Blandon MA. Molecular and expression analysis of a family of the Amblyomma americanum tick Lospins. J. Exp. Biol. 2007;210:3188–3198. doi: 10.1242/jeb.006494. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Chalaire KC. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics. 2009;10:217. doi: 10.1186/1471-2164-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Kim T, Ibelli AM. Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol. Biol. 2013;22:306–319. doi: 10.1111/imb.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Sugino M, Nakajima M, Sugimoto C, Onuma M. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J Vet Med Sci. 2001;63:1063–1069. doi: 10.1292/jvms.63.1063. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Tsuda A, Onuma M, Sugimoto C. Four serine proteinase inhibitors (serpin) from the brown ear tick, Rhiphicephalus appendiculatus; cDNA cloning and preliminary characterization. Insect Biochem. Mol. Biol. 2003;33:267–276. doi: 10.1016/s0965-1748(02)00240-0. [DOI] [PubMed] [Google Scholar]
- Muta T, Iwanaga S. Clotting and immune defense in Limulidae. Prog. Mol. Sub cell Biol. 1996;15:154–189. doi: 10.1007/978-3-642-79735-4_8. [DOI] [PubMed] [Google Scholar]
- Nene V, Lee D, Kang'a S, Skilton R, Shah T, de Villiers E, Mwaura S, Taylor D, Quackenbush J, Bishop R. Genes transcribed in the salivary glands of female Rhipicephalus appendiculatus ticks infected with Theileria parva. Insect Biochem. Mol. Biol. 2004;34:1117–1128. doi: 10.1016/j.ibmb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Nene V, Lee D, Quackenbush J, Skilton R, Mwaura S, Gardner MJ, Bishop R. AvGI, an index of genes transcribed in the salivary glands of the ixodid tick Amblyomma variegatum. Int. J. Parasitol. 2002;32:1447–1456. doi: 10.1016/s0020-7519(02)00159-5. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Sonenshine DE, Lane RS, Uilenberg G. Ticks (Ixodida) Medical and Veterinary Entomology. In: Mullen GR, Durden LA, editors. 2nd edition Academic Press; San Diego, California: 2009. pp. 493–541. [Google Scholar]
- Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Tamura K, Tanaka A, Matsuda J, Atsumi G. Experimental study on the hemostatc activity of Pollen Typhae, a traditional folk medicine used by external and oral application. Blood Coagul. Fibrinolysis. 2011;22:631–636. doi: 10.1097/MBC.0b013e328349a22c. [DOI] [PubMed] [Google Scholar]
- Oliveira DG, Alvarez-Flores MP, Lopes AR, Chudzinski-Tavassi AM. Functional characterisation of vizottin, the first factor Xa inhibitor purified from the leech Haementeria vizottoi. Thromb. Haemost. 2012;108:570–578. doi: 10.1160/TH12-04-0235. [DOI] [PubMed] [Google Scholar]
- Ong PC, McGowan S, Pearce MC, Irving JA, Kan WT, Grigoryev SA, Turk B, Silverman GA, Brix K, Bottomley SP, Whisstock JC, Pike RN. DNA accelerates the inhibition of human cathepsin V by serpins. J. Biol. Chem. 2007;282:36980–36986. doi: 10.1074/jbc.M706991200. [DOI] [PubMed] [Google Scholar]
- Pagel VZJ, Geraci NS, Guerrero FD, Wikel SK, Stuart JJ, Nene VM, Hill CA. Tick genomics, the Ixodes genome project and beyond. Int. J. Parasitol. 2007;37:1297–1305. doi: 10.1016/j.ijpara.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Beschin A, Lins L, Beaufays J, Grosjean A, Bruys L, Adam B, Brossard M, Brasseur R, Zouaoui Boudjeltia K, Vanhamme L, Godfroid E. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J. 2009;276:3235–3246. doi: 10.1111/j.1742-4658.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, Brasseur R, Vanhaeverbeek M, Vanhamme L, Godfroid E. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J. Biol. Chem. 2006;281:26361–2639. doi: 10.1074/jbc.M604197200. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine. 2007;25:3284–3292. doi: 10.1016/j.vaccine.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, haemostasis and fibrinolysis. J. Thromb. Haemost. 2007;1:102–115. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud S. The recalcification plasma clotting time. A valuable general clotting test in man and rats. Can J Physiol Pharmacol. 1969;47:689–693. doi: 10.1139/y69-118. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Anderson JM, Manoukis NC, Meng Z, Francischetti IM. A further insight into the sialome of the tropical bont tick, Amblyomma variegatum. BMC Genomics. 2011;12:136. doi: 10.1186/1471-2164-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Valle M, Vance M, Moolhuijzen P, M., Tao X, Lew-Tabor AE. Differential recognition by tick-resistant cattle of the recombinantly expressed Rhipicephalus microplus serine protease inhibitor-3 (RMS-3). Ticks Tick Borne Dis. 2012;3:159–69. doi: 10.1016/j.ttbdis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012;34:133–149. doi: 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- Růžek D, Yakimenko VV, Karan LS, Tkachev SE. Omsk haemorrhagic fever. Lancet. 2010;376:2104–2113. doi: 10.1016/S0140-6736(10)61120-8. [DOI] [PubMed] [Google Scholar]
- Schechter NM, Plotnick MI. Measurement of the kinetic parameters mediating protease-serpin inhibition. Methods. 2004;32:159–68. doi: 10.1016/s1046-2023(03)00207-x. [DOI] [PubMed] [Google Scholar]
- Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect. Genet. Evol. 2012;12:1788–1809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Siller-Matula JM, Schwameis M, Blann A, Mannhalter C, Jilma B. Thrombin as a multi-functional enzyme. Focus on in vitro and in vivo effects. Thromb. Haemost. 2011;106:1020–1033. doi: 10.1160/TH10-11-0711. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Bird PI. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J. Biol. Chem. 2010;285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE, Bissinger BW, Egekwu N, Donohue KV, Khalil SM, Roe RM. First transcriptome of the testis-vas deferens-male accessory gland and proteome of the spermatophore from Dermacentor variabilis (Acari: Ixodidae). PLoS One. 2011;6:e24711. doi: 10.1371/journal.pone.0024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of ticks. Oxford University Press; Oxford: 1993. [Google Scholar]
- Stalker TJ, Newman DK, Ma P, Wannemacher KM, Brass LF. Platelet signaling. Handb. Exp. Pharmacol. 2012;210:59–85. doi: 10.1007/978-3-642-29423-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian G, Sekeyova Z, Raoult D, Mediannikov O. Multiple tick-associated bacteria in Ixodes ricinus from Slovakia. Ticks Tick Borne Dis. 2012;3:406–410. doi: 10.1016/j.ttbdis.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Sugino M, Imamura S, Mulenga A, Nakajima M, Tsuda A, Ohashi K, Onuma MA. Serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine. 2003;21:2844–2851. doi: 10.1016/s0264-410x(03)00167-1. [DOI] [PubMed] [Google Scholar]
- Theopold U, Li D, Fabbri M, Scherfer C, Schmidt O. The coagulation of insect hemolymph. Cell Mol. Life Sci. 2002;59:363–372. doi: 10.1007/s00018-002-8428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Mather TN, Ribeiro JM. Exploring the sialome of the tick Ixodes scapularis. J Exp Biol. 2002;205:2843–2864. doi: 10.1242/jeb.205.18.2843. [DOI] [PubMed] [Google Scholar]
- Vannier E, Krause PJ. Human babesiosis. N. Engl. J. Med. 2012;366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- Waldo ED, Sidhu GS. The spirochete in erythema chroniicum mnigrans. Am. J. Dermatopathol. 1983;5:125–127. doi: 10.1097/00000372-198304000-00009. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Q, Wu L, Liu S, Zhang Y, Yang X, Zhu P, Zhang H, Zhang K, Lou J, Liu P, Tong L, Sun F, Fan Z. Identification of SERPINB1 as a physiological inhibitor of human granzyme H. J Immunol. 2013;190:1319–1330. doi: 10.4049/jimmunol.1202542. [DOI] [PubMed] [Google Scholar]
- Whisstock JC, Silverman GA, Bird PI, Bottomley SP, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Huntington JA. Serpins flex their muscle: II. Structural insights into target peptidase recognition, polymerization, and transport functions. J. Biol. Chem. 2010;285:24307–24312. doi: 10.1074/jbc.R110.141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJ, Glenn M, Gomer RH. Trypsin potentiates human fibrocyte differentiation. PLoS One. 2013;8:e70795. doi: 10.1371/journal.pone.0070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willadsen P. Tick control: thoughts on a research agenda. Vet. Parasitol. 2006;138:161–168. doi: 10.1016/j.vetpar.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Yano Y, Saito-Ito A, Anchalee D, Takada N. Japanese Babesia microti cytologically detected in salivary glands of naturally infected tick Ixodes ovatus. Microbiol. Immunol. 2005;49:891–897. doi: 10.1111/j.1348-0421.2005.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Cao J, Zhou Y, Zhang H, Zhou J. Isolation and characterization of two novel serpins from the tick Rhipicephalus haemaphysaloides. Ticks Tick Borne Dis. 2013;4:297–303. doi: 10.1016/j.ttbdis.2013.02.001. [DOI] [PubMed] [Google Scholar]