Abstract
There is increasing recognition that exposures to infectious agents evoke fundamental effects on the development and behaviour of the immune system. Moreover, where infections (especially parasitic infections) have declined, immune responses appear to be increasingly prone to hyperactivity. For example, epidemiological studies of parasite-endemic areas indicate that prenatal or early-life experience of infections can imprint an individual's immunological reactivity. However, the ability of helminths to dampen pathology in established inflammatory diseases implies that they can have therapeutic effects even if the immune system has developed in a low-infection setting. With recent investigations of how parasites are able to modulate host immune pathology at the level of individual parasite molecules and host cell populations, we are now able to dissect the nature of the host–parasite interaction at both the initiation and recall phases of the immune response. Thus the question remains – is the influence of parasites on immunity one that acts primarily in early life, and at initiation of the immune response, or in adulthood and when recall responses occur? In short, parasite immunosuppression – sooner or later?
Keywords: allergy, autoimmunity, immunoregulation, infection, therapy
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis. Clinical and Experimental Immunology 2014, 177: 13–23.
Microbial ‘old friends’, immunoregulation and socioeconomic status. Clinical and Experimental Immunology 2014, 177: 1–12.
Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clinical and Experimental Immunology 2014, 177: 24–9.
The intestinal microbiome in type 1 diabetes. Clinical and Experimental Immunology 2014, 177: 30–7.
Introduction
The ability of infectious agents to regulate the immune system of their host is an increasingly fascinating topic. In particular, the question is often raised as to whether the ‘diseases of modernity’ (such as allergies, autoimmunity and the metabolic syndrome) are a consequence of our altered, and diminished, exposure to infectious diseases 1–3. Following a wide range of studies in humans and model systems, good evidence has now emerged that both microbes and macroparasites can sufficiently distract or depress immune reactivity to alleviate allergic and autoimmune pathologies 4–7.
The ‘hygiene hypothesis’ takes a number of forms which are not exclusive, but have yet to be articulated as a unifying concept. An inverse relationship between parasite infection and immune disorders was first suggested by Greenwood, who noted the low incidence of rheumatoid arthritis in West Africa 8, and then showed that mice and rats infected with rodent malaria were protected from autoimmune disease 9,10. Subsequently, the hygiene hypothesis became linked explicity to the setting of more developed countries when Strachan postulated that early-life exposure to common childhood infections protected younger siblings in larger families from developing allergies such as hay fever 11,12. At that time, soon after the emergence of the paradigm of opposing T helper type 1 (Th1) and Th2 arms of the immune system 13, this finding was interpreted as Th1-promoting viral and bacterial infections ‘educating’ the young immune system away from excessive and allergy-promoting Th2 responses. The core concept of infections imprinting the developing immune system has become embedded in most versions of the hygiene hypothesis, but the mechanistic explanation of opposing Th1/Th2 lineages has, over time, proved untenable.
In contrast to the Th2-mediated allergies [mediated through immunoglobulin (Ig)E, mast cells and eosinophils], other modern maladies such as type I diabetes, multiple sclerosis and Crohn's disease are driven by Th1 responses, or by the more recently defined Th17 cells 14. Critically, these Th1/17 conditions have been increasing in prevalence in high-income countries as sharply as Th2-dependent allergies. For example, the incidence of type I diabetes is increasing year-on-year at the rate of 3·4% 15, in tandem with the rise in asthma 16. Tellingly, the age of diabetes onset has become significantly younger, indicating that this disease is gaining in force within the population. Similar patterns have been observed for multiple sclerosis and Crohn's disease, and it is difficult to reconcile the accentuation of these diseases with reduced exposure to Th1-stimulating microbes in early life.
Globalization of the hygiene hypothesis
A new perspective on the original hygiene hypothesis was introduced by a study of children in Gabon. In this West African country there is a high prevalence of schistosomiasis, a helminth worm disease which drives a strong Th2 phenotype in the infected host 17. Remarkably, schoolchildren carrying this infection showed lower levels of allergic reactivity than their uninfected classmates, although both the parasite and the allergen evoke Th2 responsiveness 18. A key pointer from this study was that the infected children generated high levels of an immunosuppressive cytokine, interleukin (IL)-10, when their peripheral T cells were challenged with antigen from the Schistosoma parasite. Numerous other studies, such as those in Brazilian children infected with schistosomes 19 and Ecuadorians with soil-transmitted nematodes 20, support the conclusion that helminth infections in many – but not all 21,22 – settings are associated with suppression of allergic reactivity.
IL-10 is one of two central mediators, together with transforming growth factor (TGF)-β, which act to dampen and down-regulate the immune system 23,24. While each can be produced by a range of cell types, they are particularly associated with a T cell subset that was defined between 1995 and 2000, the regulatory T cell (Treg) 25,26. Significantly, Tregs and their products (including IL-10 and TGF-β) were found to control both Th2 allergies 27,28 and the Th1/17 suite of autoimmune and inflammatory pathologies 29. Hence, the hygiene hypothesis has been modified and updated to posit that infections, and infection experience, may set the balance between Tregs, on one side, and active Th1, Th2 and Th17 populations on the other 1,6,30,31.
This emerging paradigm has been strengthened by a study of multiple sclerosis patients in Argentina, 12 of whom were found to have adventiously acquired asymptomatic gastrointestinal helminth infections. All 12 remained in remission for 5 years, while uninfected patients with similar disease severity at the outset of the study suffered multiple relapses 32. Infected patients showed strong IL-10 and TGF-β responses, unlike the relapsing uninfected individuals, but production of these cytokines declined in four patients subsequently given anthelminthic treatment who went on to develop exacerbation of disease 33. In a related example, an ulcerative colitis patient who deliberately self-infected with the human whipworm, Trichuris trichiura, reported significant alleviation of pathology 34. These reports have fuelled greater interest in the possibilities of therapy of inflammatory disorders with live helminths, or with products derived from these parasites, as discussed further below.
Other key studies have reported that anthelmintic treatment of children in parasite-endemic countries increases their allergic sensitivity, providing a causal link between helminth infection and protection of allergy 35. Although autoimmune disease has a relatively low incidence in these countries, measurements of anti-nuclear autoantibody in serum (a precursor but not a determinant of overt autoimmunity) showed reduced reactivity in schistosome-infected individuals and an increase following curative drug therapy 36.
Reports such as these have taken the hygiene hypothesis onto a global stage, and highlighted the contrast in immunological status and reactivity between the affluent developed world and the low-income countries in which helminth parasites are still highly prevalent. Furthermore, although more than 25% of the world's populations are infected with helminth parasites 37, it should be remembered that until the last century most humans would have frequently carried helminth parasites and that our immune system has intimately co-evolved with these organisms, a point we will return to subsequently.
Helminths and regulation of the immune system
The finding that helminth infection is associated with reduced allergic reactivity chimed with a range of immunological studies on both mice and humans which demonstrated enhanced activity of Tregs in infected hosts. Humans carrying long-lived chronic infections with schistosomes or mosquito-borne filarial nematodes displayed a clear phenotype of immunological down-regulation, with high levels of IL-10 38 and stronger suppressive activity of Tregs 39. In particular, asymptomatic carriers who were effectively immunologically tolerant to the parasite presented a ‘modified’ Th2 in which IL-4 is produced but the pro-eosinophilic cytokine IL-5 is suppressed 40. However, in cases which do not tolerate helminths well, and which progress to various forms of pathology, Tregs are deficient and Th1/17 dominates 41.
The implication that the ability of helminths to suppress immunopathologies is mediated by Tregs has been validated in experimental animals. Mice infected with the intestinal nematode Heligmosomoides polygyrus show expanded Treg numbers and function 42–45 and are resistant to allergic pathology in mouse models 46. Moreover, protection against allergy can be transferred by Tregs from an infected animal into uninfected but allergen-sensitized hosts 46,47. Just as humans appear to be protected from autoimmunity as well as allergy by some helminth infections, so too do mice show diminished levels of colitis 48,49 and type I diabetes 50–52 when carrying schistosome or intestinal nematode infestations.
Helminths are thought to promote Tregs to prolong their own survival in the host by defusing key elements of the immune system that would otherwise attack them 53. To test this supposition, strategies to experimentally deplete Tregs in helminth-infected mice have been studied, showing more rapid parasite killing following depletion 54,55. In addition, Treg depletion can result in more severe pathology resulting directly from the presence of helminth worms in the intestinal tract 56,57. As well as Tregs, however, helminths can drive regulatory B cell populations 58–60, natural killer T cells 61,62 and suppressive macrophage responses 63,64, each contributing to a profoundly down-modulated state.
Early in life
The importance of early-life exposure in defining the set-point of immunological reactivity of the individual is now widely recognized 65. The window of sensitivity extends prenatally to the developing foetus, as the propensity of the newborn to develop allergic eczema is altered by maternal infection or exposure to probiotic bacteria. After parturition, exposure to microbial products from environmental organisms may also be sufficient to limit allergic reactivity 66.
Helminth parasites are certainly an important element of the environmental education imparted to the developing immune system. Offspring of mothers harbouring a filarial infection (Wuchereria bancrofti) during pregnancy were found in adulthood to be immunologically tolerant to the parasite 67. A broader effect, beyond antigen-specific tolerance, has now been reported in which helminth infection during pregnancy protects the newborn from allergic eczema in childhood 68,69. It will be fascinating to follow these cohorts of well-characterized children into adulthood, to evaluate more clearly the relative importance of preterm, infant and adult infectious exposure to the functioning of the immune system.
It is important to take note of the harmful, as well as beneficial, effects of helminth infection on the immune system in early life. In helminth-endemic areas of developing countries, vaccination is less effective than in the developed world, with the polio vaccine showing only 70–90% seroconversion rates in the former, compared to 97–100% in the latter 70. Furthermore, anthelmintic treatment of infected populations prior to vaccination leads to increased efficacy of the cholera and bacilli Calmette–Guérin (BCG) vaccines 71–73. To test if maternal imprinting was depressing early-life responsiveness, anthelmintic treatment of pregnant mothers was combined with subsequent testing of vaccination responses in their offspring. However, maternal treatment had no effect on vaccine efficacy in children 74, implying that infections of children themselves are most important for suppression of vaccine responses. In this same trial, however, levels of childhood eczema were again shown to increase in the children of anthelmintic-treated mothers, implying a maternal imprinting role on the development of allergic but not vaccine responses 75.
Later in life – helminth therapy
A distinct strand of the hygiene hypothesis has developed which postulates that responses of the mature immune system can also be modulated significantly by infectious organisms, sufficiently so for particular commensal microbes or organisms of limited pathogenicity to be considered as potential therapies or prophylactics. Most of the experimental evidence for the hygiene hypothesis is also derived from infections of adult animals prior to or during the induction of an allergic or autoimmune inflammatory disease.
The principle that infectious agents can dampen inflammation in the adult immune system is currently being put to the test with a number of clinical trials utilizing live Trichuris suis or Necator americanus parasites for various indications, including Crohn's disease 76, coeliac disease 77 and multiple sclerosis 78, among others. Not all trials to date have proved successful, however, with treatment of allergic rhinitis found to be not beneficial 79,80, and most recently with Crohn's disease reporting benefits for some patients but failing to achieve statistical significance 81. Similarly, while the human hookworm N. americanus was found to dampen responses in coeliac disease patients, it did not achieve a level that significantly alleviated symptoms 77,82. While discouraging, these trials have been carried out on unstratified patient groups and there may well be subsets within each disease who are most likely to show improvement during infection. In the longer term, it may also prove desirable to identify immunosuppressive molecules from these parasites that can serve as future drug leads, thereby dissociating any beneficial properties of helminths from the need to impose active infection on patients.
An interesting parallel exists between the effect of helminth infection and specific immunotherapy (SIT) in which the patient is desensitized against particular allergens 83. In both, the immune response switches to the IgG4 isotype 84,85. Moreover, anthelmintic clearance of parasites results in rapid loss of IgG4 (and in other settings, exacerbation of allergic and autoimmune reactivity). These instances argue that the mode and degree of immune responsiveness remains relatively plastic in adult life, and that contemporary interactions with extant infection is as much or more influential on disease outcome than historical experience.
Early and late in the immune response
Immunopathologies require both initiation and sustenance of the immune response against target allergens, autoantigens or bystander antigens (such as those expressed by harmless commensals). Hence, interventions may be either or both prophylactic or therapeutic, and the appropriate choice depends on correctly identifying the initiating factors (for example, antigen uptake) and those which maintain responsiveness and are responsible for progressive tissue damage (such as inflammatory cytokines).
Much interest is therefore focused on the cell type(s) involved in kick-starting the inflammatory response, in particular the prototypical antigen-presenting cell, the dendritic cell (DC) which, following infection, both takes up pathogen products for presentation to T cells and up-regulates a range of stimulatory molecules to drive T cell activation (Fig. 1). However, helminths can interfere dramatically with this critical process, suppressing the maturation of DCs following Toll-like receptor (TLR) ligation, reducing their production of inflammatory cytokines and reducing T cell responsiveness 86–88. In the case of schistosome egg antigen, this effect is due to omega-1, a glycoprotein which degrades intracellular host RNA, resulting in defective up-regulation of T cell-activating inflammatory cytokines such as IL-12 89. Some helminths may divert DC function entirely, as in the case of H. polygyrus infection, which expands a CD11cloCD103− dendritic cell phenotype which induces Tregs through retinoic acid release 90. Helminth inhibition of DCs is thus a potent method to block both initiation and recall of immune responses by naive or memory T cells.
Fig. 1.
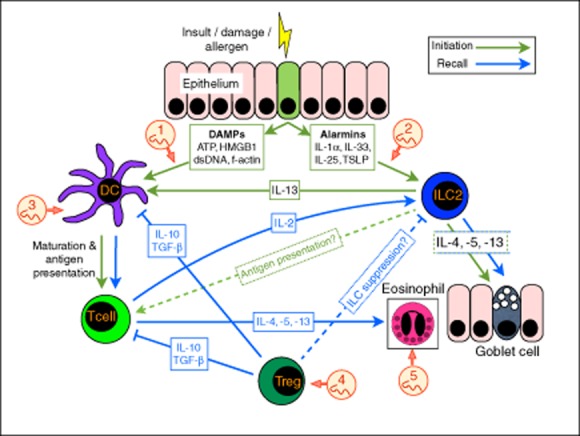
Sooner and later. The immediate response to immunological insult (in the form of injury, allergens or other toxic products) that causes epithelial cell stress and death, is the release of damage-associated molecular patterns (DAMPs), such as adenosine triphosphate (ATP), dsDNA, high-mobility group box 1 (HMGB1) and f-actin. Concurrently with, or consequent to DAMP release, the alarmin cytokines interleukin (IL)-1α, IL-33, IL-25 and thymic stromal lymphopoietin (TSLP) are induced. Both DAMPs and alarmins result in activation of dendritic cells (DCs) and group 2 innate lymphoid cells (ILC2). The responses occurring soon after immune stimulus are coloured green. ILC2 produce the type 2 cytokines interleukin (IL)-4, IL-5 and IL-13, resulting in subsequent expansion of effector cell populations such as eosinophils and goblet cells which can occur at both early (green arrows) and late (blue arrows) phases. ILC2-derived IL-13 also induces maturation and migration of DCs to the draining lymph node, where DCs present antigen to naive T cells. Although ILC2 express major histocompatibility complex (MHC) class II, it is unknown whether they present antigen directly to T cells in vivo. Through the combined efforts of ILC2 and DC, antigen-specific T helper type 2 (Th2) cells differentiate, and produce type 2 cytokines, further stimulating, recruiting and activating type 2 effector cells, as well as IL-2, activating and expanding ILC2 cells (blue arrows). Regulatory T cells (Tregs) produce immunosuppressive cytokines such as IL-10 and transforming growth factor (TGF)-β, suppressing T cells and DC responses (blue solid line), and may also suppress ILC2 responses (blue dashed line). Helminths and their products are known to suppress through at least five key mechanisms (indicated in numbered red circles): (1) secretion of apyrases, enzymes which can degrade the inflammatory DAMP ATP to non-inflammatory adenosine monophosphate (AMP) 99,100; (2) secreted products which inhibit the release of IL-33 96; (3) a range of products suppress DC maturation to Toll-like receptor (TLR) signals 86–89; (4) secretions induce Tregs through the transforming growth factor (TGF)-β pathway 107; and (5) secreted enzymes degrade eotaxin, a chemokine required for eosinophil attraction 103.
As well as DCs, the recent recognition of a new category of immunocyte, the innate lymphoid cell (ILC), has expanded our knowledge of immune response initiators. While present in only small numbers, ILCs are capable of initiating type 2 allergy (in the case of the ILC2) 91 or type 1 colitis (in the case of ILC1/3 92). ILCs are activated by early innate cytokines such as IL-12 and IL-18 (ILC1); IL-25, IL-33 and TSLP (ILC2); and IL-1β and IL-23 (ILC3) 93. Their contribution forms part of an integrated sensory system reliant on epithelial and stromal cell signalling, which acts to raise the first alarm when the body is invaded by pathogens or subject to other traumas. Recent data show further that they can be integral to the initiation of T cell responses through provision of IL-13 and DC recruitment 91, as well as the amplification of T cell responses, as ILCs are activated through T cell provision of IL-2 94. Furthermore, several authors have established that ILCs can express class II major histocompatibility complex (MHC) and may thereby be able to present antigen to T cells 94,95. Thus, the ILC2 compartment presents an important target for helminth immunoregulation (Fig. 1).
A link between the ILC2 network and helminth immunomodulation has emerged very recently through the blockage of IL-33 production by secreted products of H. polygyrus (HES; Fig. 1); as IL-33 is a key driver of ILC2 activation, its loss thereby ablates the immediately subsequent ILC response required for induction and amplification of allergic responses 96. Furthermore, both H. polygyrus infection and HES induce the release of IL-1β from macrophages, which counter-regulates IL-25 and IL-33, resulting in diminished type 2 responses and greater susceptibility to chronic helminth infection 97. IL-33 itself is induced by the damage-associated molecular pattern (DAMP) extracellular adenosine triphosphate (ATP), which is released from stressed and damaged cells 98. Many parasite secretions, including HES, contain multiple apyrase enzymes 99,100 [which degrade inflammatory ATP to non-inflammatory adenosine monophosphate (AMP)], illustrating the multiple mechanisms through which helminths may interfere with DAMP, alarmin and ILC responses.
However, many questions remain as to the role of ILCs in long-term allergic conditions (such as steroid-resistant asthma), whether the ILC set-point is formed early in life and whether there are regulatory ILCs as well as inducers. The interdependence of T cell and ILC responses during initiation and recall of the immune response also remain to be elucidated: the potential role of ILC antigen presentation and the potential role of Treg suppression on ILCs are as yet unclear (Fig. 1), and may provide further insights into the mechanisms of immune suppression used by helminth parasites.
Downstream of the induction process, whether through DCs and/or ILCs, the adaptive immune system is activated and amplified, in turn stimulating key innate effector cell populations such as neutrophils, macrophages and granulocytes 53. It appears that helminths are no less able to also modulate these innate populations. For example, both the dog hookworm Ancylostoma caninum and the livestock parasite Haemonchus contortus export factors which inhibit neutrophil activation 101,102. Moreover, human hookworm products also cleave eotaxin, suppressing recruitment of eosinophils 103. Alternatively activated macrophages differentiate in many helminth infection settings, and are able to both directly suppress bystander T cell proliferation 63 and to induce further forkhead box protein 3 (FoxP3+) Treg differentiation 104. These type 2 macrophages mediate wound-healing effects 105 and quell inflammation in inflammatory conditions such as colitis 106.
Forward look
How do we now re-evaluate the hygiene hypothesis in the light of the updated immunological picture? A number of new conclusions can be drawn that will advance the discussion of this stimulating concept. First, there is increasing focus on the initial ‘spark’ that ignites the allergic and inflammatory pathway. It seems likely that many infectious organisms have evolved means to suppress early ‘alarm’ signals, such as IL-33, and so may minimize the likelihood of a proinflammatory response being mounted.
Secondly, although this spark initiates a preliminary round of cellular reactions from innate cells such as monocytes and granulocytes, the response cannot take hold without positive feedback and amplification through signals and cytokines from the CD4+ T cell compartment. In this sense, T cells remain in control of the outcome of all immune responses, and promotion of anti-inflammatory regulatory T cells is likely to remain a keystone of any hygiene hypothesis formulation.
Thirdly, although effector T cell involvement supports a more vigorous, extensive and long-lived reaction, this inflammatory response is composed largely of innate cell types ranging from eosinophils and other granulocytes through to cells of the monocyte lineage. In many epidemiological instances of infections modulating pathology, underlying T cell phenotypes are not greatly altered but there is a powerful dampening of innate effector populations that deserves further investigation.
Finally, if our understanding of the hygiene hypothesis is to be translated into future therapies, these will largely need to be applied to patients in whom inflammatory diseases have already taken hold. Hence, a key factor is to consider the sustenance and ongoing aggravation of inflammatory responses, and how infectious agents and their molecular products can block or even reverse these pathways. In this manner the hygiene hypothesis would be both validated and transformed into a therapeutically valuable concept for future medicine.
Acknowledgments
The authors thank the Wellcome Trust (RMM), the American Asthma Foundation (R. M. M. and H. J. McS.), Asthma UK (H. J. McS.) and the Rainin Foundation (R. M. M. and D. J. S.) for financial support.
Disclosures
The authors have no financial or commercial conflicts of interest to disclose.
References
- 1.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 2.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 3.Maizels RM, Nussey DH. Into the wild: digging at immunology's evolutionary roots. Nat Immunol. 2013;14:879–883. doi: 10.1038/ni.2643. [DOI] [PubMed] [Google Scholar]
- 4.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 5.Kamradt T, Göggel R, Erb KJ. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 2005;26:260–267. doi: 10.1016/j.it.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Rook GAW. The broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood BM. Autoimmune disease and parasitic infections in Nigerians. Lancet. 1968;2:380–382. doi: 10.1016/s0140-6736(68)90595-3. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood BM, Herrick EM, Voller A. Suppression of autoimmune disease in NZB and (NZB × NZW) F1 hybrid mice by infection with malaria. Nature. 1970;226:266–267. doi: 10.1038/226266a0. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood BM, Voller A, Herrick EM. Suppression of adjuvant arthritis by infection with a strain of the rodent malaria parasite Plasmodium berghei. Ann Rheum Dis. 1970;29:321–323. doi: 10.1136/ard.29.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strachan DP. Family size, infection and atopy: the first decade of the ‘hygiene hypothesis. Thorax. 2000;55(Suppl. 1):S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;9:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 15.EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355:873–876. [PubMed] [Google Scholar]
- 16.Stene LC, Nafstad P. Relation between occurrence of type 1 diabetes and asthma. Lancet. 2001;357:607–608. doi: 10.1016/S0140-6736(00)04067-8. [DOI] [PubMed] [Google Scholar]
- 17.Fairfax K, Nascimento M, Huang SC, Everts B, Pearce EJ. Th2 responses in schistosomiasis. Semin Immunopathol. 2012;34:863–871. doi: 10.1007/s00281-012-0354-4. [DOI] [PubMed] [Google Scholar]
- 18.van den Biggelaar A, van Ree R, Roderigues LC, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 19.Araujo MI, Lopes AA, Medeiros M, et al. Inverse association between skin response to aeroallergen and Schistosoma mansoni infection. Int Arch Allergy Immunol. 2000;123:145–148. doi: 10.1159/000024433. [DOI] [PubMed] [Google Scholar]
- 20.Cooper PJ, Chico ME, Rodrigues LC, et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111:995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- 21.Cooper PJ, Barreto ML, Rodrigues LC. Human allergy and geohelminth infections: a review of the literature and a proposed conceptual model to guide the investigation of possible causal associations. Br Med Bull. 2006;79–80:203–218. doi: 10.1093/bmb/ldl015. [DOI] [PubMed] [Google Scholar]
- 22.Smits HH, Yazdanbakhsh M. Chronic helminth infections modulate allergen-specific immune responses: protection against development of allergic disorders? Ann Med. 2007;39:428–439. doi: 10.1080/07853890701436765. [DOI] [PubMed] [Google Scholar]
- 23.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 24.Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 26.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 27.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 30.Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001;22:372–377. doi: 10.1016/s1471-4906(01)01958-5. [DOI] [PubMed] [Google Scholar]
- 31.Maizels RM. Infections and allergy – helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 33.Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Broadhurst MJ, Leung JM, Kashyap V, et al. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2010;2:60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- 35.van den Biggelaar AH, Rodrigues LC, van Ree R, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189:892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 36.Mutapi F, Imai N, Nausch N, et al. Schistosome infection intensity is inversely related to auto-reactive antibody levels. PLOS ONE. 2011;6:e19149. doi: 10.1371/journal.pone.0019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahanty S, Mollis SN, Ravichandran M, et al. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 39.Wammes LJ, Hamid F, Wiria AE, et al. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLOS Negl Trop Dis. 2012;6:e1655. doi: 10.1371/journal.pntd.0001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sartono E, Kruize YCM, Kurniawan-Atmadja A, Maizels RM, Yazdanbakhsh M. Depression of antigen-specific interleukin-5 and interferon-γ responses in human lymphatic filariasis as a function of clinical status and age. J Infect Dis. 1997;175:1276–1280. doi: 10.1086/593701. [DOI] [PubMed] [Google Scholar]
- 41.Babu S, Bhat SQ, Pavan Kumar N, et al. Filarial lymphedema is characterized by antigen-specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLOS Negl Trop Dis. 2009;3:e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finney CAM, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setiawan T, Metwali A, Blum AM, et al. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rausch S, Huehn J, Kirchhoff D, et al. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect Immun. 2008;76:1908–1919. doi: 10.1128/IAI.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hang L, Blum AM, Setiawan T, Urban JP, Jr, Stoyanoff KM, Weinstock JV. Heligmosomoides polygyrus bakeri infection activates colonic Foxp3+ T cells enhancing their capacity to prevent colitis. J Immunol. 2013;191:1927–1934. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson MS, Taylor M, Balic A, Finney CAM, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaiss DMW, van Loosdregt J, Gorlani A, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 49.Sutton TL, Zhao A, Madden KB, et al. Anti-Inflammatory mechanisms of enteric Heligmosomoides polygyrus infection against trinitrobenzene sulfonic acid-induced colitis in a murine model. Infect Immun. 2008;76:4772–4782. doi: 10.1128/IAI.00744-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q, Sundar K, Mishra PK, et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun. 2009;77:5347–5358. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaccone P, Cooke A. Helminth mediated modulation of Type 1 diabetes (T1D) Int J Parasitol. 2013;43:311–318. doi: 10.1016/j.ijpara.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 54.Taylor M, Le Goff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 55.Taylor MD, Harris A, Babayan S, et al. CTLA-4+ and CD4+CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 56.D'Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T cells: a role in the control of helminth driven intestinal pathology and worm survival. J Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rausch S, Huehn J, Loddenkemper C, et al. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol. 2009;39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 58.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346–6356. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 59.Smits HH, Hammad H, van Nimwegen M, et al. Protective effect of Schistosoma mansoni infection on allergic asthma depends on intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120:932–940. doi: 10.1016/j.jaci.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 60.Wilson MS, Taylor MD, O'Gorman MT, et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaccone P, Fehervari Z, Jones FM, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 62.Mallevaey T, Fontaine J, Breuilh L, et al. Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infect Immun. 2007;75:2171–2180. doi: 10.1128/IAI.01178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loke P, MacDonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell to cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 64.Reyes JL, Terrazas LI. The divergent roles of alternatively activated macrophages in helminthic infections. Parasite Immunol. 2007;29:609–619. doi: 10.1111/j.1365-3024.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 65.Djuardi Y, Wammes LJ, Supali T, Sartono E, Yazdanbakhsh M. Immunological footprint: the development of a child's immune system in environments rich in microorganisms and parasites. Parasitology. 2011;138:1508–1518. doi: 10.1017/S0031182011000588. [DOI] [PubMed] [Google Scholar]
- 66.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 67.Steel C, Guinea A, McCarthy JS, Ottesen EA. Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial antigens. Lancet. 1994;343:890–893. doi: 10.1016/s0140-6736(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 68.Elliott AM, Mpairwe H, Quigley MA, et al. Helminth infection during pregnancy and development of infantile eczema. JAMA. 2005;294:2032–2034. doi: 10.1001/jama.294.16.2032-c. [DOI] [PubMed] [Google Scholar]
- 69.Mpairwe H, Webb EL, Muhangi L, et al. Anthelminthic treatment during pregnancy is associated with increased risk of infantile eczema: randomised-controlled trial results. Pediatr Allergy Immunol. 2011;22:305–312. doi: 10.1111/j.1399-3038.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLOS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper PJ, Chico ME, Losonsky G, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000;182:1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 72.Cooper PJ, Chico M, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette–Guérin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Webb EL, Mawa PA, Ndibazza J, et al. Effect of single-dose anthelmintic treatment during pregnancy on an infant's response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:52–62. doi: 10.1016/S0140-6736(10)61457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ndibazza J, Mpairwe H, Webb EL, et al. Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial. PLOS ONE. 2012;7:e50325. doi: 10.1371/journal.pone.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinstock JV, Elliott DE. Translatability of helminth therapy in inflammatory bowel diseases. Int J Parasitol. 2013;43:245–251. doi: 10.1016/j.ijpara.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McSorley HJ, Gaze S, Daveson J, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLOS ONE. 2011;6:e24092. doi: 10.1371/journal.pone.0024092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleming JO. Helminth therapy and multiple sclerosis. Int J Parasitol. 2013;43:259–274. doi: 10.1016/j.ijpara.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 79.Bager P, Arnved J, Rønborg S, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Croft AM, Bager P, Kumar S. Helminth therapy (worms) cei_v176_i3_fig for allergic rhinitis. Cochrane Database Syst Rev. 2012;(4) doi: 10.1002/14651858.CD009238.pub2. CD009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garg SK, Croft AM, Bager P. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev. 2014;(1) doi: 10.1002/14651858.CD009400.pub2. CD009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daveson AJ, Jones DM, Gaze S, et al. Effect of hookworm infection on wheat challenge in celiac disease – a randomised double-blinded placebo controlled trial. PLOS ONE. 2011;6:e17366. doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 84.Kwan-Lim G-E, Forsyth KP, Maizels RM. Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. J Immunol. 1990;145:4298–4305. [PubMed] [Google Scholar]
- 85.Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol. 2010;104:455–464. doi: 10.1179/136485910X12786389891407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balic A, Harcus Y, Holland MJ, Maizels RM. Selective maturation of dendritic cells by Nippostrongylus brasiliensis secreted proteins drives T helper type 2 immune responses. Eur J Immunol. 2004;34:3047–3059. doi: 10.1002/eji.200425167. [DOI] [PubMed] [Google Scholar]
- 87.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172:2016–2020. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 88.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory–secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 89.Everts B, Hussaarts L, Driessen NN, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med. 2012;209:1753–1767. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith KA, Hochweller K, Hämmerling GJ, Boon L, Macdonald AS, Maizels RM. Chronic helminth infection mediates tolerance in vivo through dominance of CD11clo CD103– DC population. J Immunol. 2011;186:7098–7109. doi: 10.4049/jimmunol.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells Are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu W, Di Santo JP. Taming the beast within: regulation of innate lymphoid cell homeostasis and function. J Immunol. 2013;191:4489–4496. doi: 10.4049/jimmunol.1301759. [DOI] [PubMed] [Google Scholar]
- 94.Mirchandani AS, Besnard AG, Yip E, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 95.Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McSorley HJ, Blair NF, Smith KA, McKenzie ANJ, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014 doi: 10.1038/mi.2013.123. doi: 10.1038/mi.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zaiss MM, Maslowski KM, Mosconi I, Guenat N, Marsland BJ, Harris NL. IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLOS Pathog. 2013;9:e1003531. doi: 10.1371/journal.ppat.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gounaris K. Nucleotidase cascades are catalyzed by secreted proteins of the parasitic nematode Trichinella spiralis. Infect Immun. 2002;70:4917–4924. doi: 10.1128/IAI.70.9.4917-4924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hewitson JP, Harcus Y, Murray J, et al. Proteomic analysis of secretory products from the model gastrointestinal nematode Heligmosomoides polygyrus reveals dominance of Venom Allergen-Like (VAL) proteins. J Proteomics. 2011;74:1573–1594. doi: 10.1016/j.jprot.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moyle M, Foster DL, McGrath DE, et al. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J Biol Chem. 1994;269:10008–10015. [PubMed] [Google Scholar]
- 102.Anbu KA, Joshi P. Identification of a 55 kDa Haemonchus contortus excretory/secretory glycoprotein as a neutrophil inhibitory factor. Parasite Immunol. 2008;30:23–30. doi: 10.1111/j.1365-3024.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 103.Culley FJ, Brown A, Conroy DM, Sabroe I, Pritchard DI, Williams TJ. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. J Immunol. 2000;165:6447–6453. doi: 10.4049/jimmunol.165.11.6447. [DOI] [PubMed] [Google Scholar]
- 104.Lund ME, O'Brien BA, Hutchinson AT, et al. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLOS ONE. 2014;9:e86289. doi: 10.1371/journal.pone.0086289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jenkins SJ, Allen JE. Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes. J Biomed Biotechnol. 2010;2010:262609. doi: 10.1155/2010/262609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hunter MM, Wang A, Parhar KS, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 107.Grainger JR, Smith KA, Hewitson JP, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]


