Abstract
Background:
Polycythemia (PC) is defined as venous hematocrit (hct) ≥65%. Its incidence is high among certain risk factors (RFs). Its management is controversy.
Aims:
To determine: (1) The incidence of PC in our neonatal intensive care unit (NICU). (2) Most common RF, symptoms, and laboratory abnormalities (LA) associated with PC and their effect on the length of hospital stay (LOS). (3) Whether noninvasive interventions are effective in reducing hct. (4) Hct pattern of PC neonates.
Design:
Retrospective cohort study.
Setting:
NICU at a maternity and children hospital.
Materials and Methods:
Records review of all neonates from March 2011 to August 2013. Inclusions criteria were: (1) Venous hct ≥65%. (2) Neonates born in our institution. (3) Early umbilical cord clamping. (4) Gestational age ≥34 weeks.
Statistical Analysis:
Chi-square and multiple regression analysis.
Results:
One hundred and one PC neonates were eligible. Incidence of PC in our NICU is 14.5%. The most common RF, symptoms, and LA were: Small for gestational age, jaundice and hypoglycemia respectively. Tachypnea ( P - 0.04) and oliguria (P - 0.03) significantly prolonged LOS. Noninvasive interventions or observation could not reduce the hct significantly (P - 0.24). The hcts mean peaked maximally at a mean of 2.8 h of age.
Conclusion:
PC incidence in our NICU is higher than the reported incidence in healthy newborns. Most of the PC neonates were either symptomatic or having LA. Noninvasive interventions or observation were not effective in reducing hct in polycythemic neonates. Hct in both healthy and PC neonates peaked at the same pattern.
Keywords: Hematocrit, length of hospital stay, neonatal intensive care unit, neonate, noninvasive, observation, overhydration, pattern, polycythemia
INTRODUCTION
The incidence of polycythemia (PC) in healthy newborns is 0.4-5%. [1,2] Symptoms of PC are thought to be due to hyperviscosity that occur in 47.4% of PC neonates. [3] The choice between partial exchange transfusion (PET) and noninvasive management is controversial. [4,5] The study objective is to determine: (1) The incidence of PC in our neonatal intensive care unit (NICU). (2) Common risk factors (RFs), symptoms, and laboratory abnormalities (LA) of PC neonates admitted to NICU and their effect on the length of hospital stay (LOS). (3) Whether noninvasive interventions are effective in reducing hematocrit (hct) in PC neonates. (4) The pattern of hct in PC neonates.
MATERIALS AND METHODS
We conducted a retrospective cohort study by screening the records of all neonates who had been admitted to our NICU during the past 28 months, from March 2011 to August 2013 at a maternity and children hospital, which has around 2700 deliveries per year and average of 370 admissions to NICU per year. All polycythemic neonates were discovered incidentally, either well babies from the nursery whom complete blood counts had been done as a part of premature rupture of membrane (PROM) work-up, or as a part of Rh-ABO incompatibility screening, or ill neonates admitted to NICU for other reasons and discovered incidentally to have PC. The research was approved by the Research and Ethic Committee of the Ministry Of Health. Inclusion criteria were: (1) Venous hct ≥65% in the first 7 days of life. (2) Neonates born in our institution. (3) Early umbilical cord clamping (<30 s), which is our hospital's routine. (4) Gestational age ≥34 weeks. Exclusion criteria: (1) Neonates who had been admitted to our NICU from the emergency department (home delivery, car delivery, transfer from other hospitals). (2) Neonates who died in the first 7 days of life. The first exclusion was set because, we cannot insure early cord clamping in those neonates who were not born in our hospital. The second exclusion was set because, those neonates were sick and their symptoms could not be attributed to PC. Equipment: Blood drawn into purple top test tubes contained anticoagulant EDTA. All hcts were measured by cell counter method using (CELL-DYN emerald analyze r–09H39–01 device, France). The data included: General patient profile (birth weight, sex, gestational age, and delivery mode), RFs (small for gestational age [SGA], large for gestational age [LGA], twins, pregnancy induced hypertension [PIH], infant of diabetic mother [IDM], asphyxia, and chromosomal abnormalities). Symptoms (jaundice, tachypnea, poor feeding, vomiting, lethargy, apnea, cyanosis, and oliguria), LA: (Hypoglycemia, hypocalcemia, and thrombocytopenia). The following definitions were used: Hypoglycemia – Capillary or serum glucose value <50 mg/dl; thrombocytopenia – Platelets count <150 × 103/mm3; hypocalcemia – total serum calcium <8.5 mg/dl. Birth weights were recorded to the nearest 10 g. Gestational age was determined from the maternal menstrual history and/or fetal ultrasound assessment. The patient was considered jaundiced if he/she was clinically jaundiced and required phototherapy. Asphyxia was defined as Apgar score ≤5 at 5 min.
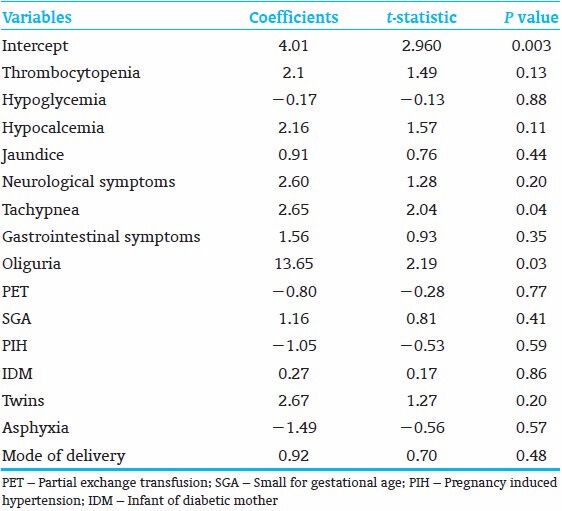
Multiple regression analysis was used to reveal the effect of the variables (thrombocytopenia, hypoglycemia, hypocalcemia, jaundice, neurological symptoms, tachypnea, gastrointestinal symptoms, oliguria, PET, SGA, PIH, IDM, twins, asphyxia, and mode of delivery) on LOS in PC neonates.
Each hct ≥65% (a single patient could have more than one blood sample of hct ≥65%) had one or more of the following interventions: (1) Observation. (2) Intravenous normal saline (IVNS) bolus (10-20 ml/kg). (3) Increase total fluid intake (ITFI) (10-20 ml/kg/day). (4) Combination of (IVNS) bolus and (ITFI). The intervention considered successful if at least one of the following criteria was fulfilled provided that no rebound of hct within the next 48 h of the intervention: (1) Drop of hct ≥3% within 12 h of an intervention. (2) Drop of hct ≥4% from 12 to 24 h of an intervention. (3) Drop of hct ≥5% 24-48 h of an intervention. The data were interpreted for significance using Chi-square. P <0.05 was considered as significant. All data were analyzed by Microsoft excel 2010.
To determine the hct pattern, we documented all hcts (<65% and >65%) with their corresponding sampling times in hours after birth of any neonate who had PC, until a maximum of 10 days of age. The means of blood sampling time ± 2standard deviation (2SD) were plotted against their hcts means ± 2SD; to create a diagram that represented the hct pattern of all PC neonates in the study.
RESULTS
A total of 768 records were screened, 101 PC neonates were eligible for the study out of 112 PC neonates. The incidence of PC in our NICU is 14.5%. The mean birth weight was 2742 (g) (ranges from 1350 to 4300 g). The mean gestational age was 38.1 weeks (ranges from 34 to 43 weeks). Although the majority of PC infants were term, the number of preterm infants was 22/101 (21.7%), versus 3/101 (2.9%) in postterm infants. Fifty-seven percent (58/101) PC neonates were male, and 57% (58/101) PC neonates were born by caesarian section. Thirty four percent (35/101) neonates were SGA, 18% (19/101) were IDM, and 18% (19/101) were neonates whose mothers had PIH. Growth status, gestational ages and RFs are shown in Table 1. Forty six percent (47/101) neonates had jaundice, 24% (25/101) neonates had tachypnea, 14% (15/101) neonates had poor feeding, and 13% (14/101) neonates had lethargy. Twenty-eight percent (29/101) neonates had hypoglycemia, 24% (25/101) neonates had hypocalcemia, and 21% (22/101) neonates had thrombocytopenia. Thirty nine percent (40/101) neonates had no symptoms; 47% (48/101) neonates had normal laboratory results. Seventeen percent (18/101) neonates were asymptomatic with normal laboratory results, those PC neonates were discovered incidentally at the nursery as a part of PROM work up or ABO-Rh screening, our protocol is to observe any polycythemic neonate in the NICU [Table 2]. Independent variables were regressed on the LOS (the dependent variable) in a multiple regression analysis. The results showed R2 of 0.29, adjusted R2 of 0.17. ANOVA analysis showed (F - 2.3 and P - 0.007), the variables that significantly affect LOS were tachypnea (P - 0.043) and oliguria (P - 0.031) [Table 3].
Table 1.
Patient characteristics and risk factors for 101 polycythemic neonates
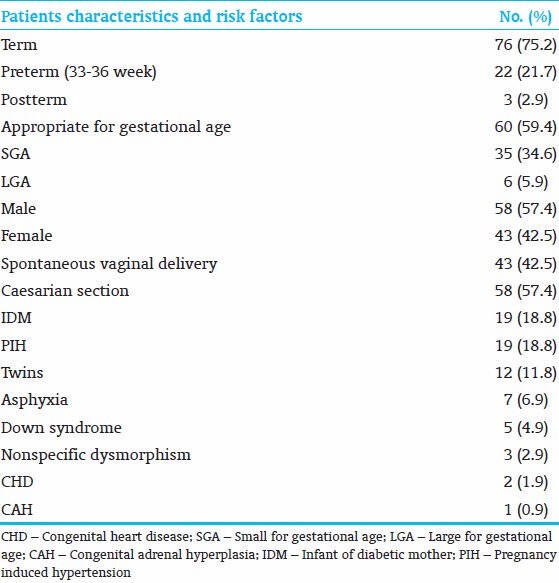
Table 2.
Symptoms and laboratory abnormalities of 101 polycythemic neonates
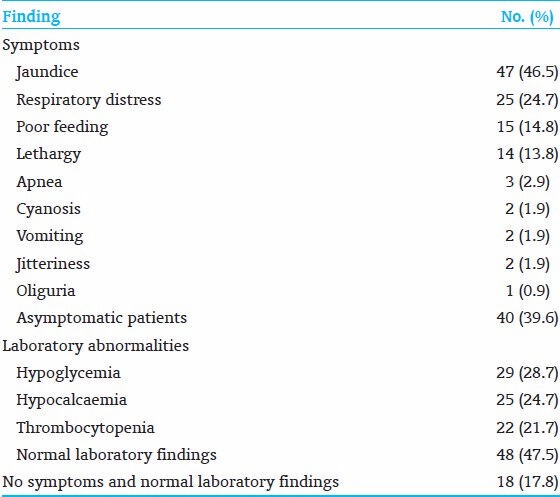
Table 3.
The coefficients, P values, t-statistics of the independent variables that affect length of hospital stay (dependent variable)
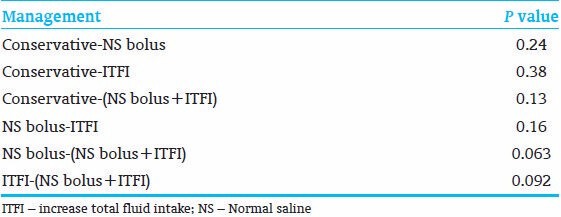
Total samples of hct ≥65% were 181. Sixty-seven percent (18/27) of (IVF bolus + ITFI) were successful. Sixty-one percent (19/31) of ITFI interventions were successful. Fifty percent (22/44) of conservative treatment were successful; About 46% (36/78) of IVF bolus interventions were successful. P value is 0.24 of the Chi-square test for the total results [Table 4]. The P values between different interventions are shown in Table 5.
Table 4.
Results (numbers) of different noninvasive interventions to reduce hct, with the P value of the Chi-square test of all interventions
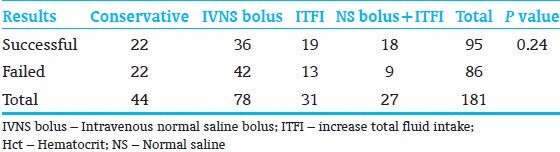
Table 5.
P values of the Chi-square of an intervention versus an intervention
Five hundred and thirty-five hct samples were obtained. At the mean of 2.8 h, the mean hct peaked maximally to (67.34 ± 7.9%) from (67.29 ± 8.9%) at the mean time of 1 h, after that; gradually descended until the mean time of 77 h (hct: 61.1 ± 9%) where steeper descent started [Table 6 and Figure 1].
Table 6.
Means time of hct sampling in hours±SD with their means of hct±2SD

Figure 1.
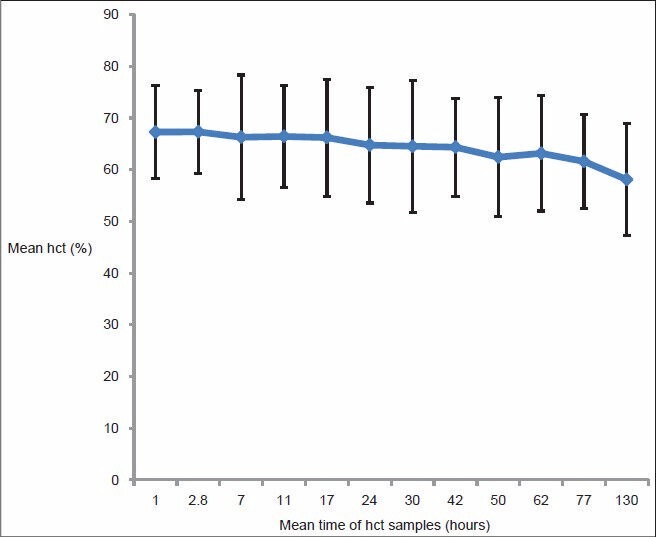
Pattern of hematocrit (hct) of 101 polycythemia neonates, mean time was plotted against mean hct ± 2 standard deviation
DISCUSSION
the study showed that the incidence of PC in our NICU is much higher than the reported incidence of PC in healthy newborns; [6] this can be explained by that NICU population is sicker and have more RFs for PC than healthy newborns.
In our study, cesarean deliveries were more predominant than vaginal deliveries; this can be attributed to the large number of IDM, twins, and neonates whose mother had PIH that had been delivered by cesarean section. Although the majority of PC neonates were term, the number of late preterm neonates with PC is significant, indicating that PC is not uncommon among late preterm neonates, one cohort study showed more than 6% of PC neonates were preterm. [6,7]
Increased intrauterine erythropoiesis usually results from placental insufficiency and chronic intrauterine hypoxia that is seen in infants who are SGA or whose mothers have PIH. [7,8] In our study, SGA is the commonest RF for PC followed by PIH and IDM. PC due to erythropoiesis occurs in 13-33% of IDM and may be seen in other infants who are LGA, we found that IDM represented a significant RF for PC, maternal hyperglycemia is proposed to increase fetal erythropoiesis through fetal hyperinsulinemia, tissue hypoxia, and increased erythropoietin concentrations. [9]
Four percent PC neonates were Down syndrome. The incidence of PC in infants who have Down syndrome is 15-33%. In affected infants, high cord blood erythropoietin concentration is thought to be the cause, which has led to the speculation that intrauterine hypoxemia may play a role. [10,11]
Six percent of PC neonates in our study had asphyxia; placental transfusion is augmented in those patients, which causes an active shift of the blood volume from the placenta to the fetus. Eleven percent of PC neonates were twins; the mechanism of PC in twins is through twin-to-twin transfusion syndrome due to a vascular communication which occurs in approximately 10% of monozygotic twin pregnancies. [12]
The clinical manifestations of PC occur with many other neonatal disorders and may be associated with, but not caused by PC. Around half of the PC infants in the study had jaundice; 40% of them had ABO or Rh incompatibility, which may explain such high incidence. Jaundice has been reported in at least one-third of infants with PC, most probably due to the breakdown of an increased number of circulating red blood cells.
Neurological manifestations (jitteriness, poor feeding and lethargy) occurred in one-third of the PC infants in our study; neurologic symptoms occurred in approximately 60% of affected patients. [13] The cause of these symptoms is probably due to reduced cerebral blood flow and altered tissue metabolism. [13,14,15] Neurologic manifestations may also be related to metabolic complications such as hypoglycemia and hypocalcaemia.
The respiratory symptoms were unexpectedly high, which may be attributed to the significant number of PC premature infants and the large number of PC infants born by cesarean section, hyperviscosity resulted from PC also can reduce pulmonary blood flow that, in turn, can cause systemic hypoxia. [16] Our study revealed that tachypnea significantly prolong LOS of PC neonates in NICU [Table 3].
In our study, two patients had congenital heart disease. One patient had oliguria, which significantly prolonged LOS as shown by multiple regression analysis [Table 3]. One patient had congenital adrenal hyperplasia. None of PC neonates had necrotizing enterocolitis (NEC), although PET had been done for six PC neonates in the study.
Most of the studies state that most of PC infants are asymptomatic including two large prospective case control studies. In contrast, one cohort study reported only 14% of PC neonates were asymptomatic and having normal laboratory results. [6,17,18] Our study revealed that, 60% of the PC neonates were symptomatic, and only 17% had no symptoms or abnormal LA.
Most common LA in the study was hypoglycemia; which was reported as the most common metabolic complication that occurs in 12-40% of cases. [17,19,20] Hypocalcemia is the second most common lab abnormality; which is reported in 1-11% of neonates who have PC, possibly related to elevated concentrations of calcitonin gene-related peptide in affected infants. [21] Thrombocytopenia is third most common complication in our study, which is reported in 20% of PC neonates, thrombocytopenia may be related to the diversion of hematopoietic progenitors toward erythropoiesis at the expense of other lineages. [22]
About 17% of the variability (adjusted R2: 0.17) of LOS was explained by the variables listed in Table 3. Other variables such as sepsis, duration of antibiotics use, time to reach full feed, etc., that influence LOS were not included in the study, this could explain such a low variability of our variables on LOS.
Blood viscosity and hct have a linear relationship when the hct is <60%. [2,23] This relationship becomes exponential when the hct exceeds 65%. The use of PET in the management of PC is controversial. Although PET improves cerebral blood flow and hemodynamic parameters in infants with hyperviscosity, there is no evidence that the procedure improves long-term outcome of these infants, and it may be associated with NEC. [24,25] The optimal management of PC infants with or without symptoms has not been established and varies among providers, the choices between observations or aggressive hydration is still controversial. The aim of reducing hct in PC infant in our study is to reduce blood viscosity in a symptomatic patient, and to provide early discharge from NICU for asymptomatic PC neonates. In our study, (IVNS bolus + ITFI) was the most successful intervention to reduce hct, followed by ITFI, half of the conservative treatments were successful, although IVF bolus intervention was the most common intervention, it had been the least successful intervention, most of IVF bolus failures were due to rebounds of hcts after initial drop of hct, which may be explained by redistribution of fluid between intracellular and extracellular spaces after sudden hydration with IVF bolus. All of these intervention results were not significant as the P < 0.05 (0.24), also, the results of comparison between different methods of overhydration with each other were not statistically significant [Table 5]. This led to acceptance of the null hypothesis that there was no difference between observation and different intervention groups in reducing the hct according to our study's criteria.
The study revealed that the mean hct peaked maximally at around the mean of 2.8 h of age followed by slow gradual descend until a mean of 77 h where steeper descent started. Similar pattern of hct peak occurs in healthy newborns, where hct increases from birth, reaching a maximum at approximately 2 h of age, then decreases to levels in cord blood by 18 h of age. [26]
One limitation of the study is that because the study was not controlled, and most of PC infants were admitted to NICU because of other conditions associated with PC, the symptoms of PC could not be attributed solely to PC. Other limitation is that more than one noninvasive intervention could occur for the same patient, we suggest that prospective randomized study that provides a patient with a single noninvasive intervention, and fixed follow-up blood sampling time for all the PC neonates can detect if overhydration is better than observation and routine care in decreasing hct.
ACKNOWLEDGMENT
We thank all the employees in the records department, especially Ali Alghamdi, Wajdi Almitiry, and Eissa Alharbi. We thank also Mrs. Alyousef who helped as with the statistics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rkin SH, Nathan DG, Ginsburg D, Look AT, Brugnara C, Platt OS. The neonatal erythrocyte and its disorders. In: Orkin SH, Nathan DG, Ginsburg D, Look AT, editors. Nathan and Oski's Hematology of Infancy and Childhood. 7th ed. Vol. 1. Philadelphia PA: Elsevier; 2009. pp. 36–66. [Google Scholar]
- 2.Gross GP, Hathaway WE, McGaughey HR. Hyperviscosity in the neonate. J Pediatr. 1973;82:1004–12. doi: 10.1016/s0022-3476(73)80433-0. [DOI] [PubMed] [Google Scholar]
- 3.Drew JH, Guaran RL, Grauer S, Hobbs JB. Cord whole blood hyperviscosity: Measurement, definition, incidence and clinical features. J Paediatr Child Health. 1991;27:363–5. doi: 10.1111/j.1440-1754.1991.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 4.Schimmel MS, Bromiker R, Soll RF. Neonatal polycythemia: Is partial exchange transfusion justified? Clin Perinatol. 2004;31:545–53. doi: 10.1016/j.clp.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Committee on Fetus and Newborn: Routine evaluation of blood pressure, hematocrit, and glucose in newborns. Pediatrics. 1993;92:474–6. [PubMed] [Google Scholar]
- 6.Wiswell TE, Cornish JD, Northam RS. Neonatal polycythemia: Frequency of clinical manifestations and other associated findings. Pediatrics. 1986;78:26–30. [PubMed] [Google Scholar]
- 7.Wirth FH, Goldberg KE, Lubchenco LO. Neonatal hyperviscosity: I. Incidence. Pediatrics. 1979;63:833–6. [PubMed] [Google Scholar]
- 8.Rawlings JS, Pettett G, Wiswell TE, Clapper J. Estimated blood volumes in polycythemic neonates as a function of birth weight. J Pediatr. 1982;101:594–9. doi: 10.1016/s0022-3476(82)80716-6. [DOI] [PubMed] [Google Scholar]
- 9.Mimouni F, Miodovnik M, Siddiqi TA, Butler JB, Holroyde J, Tsang RC. Neonatal polycythemia in infants of insulin-dependent diabetic mothers. Obstet Gynecol. 1986;68:370–2. doi: 10.1097/00006250-198609000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger MM, Oleinick A. Congenital marrow dysfunction in Down's syndrome. J Pediatr. 1970;77:273–9. doi: 10.1016/s0022-3476(70)80335-3. [DOI] [PubMed] [Google Scholar]
- 11.Miller M, Cosgriff JM. Hematological abnormalities in newborn infants with Down syndrome. Am J Med Genet. 1983;16:173–7. doi: 10.1002/ajmg.1320160207. [DOI] [PubMed] [Google Scholar]
- 12.Chalouhi GE, Stirnemann JJ, Salomon LJ, Essaoui M, Quibel T, Ville Y. Specific complications of monochorionic twin pregnancies: Twin-twin transfusion syndrome and twin reversed arterial perfusion sequence. Semin Fetal Neonatal Med. 2010;15:349–56. doi: 10.1016/j.siny.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkrantz TS. Polycythemia and hyperviscosity in the newborn. Semin Thromb Hemost. 2003;29:515–27. doi: 10.1055/s-2003-44558. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar S, Rosenkrantz TS. Neonatal polycythemia and hyperviscosity. Semin Fetal Neonatal Med. 2008;13:248–55. doi: 10.1016/j.siny.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Dempsey EM, Barrington K. Short and long term outcomes following partial exchange transfusion in the polycythaemic newborn: A systematic review. Arch Dis Child Fetal Neonatal Ed. 2006;91:F2–6. doi: 10.1136/adc.2004.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herson VC, Raye JR, Rowe JC, Philipps AF. Acute renal failure associated with polycythemia in a neonate. J Pediatr. 1982;100:137–9. doi: 10.1016/s0022-3476(82)80254-0. [DOI] [PubMed] [Google Scholar]
- 17.Black VD, Lubchenco LO, Koops BL, Poland RL, Powell DP. Neonatal hyperviscosity: Randomized study of effect of partial plasma exchange transfusion on long-term outcome. Pediatrics. 1985;75:1048–53. [PubMed] [Google Scholar]
- 18.Black VD, Lubchenco LO, Luckey DW, Koops BL, McGuinness GA, Powell DP, et al. Developmental and neurologic sequelae of neonatal hyperviscosity syndrome. Pediatrics. 1982;69:426–31. [PubMed] [Google Scholar]
- 19.Goldberg K, Wirth FH, Hathaway WE, Guggenheim MA, Murphy JR, Braithwaite WR, et al. Neonatal hyperviscosity. II. Eff ect of partial plasma exchange transfusion. Pediatrics. 1982;69:419–25. [PubMed] [Google Scholar]
- 20.Black VD, Lubchenco LO. Neonatal polycythemia and hyperviscosity. Pediatr Clin North Am. 1982;29:1137–48. doi: 10.1016/s0031-3955(16)34251-1. [DOI] [PubMed] [Google Scholar]
- 21.Saggese G, Bertelloni S, Baroncelli GI, Cipolloni C. Elevated calcitonin gene-related peptide in polycythemic newborn infants. Acta Paediatr. 1992;81:966–8. doi: 10.1111/j.1651-2227.1992.tb12155.x. [DOI] [PubMed] [Google Scholar]
- 22.Acunas B, Celtik C, Vatansever U, Karasalihoglu S. Thrombocytopenia: An important indicator for the application of partial exchange transfusion in polycythemic newborn infants? Pediatr Int. 2000;42:343–7. doi: 10.1046/j.1442-200x.2000.01244.x. [DOI] [PubMed] [Google Scholar]
- 23.Ramamurthy RS. Neonatal polycythemia and hyperviscosity; State of the art. Perinatol Neonatology. 1979;3:38. [Google Scholar]
- 24.Mimouni FB, Merlob P, Dollberg S, Mandel D. Israeli Neonatal Association. Neonatal polycythaemia: Critical review and a consensus statement of the Israeli Neonatology Association. Acta Paediatr. 2011;100:1290–6. doi: 10.1111/j.1651-2227.2011.02305.x. [DOI] [PubMed] [Google Scholar]
- 25.Black VD, Rumack CM, Lubchenco LO, Koops BL. Gastrointestinal injury in polycythemic term infants. Pediatrics. 1985;76:225–31. [PubMed] [Google Scholar]
- 26.Ramamurthy RS, Berlanga M. Postnatal alteration in hematocrit and viscosity in normal and polycythemic infants. J Pediatr. 1987;110:929–34. doi: 10.1016/s0022-3476(87)80417-1. [DOI] [PubMed] [Google Scholar]


