Abstract
Few concepts in recent years have garnered more disease research attention than that of the intestinal (i.e. ‘gut’) microbiome. This emerging interest has included investigations of the microbiome's role in the pathogenesis of a variety of autoimmune disorders, including type 1 diabetes (T1D). Indeed, a growing number of recent studies of patients with T1D or at varying levels of risk for this disease, as well as in animal models of the disorder, lend increasing support to the notion that alterations in the microbiome precede T1D onset. Herein, we review these investigations, examining the mechanisms by which the microbiome may influence T1D development and explore how multi-disciplinary analysis of the microbiome and the host immune response may provide novel biomarkers and therapeutic options for prevention of T1D.
Keywords: autoimmunity, immunology, insulin, intestinal physiology, microbiome
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis. Clinical and Experimental Immunology 2014, 177: 13–23.
Microbial ‘old friends’, immunoregulation and socioeconomic status. Clinical and Experimental Immunology 2014, 177: 1–12.
Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clinical and Experimental Immunology 2014, 177: 24–9.
Helminths in the hygiene hypothesis: sooner or later? Clinical and Experimental Immunology 2014, 177: 38–46.
Overview of type 1 diabetes and involvement of the microbiome in disease development
T1D is a disorder resulting from an immune-mediated destruction of pancreatic beta cells in genetically predisposed individuals 1,2. Poorly understood environmental factors probably contribute to disease development through mechanisms that either trigger the initial autoimmune response, or modify this destructive process at various points throughout the natural history of the disease 3,4. A variety of putative environmental factors have been posited as potential candidates for serving in such a role, the majority being dietary 5–7. However, the specific environmental constituents responsible for eliciting or driving beta cell autoimmunity are unknown.
The intestinal microbiome represents a complex, symbiotic ecological community that influences human health and development, including the education and maintenance of the immune system 8–10. While considered to impact upon a wide array of physiological activities and disease, the role of the intestinal microbiome in autoimmunity has recently garnered significant attention, with evidence suggesting a role for impacting upon the development of a number of disorders including T1D 11–14.
Based on the available body of literature, it is feasible to suggest that the well-described increased incidence in T1D over the past 50 years 15,16 arises, at least in part, from one of two primary mechanisms related to the intestinal microbiome. In the first notion (Fig. 1), defective development and/or alteration of healthy microbiota in an individual at genetic risk for T1D may result in abnormal immunoregulation that enables autoimmune destruction of insulin-producing β cells. This notion is supported by evidence suggesting that immune education required for self/non-self immunoregulation is, to a large degree, conferred early in life, through maturation and education of the immune system by microbiota that colonize the gastrointestinal tract, living symbiotically with the host 18,19. The second concept (Fig. 1), acting either independently of or co-incident with the first, is that enhanced leakiness of the gut epithelial barrier (observed in both human patients and animal models of T1D) either results from an altered microbiome or is a key determinant of an altered microbiome, or ‘dysbiosis’ 17,20. Either type of microbiome-mediated mechanism could underlie the observed combination of increasing disease incidence as well as the younger age of onset 21, resulting from less robust or delayed maturation of immunoregulation in early childhood. Understanding such mechanisms is an important consideration. Indeed, if a central role for the microbiome in T1D risk was confirmed, as will be discussed later, the disease might be preventable by augmenting or accelerating healthy microbiota-induced immunoregulation, as well as by attenuating intestinal leakiness. However, before undertaking such therapeutic efforts, it would appear critical to determine first whether and how an altered microbiome contributes to either defective immunoregulation and/or gut leakiness in T1D.
Fig. 1.
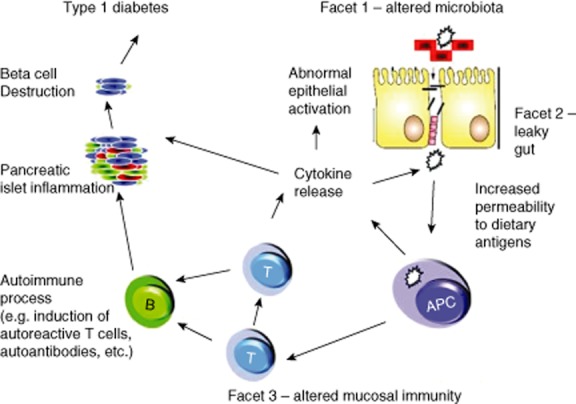
‘The perfect storm’ – a hypothetical model of the contribution of various gut components (including altered immune regulation and gut leakiness) to the pathogenesis of type 1 diabetes. Reproduced with permission 17.
Apart from studies of T1D pathogenesis, longitudinal sampling of the intestinal microbial flora from birth to early childhood has demonstrated a dynamic pattern of microbial colonization, with alterations in the composition of intestinal bacterial communities associated with various life and developmental events, beginning with mode of delivery 9,22. Specifically, clear changes in microbial composition have been observed with weaning and the introduction of cow's milk-based formula or solid foods, illnesses including viral infections and antibiotic treatment, among others 7,23–25. While intellectually intriguing, the role for each of these aspects in T1D pathogenesis remains unclear, but continues to emerge.
Current knowledge of the microbiome in T1D
While the exact genesis for the role of the microbiome in T1D may be of some debate, some of the earliest evidence for such an association arose from studies of BioBreeding Diabetes Prone (BB-DP) rats, where antibiotic treatment decreased the risk for the disease 26,27. This was quickly followed by a similar study utilizing the non-obese diabetic (NOD) mouse 8. Rederivation of NOD mice from conventional to a specific pathogen-free (SPF) setting increased diabetes incidence, suggesting that some microbial exposures may protect against T1D 28. Moreover, experimental exposure to bacterial antigens and infections decreases the risk for T1D in the NOD model 29. Feeding either Lactobacillus reuteri or L. johnsonii to BB-DP rats increased or decreased the risk for diabetes, respectively 30. NOD mice carrying a null mutation in an adaptor for innate immune receptors [myeloid differentiation primary response gene (MYD88)] are protected from T1D and have an altered microbiome compared to wild-type controls 8. Taken collectively, these results suggest that microbial exposures may play a role in the onset of the disease.
With respect to humans, an early microbial 16S rRNA gene sequencing study of faecal bacterial composition in a small Finnish cohort of four pairs of T1D cases and controls demonstrated a higher level of Bacteroidetes relative to Firmicutes approximately 6 months after birth in those who eventually developed T1D, and suggested that this ratio of Bacteroidetes to Firmicutes increased over time in autoimmune cases, but declined in those who did not develop the disorder 14. The case (i.e. T1D) microbiomes were also much less diverse than the control microbiomes. In addition, the case microbiomes were much less similar to each other than were the control microbiomes. These results suggested that the microbiomes of subjects with T1D autoimmunity were much less stable over time than those from control subjects.
This study was followed by a metagenomic analysis of faecal samples of the same four case–control pairs taken at the time that the cases displayed seroconversion to serum anti-islet autoantibodies 11. Bacterial genes associated with production of short chain fatty acids and gut integrity were more abundant in healthy controls than autoantibody-positive cases (i.e. people at increased risk for T1D). This work led to the hypothesis that the fate of lactate is important to gut health.
A more recent study was conducted in Spain, where 16 children with T1D showed increased numbers of Clostridium, Bacteroides and Veillonella and decreased numbers of Bifidobacterium and Lactobacillus compared to 16 healthy children 31. However, unlike the studies described previously that were performed by 16S rRNA gene sequencing, these investigators used polymerase chain reaction (PCR) primer sets designed for certain bacterial genera followed by denaturing gradient gel electrophoresis (DGGE). The discrepancy between studies raises the important caveat that apparent variance in the data can reflect dependency on the methods of detection and analysis.
To exclude the potential impact of the major histocompatibility complex genotype on microbiome composition and examine potential microbiome differences that precede disease onset, a recent study using 16S rRNA gene sequencing examined the faecal microbiome of healthy children matched for age, sex and high genetic risk and discordant for islet autoantibodies 13. This study showed that certain bacteria correlated with the number of positive autoantibodies, potentially indicating a role of dysbiosis as a regulator of β cell autoimmunity in the progression of the autoimmune process towards β cell destruction and clinical disease. In yet another effort 11, a lower abundance of lactate- and butyrate-producing bacterial species was associated with autoantibody status, confirming a relationship of bacterial harbouring specific to beta cell autoimmunity prior to disease onset. Interestingly, several associations between autoantibody status and specific bacterial taxa were significant in only one sex (Bacteroides in males, and Bacteroides fragilis in females). Larger cohorts will be needed to validate potential gender influences on the T1D-associated microbiome.
Although the number and size of studies of the gut microbiome of T1D remains small, they suggest some interesting trends (Table 1). At the taxonomic level, Bacteroides is associated positively with islet autoimmunity for T1D while Firmicutes is associated negatively. Butyrate-producing bacteria may be protective, while those that produce other short chain fatty acids may lead to autoimmunity. These observations may relate to the effects of bacterial fermentation products on gut epithelial integrity. Fermentation of lactate to butyrate is associated with tight junction formation and mucin synthesis and increased integrity of the gut epithelium. In contrast, lactate fermentation to other short chain fatty acids such as propionate, acetate or succinate is not associated with mucin synthesis and tight junction formation and sustenance of an intact epithelial layer 11. Thus, gut health may be favoured when the intestinal microbial community is dominated by butyrate producers and disrupted by dominance of propionate producers. Such findings are, in general, supportive of the aforementioned ‘gut leakiness’ hypothesis, but do not include any functional assessment of these organisms on gut epithelial function. Larger metagenomic and affiliated functional studies will be needed to resolve the many confounding factors affecting the microbial composition, and to validate and refine these trends beyond the initial findings of genus-level associations.
Table 1.
Putative differences in the microbiome of seroconverted versus high-risk non-diabetic people 11,13,14,31).
| Property | Seroconverted subjects | High-risk control subjects |
|---|---|---|
| Dominant phylum | Bacteroidetes | Firmicutes |
| SCFA producers | Succinate, acetate | Butyrate |
| Bacterial diversity | Low | High |
| Functional diversity | Low | High |
| Genus differences | Bacteroides | Bifidobacterium |
| Clostridium | Faecalibacterium | |
| Veillonella | Lactobacillus | |
| Community stability | Low | High |
SCFA = short chain fatty acid.
Uncovering a pathogenic role for the microbiome in T1D – a proposed pathway forward
As mentioned previously, interactions between susceptibility genes and environmental determinants of T1D remain poorly defined 16. The most pressing outstanding questions regarding the microbiome as an environmental determinant in T1D are (i): does the microbiome hold any additional clues into disease aetiology, including potential viral or bacterial antigens and metabolites; (ii) is there a microbiome-wide dysbiosis linked to pathogenesis (i.e. development of autoimmunity, progression of autoimmunity, onset of clinical disease); and (iii) is defective microbiome-induced immunoregulation contributing to pathogenesis of T1D?
Identifying the environmental determinants of disease is quite challenging, and poses a number of requirements on study design. First, subjects need to be studied before the onset and throughout all phases of the disease to capture important transition points. Setting up a prospective cohort for a disease with an incidence rate of ∼10–30 (i.e. intermediate to high) new cases per 100 000 per year in a cost-effective way requires an approach to screen and follow a large number of subjects. One approach is to enrol first-degree relatives (FDR) of T1D patients, who have an elevated risk and are straightforward to identify. However, they represent a fraction of all cases of T1D, as 85–90% of diagnosed patients do not have an FDR with the disease. An alternative is general population screening for T1D genetic risk. Such screening focuses upon the human leucocyte antigen (HLA) class II genes (DRB1, DQA1 and DQB1), which account for approximately 50% of the total genetic contribution to T1D 32. Secondly, in order to capture environmental determinants comprehensively, subjects will need to be examined frequently for a variety of potentially impactful exposures. This includes the collection of diverse sample types (e.g. blood, stool, saliva) and a broad spectrum of information on other environmental factors, such as dietary patterns, psychosocial factors, allergies, living habits, antibiotic use and vaccinations. Because T1D onset can occur very early on in life, such longitudinal screening should start from birth or, preferably, during the prenatal period, including collection of maternal samples. Lastly, the human microbiome is both complex and variable between individuals and within individuals due to developmental and environmental events 33. Study design and analyses need to anticipate controlling or accounting for host genetic, developmental and environmental influences 34 requiring a large enough cohort or cohorts to retain statistical power for multiple interactions.
A number of cohort studies designed to partially address the needs outlined above have ongoing or recently completed sample collections suited to address several aspects of microbiome-targeted questions. These include, but are not limited to, the Finnish studies Diabetes Prediction and Prevention Project (DiPP) 35, the Pathogenesis of Type 1 Diabetes – Testing the Hygiene Hypothesis (DIABIMMUNE) study 36,37, the German studies TEENDIAB 38 and ImmunDiabRisk, the Australian Environmental Determinants of Islet Autoimmunity (ENDIA) study 39 and the international Environmental Determinants of Diabetes in the Young study (TEDDY) study 40. The characteristics of each cohort are tabulated here (Table 2), including the location of the study and age at enrolment, and highlight the shared and unique contributions of each study. For example, DIABIMMUNE can address the relationship between T1D and the hygiene hypothesis by comparing subjects in several countries with different T1D incidence rates; TEENDIAB will identify factors that influence T1D development specifically during puberty; and ENDIA is the largest cohort that enrols subjects prior to birth and from the southern hemisphere.
Table 2.
Overview of cohort characteristics in ongoing microbiome studies.
| Cohort | Location | Enrolment | Cohort size | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | North America | Australia | Prospective | FDR | GP | Pregnancy | Birth | Young children | Teen | Screening | Enrolling | T1D cases | |
| DiPP | ✓ | ✓ | ✓ | ✓ | ✓ | 180 000 | 8500 | 300 | |||||
| DIABIMMUNE | ✓ | ✓ | ✓ | ✓ | ✓ | 12 000 | 4400 | 4 | |||||
| TEENDIAB | ✓ | ✓ | ✓ | n.a. | 1500 | ? | |||||||
| ImmunDiabRisk | ✓ | ✓ | ✓ | ✓ | ✓ | n.a. | 200 | ? | |||||
| TEDDY | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 500 000 | 12 000 | 800 | ||||
| ENDIA | ✓ | ✓ | ✓ | ✓ | n.a. | 1400 | 50 | ||||||
DiPP = Diabetes Prediction and Prevention Project; DIABIMMUNE = Pathogenesis of Type 1 Diabetes – Testing the Hygiene Hypothesis; TEDDY = The Environmental Determinants of Diabetes in the Young study; ENDIA = Environmental Determinants of Islet Autoimmunity; FDR = first-degree relatives; GP = general practitioner; n.a. = not applicable; T1D = type 1 diabetes.
Therapeutic targeting of the gut microbiome to block T1D progression
Experimental microbiome manipulation in young T1D prone rodents provides robust protection from islet-autoimmunity and disease, providing proof of principle that microbial therapy could provide effective protection of individuals with high genetic risk 12. The gut microbiome is extensively remodelled during early postnatal development and throughout childhood and puberty 9,41,42. This natural fluctuation in microbial colonization provides a window of opportunity to modify this risk factor in children with risk markers of anti-islet autoimmunity.
The emerging vanguard in microbial therapeutics is in the treatment of refractory Clostridium difficile infection, a serious problem for patients who are repeatedly hospitalized and treated with antibiotics or living in chronic care facilities, many of them elderly. Faecal microbial therapy (FMT; stool transplant) is a strikingly effective and safe treatment for C. difficile 43,44. An alternate approach could be the use of defined human commensal ecosystems cultured in conditions that recapitulate the human colon 45,46. A recent report demonstrates that such cultured human bacterial consortia substitutes effectively for transplant of an intact faecal community to treat C. difficile infection 47. These successes demonstrate that this infection is caused by disruption of the normal microbiome. However, as the disease aetiology, progression and pathogenesis for C. difficile and T1D are markedly different, the likelihood of FMT being successful in T1D or other inflammatory diseases is unclear. For example, in inflammatory bowel disease, which has a different aetiology from C. difficile, outcomes have been mixed in early clinical studies of FMT for treatment 44,48,49. These clinical results underscore the nascent stage of this therapeutic approach and need for a better understanding of the status of the gut epithelial, gut commensal community and immune response disturbances associated with specific disease to design microbial therapeutic approaches. Furthermore, an FMT approach is probably unsuitable at present for T1D prevention trials in children because of the problems of standardizing stool composition that could include communicable pathogens. Rather, microbial therapeutics for T1D prevention will require the development and manufacture of reproducible, bacterial consortia with well-defined genetic content and metabolic output, such as an adult synthetic community used recently for C. difficile treatment 47. Synthetic culture microbial consortia can be grown to steady state, carefully characterized and monitored. Although these culture consortia will persist in treated subjects, their genome content, metabolic output and antibiotic response profiles will be known, facilitating their removal if necessary. Given the developmental dynamics of the gut microbiome, microbial therapeutics to treat young, healthy children with T1D risk markers will probably be derived from healthy paediatric stool samples. An alternative focus has been on probiotic preparations composed of small numbers of well-characterized strains that can be bulk-produced, carefully monitored and have long shelf-life. The safety record of these preparations is excellent; these organisms are excreted from treated subjects and do not become part of the commensal landscape. However, there is minimal evidence of the effectiveness of probiotic strains to treat existing human diseases associated with dysbiosis or aberrant immunoregulation.
Manipulating microbiota-induced immunoregulation through non-bacterial means is also being considered. For example, while the BABYDIET study, which studied the delayed introduction of gluten in infants with a genetic risk of islet autoimmunity, was shown to be safe, it had no effect on developing autoimmunity 50. However, in a recent study, NOD mice were raised on either gluten-containing chows (GCC) or gluten-free chows (GFC). GFC-fed mice had a significantly reduced incidence of hyperglycaemia, which was associated with changes in bacterial genera compared to the GCC-fed mice 51. While the effects of gluten on disease development in a human trial were different than in the NOD study, these studies show the feasibility of early dietary interventions in T1D and the further need to study whether and if these interventions alter the microbiota and associated effects on immunoregulation and disease development. Other orally delivered therapeutic approaches should be studied for their possible effects on the gut microbiome and T1D. Parasitic helminths, with their potent immunomodulatory effects, have been implicated in protection from autoimmune disease pathogenesis. Infection with helminths has been shown to reduce disease incidence in preclinical models of T1D 52. A randomized clinical trial of oral administration of helminthic eggs (Trichuris suis ova) was completed in Crohn's disease 53. While the treatment was well tolerated, there were no clinically significant changes in disease course or severity. However, such immune-modulators might show efficacy if given before the onset of disease symptoms, for example in people with genetic and immunological markers of T1D risk. Orally administered anti-CD3 monoclonal antibody is biologically active in the gut and suppresses experimental models of T1D 54. In clinical trials of intravenous anti-CD3 monoclonal antibody (mAb) given to new-onset individuals, post-hoc analysis demonstrated an improvement in insulin production and a reduction in the use of exogenous insulin in some patients 55. In future clinical studies with oral anti-CD3 mAb it would be useful to collect longitudinal stool samples for sequence analysis of potential effects on the gut microbiome.
However exciting the research and encouraging the prospects to prevent progression to diabetes in clearly defined at-risk individuals, significant microbial product development, regulatory and funding hurdles lie ahead. Drugs that are single synthetic or protein molecules dominate the pharmaceutical industry. In contrast, it is likely that the beneficial effects of commensal microbes result from a suite of metabolic compounds whose regulation requires their residence in a community ecosystem, and so may not be reducible to single molecules. Live microbes are distinct from all other classes of existing therapeutics, and their clinical development will require new regulatory frameworks. Moreover, there is no clear path to creating patented technology based on live microbes in order to wall-off proprietary technologies. Nevertheless, the advantages of microbial therapies are many: their capacity to colonize the subjects may create long windows of beneficial effects; their production would be far less costly than protein biologicals; and their administration far less invasive than surgery. As genetic markers of autoimmune disease risk are translated to use in prospective identification of high-risk infants, there will be an opportunity to evaluate the potential of exposures to non-pathogenic bacteria, or their complex products, to prevent or delay T1D or other human autoimmune diseases.
Challenges and opportunities
Our continued understanding of the role of the microbiome in T1D development is underscored by the need to build a comprehensive understanding of microbiota-induced immunoregulation. Table 3 conveys a series of current challenges that remain for deciphering the contributions of the microbiome in T1D, yet each one also forms an important if not vital opportunity for investigation. Furthermore, given that there is a strong and highly variable genetic component to T1D risk outside that of HLA 56, the contributions of the host's genetic profile should not be ignored. Even with robust collection of well-characterized samples, it may be unlikely that all environmental alterations can be determined and taken into account when analysing the interplay between the host, the external environment and the microbiome. It may be easier to begin to untangle this complex interaction in more genetically and culturally homogeneous populations where the T1D incidence is high, such as in Finland. Furthermore, as discussed above, the DIABIMMUNE study, in its study design looking at genetically similar, but environmentally disparate cohorts, may be a valuable resource into identification of putative microbiome factors that influence disease progression. We also need to understand how factors before birth and during pregnancy, including the role of the maternal environment, impact upon disease development. The ENDIA study 39 and others in development will enrol mothers during pregnancy and their infants from birth, providing essential information of the role of the maternal environment in T1D development. Despite their size, careful design and execution, individual longitudinal studies (DIABIMMUNE, TEDDY, ENDIA and others) may not provide definitive answers on their own. Larger cohort studies are needed to gain more resolution and statistical power to test the associations found from these preliminary studies. Furthermore, multiple microbiome and multi-'omic studies should be performed in parallel, with a collaborative plan for meta-analysis across analytical platforms to arrive at robust conclusions about the ability of the microbiome to confer disease susceptibility in individuals at risk for T1D.
Table 3.
Key questions for future research into the role of the microbiome in type 1 diabetes.
| • Does altered maturation or development of an adult microbiome or a dysbiotic state contribute to the pathogenesis of human type 1 diabetes, what is the mechanism(s), and when does it occur? |
| • Does an altered microbiome or dysbiosis act at the level of initiation of autoimmunity and/or progression of type 1 diabetes? |
| • What is the basis of healthy microbiome-induced immunoregulation and does the lack of such contribute to the pathogenesis of human type 1 diabetes? |
| • Is altered gut epithelial function and integrity important in the pathogenesis of type 1 diabetes, and if so, what is the mechanism(s) and relation to dysbiosis and how do we demonstrate impaired function in humans? |
| • How important are the interactions between host genetics, metabolism and the immune system in shaping the microbiome and predilection to disease? |
| • Are faecal samples an appropriate representation of the microbiome for type 1 diabetes studies? |
| • What are the most promising type 1 diabetes preventive/therapeutic opportunities targeting the microbiome, microbiome-induced immunoregulation, or microbiome-altered gut permeability? |
Important operational hurdles must be overcome to achieve multi-study data analysis and integration. First, there are no universal standard operating procedures (SOPs) for the collection, transport and storage of biological samples with the probable outcome of substantial variability between studies. Commensals are largely fastidious anaerobes that die rapidly upon contact with room air, so stool collection protocols are essential to preserve microbial complexity, particularly for metabolic and functional analyses of these organisms. Thus, even with the current large studies, such as TEDDY and DIABIMMUNE, the emerging data may only suggest effects of the microbiome on T1D development, but importantly will inform the design of future studies that begin in pregnancy to survey the prenatal period of development. As part of the second phase of the Human Microbiome Project (HMP2), SOPs will be developed for biospecimen collection, and computational tools and analytical approaches will be advanced that will guide microbiome research. As SOPs and integrative meta-analyses are developed and accepted by the community, the data generated from future programmes will provide more definitive answers to the questions addressed in this paper.
Current microbiome studies are focused heavily upon resident bacteria, with little attention to eukaryotic viruses, bacteriophage, fungi and other microorganisms that are known to be resident in the gastrointestinal tract 57. A major challenge here is that fewer than 5% of the genomic sequences of these organisms have been annotated. Considerable work is required to predict the potential functions of these sequences and begin to correlate them with disease risk and progression.
Conclusions
Determining the underlying causes of T1D have been challenging, and unravelling the contribution of the microbiome in T1D development may prove especially difficult. The field needs well-co-ordinated research efforts to define the role of the gut microbiome in T1D, with a focus upon robust data collection and analyses, to address specific objectives. The longitudinal collection of well-characterized and high quality samples, beginning in pregnancy and continuing up to and through T1D onset, should be a priority in order to understand the earliest microbial effects that may impact upon immune dysregulation, alterations in gut integrity or other undefined mechanisms contributing to this disease. Furthermore, an additional focus of future studies should be the functionality of microbes associated with T1D risk or prevention with integrated analyses of the host and microbial metabolome, transcriptome and proteome. Such efforts will enable testing hypotheses related to specific host and microbial functional pathways in T1D development. Once the functional pathways at the host–immune interface are identified and validated, rational approaches for therapeutic targeting can be identified to prevent and/or cure this disorder.
Disclosures
None to declare.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2013;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304:1020–1022. doi: 10.1136/bmj.304.6833.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 5.Klemetti P, Savilahti E, Ilonen J, Akerblom HK, Vaarala O. T-cell reactivity to wheat gluten in patients with insulin-dependent diabetes mellitus. Scand J Immunol. 1998;47:48–53. doi: 10.1046/j.1365-3083.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 6.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 8.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108(Suppl. 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown CT, Davis-Richardson AG, Giongo A, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLOS ONE. 2011;6:e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 13.de Goffau MC, Luopajärvi K, Knip M, et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 16.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl. 2):S125–136. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 17.Vaarala O, Atkinson MA, Neu J. The ‘perfect storm’ for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen CH, Nielsen DS, Kverka M, et al. Patterns of early gut colonization shape future immune responses of the host. PLOS ONE. 2012;7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 21.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patelarou E, Girvalaki C, Brokalaki H, Patelarou A, Androulaki Z, Vardavas C. Current evidence on the associations of breastfeeding, infant formula, and cow's milk introduction with type 1 diabetes mellitus: a systematic review. Nutr Rev. 2012;70:509–519. doi: 10.1111/j.1753-4887.2012.00513.x. [DOI] [PubMed] [Google Scholar]
- 24.Lempainen J, Tauriainen S, Vaarala O, et al. Interaction of enterovirus infection and cow's milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes Metab Res Rev. 2012;28:177–185. doi: 10.1002/dmrr.1294. [DOI] [PubMed] [Google Scholar]
- 25.Oikarinen S, Tauriainen S, Hober D, et al. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2013;63:655–662. doi: 10.2337/db13-0620. [DOI] [PubMed] [Google Scholar]
- 26.Brugman S, Klatter FA, Visser JT, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz RF, Neu J, Schatz D, Atkinson MA, Wasserfall C. Comment on: Brugman S et al. (2006) Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 49:2105–2108. Diabetologia. 2007;50:220–221. doi: 10.1007/s00125-006-0526-7. [DOI] [PubMed] [Google Scholar]
- 28.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 29.King C, Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLOS ONE. 2011;6:e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau K, Benitez P, Ardissone A, et al. Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6.2-mediated Th17 bias. J Immunol. 2011;186:3538–3546. doi: 10.4049/jimmunol.1001864. [DOI] [PubMed] [Google Scholar]
- 31.Murri M, Leiva I, Gomez-Zumaquero JM, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case–control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagopian WA, Erlich H, Lernmark A, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12:733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246–257. doi: 10.1016/j.trsl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Lönnrot M, Korpela K, Knip M, et al. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49:1314–1318. doi: 10.2337/diabetes.49.8.1314. [DOI] [PubMed] [Google Scholar]
- 36.Peet A, Hämäläinen AM, Kool P, et al. Early postnatal growth in children with HLA-conferred susceptibility to type 1 diabetes. Diabetes Metab Res Rev. 2013;30:60–68. doi: 10.1002/dmrr.2449. [DOI] [PubMed] [Google Scholar]
- 37.Peet A, Kool P, Ilonen J, Knip M, Tillmann V, Group DS. Birth weight in newborn infants with different diabetes-associated HLA genotypes in three neighbouring countries: Finland, Estonia and Russian Karelia. Diabetes Metab Res Rev. 2012;28:455–461. doi: 10.1002/dmrr.2303. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler AG, Meier-Stiegen F, Winkler C, Bonifacio E, Group TS. Prospective evaluation of risk factors for the development of islet autoimmunity and type 1 diabetes during puberty – TEENDIAB: study design. Pediatr Diabetes. 2012;13:419–424. doi: 10.1111/j.1399-5448.2011.00763.x. [DOI] [PubMed] [Google Scholar]
- 39.Penno MA, Couper JJ, Craig ME, et al. Environmental determinants of islet autoimmunity (ENDIA): a pregnancy to early life cohort study in children at-risk of type 1 diabetes. BMC Pediatr. 2013;13:124. doi: 10.1186/1471-2431-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8:286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 41.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLOS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 44.Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–1630. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 45.McDonald JA, Schroeter K, Fuentes S, et al. Evaluation of microbial community reproducibility, stability and composition in a human distal gut chemostat model. J Microbiol Methods. 2013;95:167–174. doi: 10.1016/j.mimet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Allen-Vercoe E, Reid G, Viner N, et al. A Canadian Working Group report on fecal microbial therapy: microbial ecosystems therapeutics. Can J Gastroenterol. 2012;26:457–462. doi: 10.1155/2012/213828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrof EO, Claud EC, Gloor GB, Allen-Vercoe E. Microbial ecosystems therapeutics: a new paradigm in medicine? Benef Microbes. 2013;4:53–65. doi: 10.3920/BM2012.0039. [DOI] [PubMed] [Google Scholar]
- 48.Kump PK, Gröchenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 49.van Nood E, Speelman P, Nieuwdorp M, Keller J. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. 2014;30:34–39. doi: 10.1097/MOG.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 50.Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301–1305. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marietta EV, Gomez AM, Yeoman C, et al. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLOS ONE. 2013;8:e78687. doi: 10.1371/journal.pone.0078687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooke A, Tonks P, Jones FM, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 53.Sandborn WJ, Elliott DE, Weinstock J, et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Aliment Pharmacol Ther. 2013;38:255–263. doi: 10.1111/apt.12366. [DOI] [PubMed] [Google Scholar]
- 54.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 55.Herold KC, Gitelman SE, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minot S, Grunberg S, Wu GD, Lewis JD, Bushman FD. Hypervariable loci in the human gut virome. Proc Natl Acad Sci USA. 2012;109:3962–3966. doi: 10.1073/pnas.1119061109. [DOI] [PMC free article] [PubMed] [Google Scholar]


