Abstract
Physiological changes during normal pregnancy are characterized by an inflammatory immune response and insulin resistance. Therefore, we hypothesize that gestational diabetes mellitus (GDM) may be caused by an inappropriate adaption of the maternal immune system to pregnancy. In this study we examined the role of regulatory T cell (Treg) differentiation for the development of GDM during pregnancy. We used six-colour flow cytometric analysis to demonstrate that the total CD4+CD127low+/−CD25+ forkhead box protein 3 (FoxP3+) Treg pool consists of four different Treg subsets: naive CD45RA+ Tregs, HLA-DR−CD45RA− memory Tregs (DR− Tregs) and the highly differentiated and activated HLA-DRlow+CD45RA− and HLA-DRhigh+CD45RA− memory Tregs (DRlow+ and DRhigh+ Tregs). Compared to healthy pregnancies, the percentage of CD4+CD127low+/−CD25+FoxP3+ Tregs within the total CD4+ T helper cell pool was not different in patients affected by GDM. However, the suppressive activity of the total CD4+CD127low+/−CD25+ Treg pool was significantly reduced in GDM patients. The composition of the total Treg pool changed in the way that its percentage of naive CD45RA+ Tregs was decreased significantly in both patients with dietary-adjusted GDM and patients with insulin-dependent GDM. In contrast, the percentage of DR−-memory Tregs was increased significantly in patients with dietary-adjusted GDM, while the percentage of DRlow+ and DRhigh+ memory Tregs was increased significantly in patients with insulin-dependent GDM. Hence, our findings propose that alterations in homeostatic parameters related to the development and function of naive and memory Tregs may cause the reduction of the suppressive capacity of the total Treg pool in GDM patients. However, as this is an exploratory analysis, the results are only suggestive and require further validation.
Keywords: pregnancy, immune suppression, subsets of regulatory T cells, gestational diabetes mellitus, inflammation
Introduction
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance with onset or first recognition during pregnancy. It represents a common metabolic complication that affects about 2–9% of all pregnant women in western countries 1. The resulting maternal hyperglycaemia is associated with numerous adverse neonatal outcomes, such as neonatal hypoglycaemia and macrosomia, and therefore with an increased risk of shoulder dystocia. Maternal complications primarily include an increased risk of pre-eclampsia 2. Furthermore, women with GDM have an increased risk of developing type 2 diabetes mellitus within 5–15 years of delivery 3, while children exposed prenatally to a diabetic milieu also have an increased risk for the development of type 2 diabetes during later life 4. Currently, GDM screening tests are used to identify women with GDM 5, who are normally treated either with diet or insulin. Despite the better detection of GDM and subsequent therapy, the underlying pathophysiology of GDM is largely unknown. Similar to patients with type 2 diabetes, GDM patients are unable to increase insulin production to compensate for the increased insulin resistance during pregnancy 6. Therefore, it is assumed that women developing GDM already suffer from chronic reduced insulin sensitivity before conception that is enhanced further by the physiological insulin resistance occurring after 20 weeks of gestation 7. Recently, several susceptible genes involved in impaired β cell function, insulin resistance and abnormal glucose utilization were shown to increase the risk of type 2 diabetes mellitus 8. Meanwhile, these novel gene variants were also detected in GDM patients 9. However, further genes participating in cell functions involving cell activation, immune response, organ development and regulation of cell death were also found to be expressed differentially in GDM patients 10. In addition to exaggerated glucose intolerance and insulin resistance, GDM is characterized by chronic systemic inflammation 11,12 and an increased humoral immune response 13. Moreover, recent studies document an important role for adipose tissue inflammasome activation in the development of insulin resistance in GDM pregnancies 14.
In particular, as the immunosuppressive regulatory T cells (Tregs) were shown to control excessive inflammation and immoderate immune responses 15 and beyond are known to be of vital importance for the successful course of pregnancy 16, it could be possible that functional deficiencies of such cells are involved in the pathogenesis of GDM. Meanwhile, it is known that the total Treg pool, currently characterized as CD4+CD127low+/−CD25+forkhead box protein 3 (FoxP3+) Treg cells, consists of less suppressive, naive CD45RA+ Tregs and strongly suppressive, antigen-experienced CD45RO+ memory Tregs 17. Thereby, the CD45RO+ Treg population comprises highly differentiated human leucocyte antigen D-related (HLA-DR)-expressing cells, which were shown to reveal higher FoxP3 expression levels and to possess stronger suppressive activity than HLA-DR− memory Tregs 18. Recently, our group demonstrated that the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool can be divided into four distinct Treg subsets: naive CD45RA+ Tregs, HLA-DR−CD45RA− memory Tregs (DR− Tregs), HLA-DRlow+CD45RA− memory Tregs (DRlow+ Tregs) and HLA-DRhigh+CD45RA− memory Tregs (DRhigh+ Tregs). The composition of the total Treg pool with these distinct subsets changed both during the normal course of pregnancy and in the presence of characteristic gestation-associated diseases 19,20. Thereby, we demonstrated that the ratio of naive CD45RA+ Tregs increased during the normal course of pregnancy, but was reduced strongly in the presence of complications such as pre-eclampsia and preterm labour. In contrast, the ratio of memory Tregs, especially of the mature HLA-DRlow+ and DRhigh+ Tregs, was found to be increased in patients affected by pre-eclampsia, while the ratio of DR− and DRlow+ Tregs was increased in patients affected by preterm labour. Although both forms of diseases revealed lower ratios of the less suppressive CD45RA+-naive Tregs and higher ratios of the well suppressive CD45RA− memory Tregs, the suppressive activity of the total Treg pool was reduced significantly in these patients 19,20. Therefore, it is conceivable that defective Treg cell differentiation, producing less suppressive CD45RA− memory Tregs, may be causatively involved in the development of gestation-associated diseases.
Currently, little information exists about the influence of the immune system on the pathogenesis of GDM. In this study, we demonstrate that the occurrence of GDM is associated with a moderate decrease of its suppressive activity while the size of the CD4+CD127low+/−CD25+FoxP3+ Treg pool among the CD4+ T cells is not reduced. Thereby, we also observed characteristic changes in its composition with the above-described Treg subsets: compared to healthy pregnancies, patients with dietary-adjusted GDM had significantly increased percentages of HLA-DR− memory Tregs, while patients diagnosed with insulin-dependent GDM had increased percentages of activated DRlow+ and DRhigh+ memory Tregs. Both patient groups exhibit decreased percentages of naive CD45RA+ Tregs. As this study is an exploratory analysis, the study is only suggestive and needs further validation.
Methods
Patient collectives and healthy volunteers
Blood samples were obtained from 64 healthy pregnant women between 24 and 41 weeks of gestation (group 1), 21 pregnant women with dietary-adjusted gestational diabetes (group 2) and 40 pregnant women with insulin-dependent gestational diabetes (group 3). Table 1 summarizes the clinical characteristics of all participants (groups 1–3). Blood samples from healthy pregnancies were collected from women who had routine ultrasonography to exclude fetal malformations and from women delivering by term elective caesarean section, due to breech presentation, cephalopelvic disproportion or status after caesarean section (group 1). Blood samples from women with gestational diabetes were obtained between 24 and 41 weeks of gestation (groups 2 and 3). The diagnosis of gestational diabetes was made between 24 and 28 weeks of gestation by a positive 2-h 75g oral glucose tolerance test (OGTT) with the following criteria: a fasting plasma glucose ≥ 5·1 mmol/l (92 mg/dl), or a 1-h plasma glucose level of ≥ 10·0 mmol/l (180 mg/dl), or a 2-h plasma glucose of ≥ 8·5 mmol/l (153 mg/dl) 21. The blood glucose levels of all affected women were checked regularly to determine the individually needed insulin dose. The insulin regimen included an individual combination of short-acting Actrapid® before meals and Protaphane® as long-acting insulin at night. The study was approved by the Regional Ethics Committee. All women were fully informed of the aim of the study and informed consent was obtained from all participants.
Table 1.
Clinical characteristics of healthy pregnancies and women with gestational diabetes mellitus (GDM).
| Group 1: healthy pregnancies median (range) | Group 2: Dietary-adjusted GDM median (range) | Group 3: insulin-dependent GDM median (range) | |
|---|---|---|---|
| n | 64 | 21 | 40 |
| Weeks gestation | 37 (24–41) | 39 (24–41) | 36 (24–42) |
| Age | 31 (21–44) | 32 (25–43) | 34 (22–43) |
| Weight before pregnancy (kg) | 66 (50–117) | 67 (45–126) | 89 (54–153) |
| Weight at delivery (kg) | 81 (59–128) | 83 (58–136) | 100 (54–160) |
| Time since diagnosis (weeks) | – | 11 (1–15) | 7 (1–15) |
| Period of treatment (weeks) | – | 11 (1–15) | 5 (1–12) |
For all participants, both the percentage of CD4+CD127low+/−CD25+FoxP3+ Tregs of total CD4+ T cells and the percentage of naive CD45RA+ Tregs, DR−, DRlow+ and DRhigh+ memory Tregs among the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool were determined by six-colour flow cytometric analysis. For a total number of 33 healthy pregnancies between 24 and 41 weeks of gestation (group 1) and 23 pregnancies with gestational diabetes mellitus (groups 2 and 3), suppression assays were performed in order to evaluate the suppressive capacity of the magnetically selected total CD4+CD127low+/−CD25 Treg cell pool.
Fluorescence-activated cell sorter (FACS) staining
Venous blood samples (10 ml) from all participants were collected into ethylenediamine tetraacetic acid (EDTA)-containing tubes. Whole peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Amersham Bioscience, Glattbrugg, Switzerland) gradient centrifugation and analysed by six-colour flow cytometric analysis. Briefly, PBMCs (4 × 106 cells) were surface-stained with allophycocyanin-cyanin 7 (APC-Cy7)-conjugated-anti-CD4 (BD Bioscience, San Jose, CA, USA), peridinin chlorophyll (PerCP)-Cy5·5-conjugated anti-CD127 (BD Bioscience), phycoerythrin (PE)-conjugated anti-CD25 (BD Bioscience), PE-Cy7-conjugated anti-HLA-DR (BD Bioscience) and APC-conjugated anti-CD45RA (BD Bioscience) mouse monoclonal antibodies. Subsequently, intracellular staining for the detection of FoxP3 was performed using a fluorescein isothiocyanate (FITC) labelled anti-human FoxP3 staining set (clone PCH101; eBioscience), according to the manufacturer's instructions. Both the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells and the percentages of the four Treg subsets (naive CD45RA+ Tregs, HLA-DR−, HLA-DRlow+ and HLA-DRhigh+ memory Tregs) within the total Treg pool and the HLA-DR mean fluorescence intensity (MFI) of the HLA-DR+ Treg subset were determined for all participants. Thereby, the HLA-DR− and HLA-DR+ memory Treg subsets were characterized as HLA-DR−CD45RA− or HLA-DR+CD45RA− memory Tregs and abbreviated as DR−, DRlow+ and DRhigh+ memory Tregs. Negative control samples were incubated with isotype-matched antibodies. Dead cells were excluded by forward- and side-scatter characteristics. Cells were analysed by a FACSCanto cytometer (BD Bioscience). Statistical analysis was based on at least 100 000 gated CD4+ T cells.
Positive selection and staining of CD4+CD127low+/−CD25+ Treg cells
Whole peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood (50 ml) drawn in EDTA tubes by Ficoll-Hypaque (Amersham Bioscience) gradient centrifugation. CD4+CD127low+/−CD25+ Tregs were purified using the CD4+CD127low+/−CD25+ Regulatory T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. First, CD4+CD127low+/− T cells were isolated by magnetic depletion of non-CD4+CD127high+ T cells. In a second step, the CD4+CD127low+/−CD25+ Treg cells were isolated by positive selection over two consecutive columns. The CD4+CD127low+/−CD25− T cells were obtained in the flow-through fraction and used as responder T cells (Tresp). The CD4+CD127low+/−CD25+ Treg cells were subsequently retrieved from the columns. The purified CD4+CD127low+/−CD25+ Treg cell fraction was analysed using four-colour flow cytometry. Briefly, 1 × 105 cells were stained with 10 μl PerCP-conjugated-anti-CD4, PE-conjugated anti-CD25, FITC-conjugated FoxP3 and biotin-conjugated anti-CD127 mouse monoclonal antibodies. Positive staining for CD127 was detected using APC-conjugated streptavidin molecules. On average, 85% of the isolated CD4+CD127low+/−CD25+ Treg cells were shown to be within the CD4+CD127low+/−CD25+FoxP3+ Treg cell population.
Co-culture suppression assay
Whole peripheral mononuclear cells (PBMCs) were isolated from peripheral blood drawn in EDTA tubes by Ficoll-Hypaque (Amersham Bioscience) gradient centrifugation. CD4+CD127low+/−CD25 Treg cells were purified using the CD4+CD127low+/−CD25+ Regulatory T cell Isolation Kit II (Miltenyi Biotec) described above. In all assays, 2 × 104 responder T cells (Tresp) were co-cultured with the purified CD4+CD127low+/−CD25+ Treg cells at ratios 1:1–1:64 in 96-well U-bottomed plates. Suppression assays were performed in a final volume of 100 μl/well of X-VIVO15 medium (BioWhittaker, Radnor, PA, USA). For T cell stimulation, the medium was supplemented with 1 μg/ml anti-CD3 and 2 μg/ml anti-CD28 antibodies (eBioscience, San Diego, CA, USA). As controls, CD4+CD127low+/−CD25+ Treg cells and Tresp cells alone were cultured both with and without any stimulus. Cells were incubated at 37°C and 5% of CO2. After 4 days, 1 μCi [3H]-thymidine was added to the cultures and cells were incubated further for 16 h. Cells were then harvested and [3H]-incorporation was measured by scintillation counting. All assays exhibited < 10% standard error of the mean (s.e.m.) and were performed a minimum of six times. In order to compare the suppressive capacity of the isolated CD4+CD127low+/−CD25+ Tregs between the different patient groups, we calculated the maximum suppressive activity (ratio of Treg cells to Tresp cells 1:1). In addition, we determined the suppressive activity of the isolated Treg cells with gradient ratios of Treg cells to Tresp cells (ratio of Treg cells to Tresp cells 1:1–1:64) and identified the ratio with which a minimum suppression of at least 15% could be achieved (ratio Treg/Tresp) 20.
Statistical analysis
As all data were not distributed normally, the statistical comparison of the percentages of CD4+ T cells within total leucocytes, CD4+CD127low+/−CD25+FoxP3+ Treg cells within total CD4+ T cells, the different Treg subsets (DRlow+ Tregs, DRhigh+ Tregs, DR− Tregs and naive CD45RA+ Tregs) within the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool and of its HLA-DR mean fluorescence intensities (MFIs) between the different patient populations (healthy control group, dietary-treated group, insulin-treated group) was performed using the non-parametric Kruskal–Wallis H test. This test is used for simultaneous comparison of more than two sample populations. In addition, dietary- and insulin-treated GDM groups were pooled and compared with the healthy control group using the Wilcoxon–Mann–Whitney U-test. Comparison of the suppressive activity of purified CD4+CD127low+/−CD25+ Treg cells was also performed using the Kruskal–Wallis H test. Each H test was followed by a Dunn test. P < 0·05 was considered significant. As this was an exploratory analysis, no adjustments for multiple testing were performed.
Results
Gestational diabetes is not associated with deviations in the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells within the total CD4+ T cell pool
In this study, peripheral blood mononuclear cells (PBMCs) were obtained from 64 healthy pregnancies (group 1), 21 patients with dietary-adjusted GDM (group 2) and 40 patients with insulin-dependent GDM (group 3) (Table 1). Their PBMCs were stained with CD4-, CD127-, CD25-, FoxP3-, HLA-DR- and CD45RA-specific monoclonal antibodies and analysed by six-colour flow cytometric analysis. Figure 1 shows the gating strategy for these measurements. First, CD4+ T cells (Fig. 1a, P1) were analysed for their simultaneous expression of CD25, CD127 and FoxP3 (Fig. 1b, P2 and Fig. 1c, P3). The percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells (Fig. 1c, P3) within the total CD4+ T cell pool, their composition with four distinct Treg subsets [DRhigh+ (P4), DRlow+ (P5), DR− memory Tregs (P6), naive CD45RA+ Tregs (P7)] and the HLA-DR MFI of the DR+ memory Treg subset (P8) were estimated for all participants (Fig. 1d). Figure 1e,f shows the dot-plots of HLA-DR versus CD45RA analysis of one representative experiment performed with PBMCs from a patient with dietary-adjusted GDM (group 2, Fig. 1e) and a patient with insulin-dependent GDM (group 3, Fig. 1f).
Fig. 1.
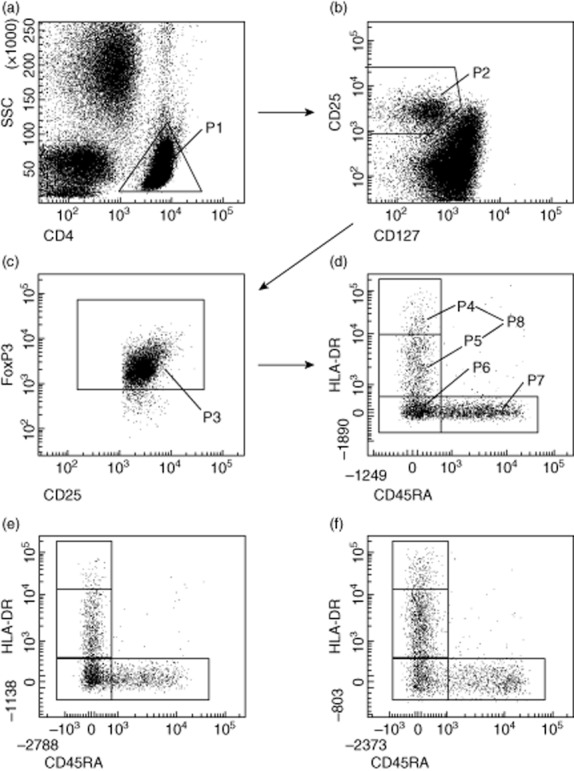
Gating strategy for six-colour flow cytometric detection of the total CD4+CD127low+/−CD25+ forkhead box protein 3 (FoxP3+) regulatory T cell (Treg) pool and its percentage of DRlow+, DRhigh+, DR− memory Tregs and naive CD45RA+ Tregs. (a) First, CD4+ T cells (P1) were gated by fluorescence intensity of CD4 versus side light scatter (SSC). (b) CD4+CD127low+/−CD25+ Treg cells were gated by fluorescence intensity of CD25 versus CD127 (P2). (c) CD4+CD127low+/−CD25+FoxP3+ Treg cells were gated by excluding cells without FoxP3 expression (P3). (d) The percentage of the DRhigh+ (P4), the DRlow+ (P5), the DR− (P6), and the naive CD45RA+ Treg subset (P7) was estimated by analysing CD4+CD127low+/−CD25+FoxP3+ Treg cells (P3) for their expression of human leucocyte antigen D-related (HLA-DR) and CD45RA. Additionally, the HLA-DR mean fluorescence intensity (MFI) of the DR+ Treg subset (P8) was determined for all participants. (a–d) Representative experiment for healthy pregnancies; (e,f) representative experiments for patients with dietary-adjusted gestational diabetes mellitus (GDM) (e) and with insulin-dependent GDM (f).
We found no significant differences in either the percentage of CD4+ T cells of total leucocytes or the percentage of CD4+CD127low+/−CD25+FoxP3+ Treg cells within the total CD4+ T cell pool between healthy pregnancies and pregnancies affected by dietary-adjusted or insulin-dependent GDM (Fig. 2a,b).
Fig. 2.
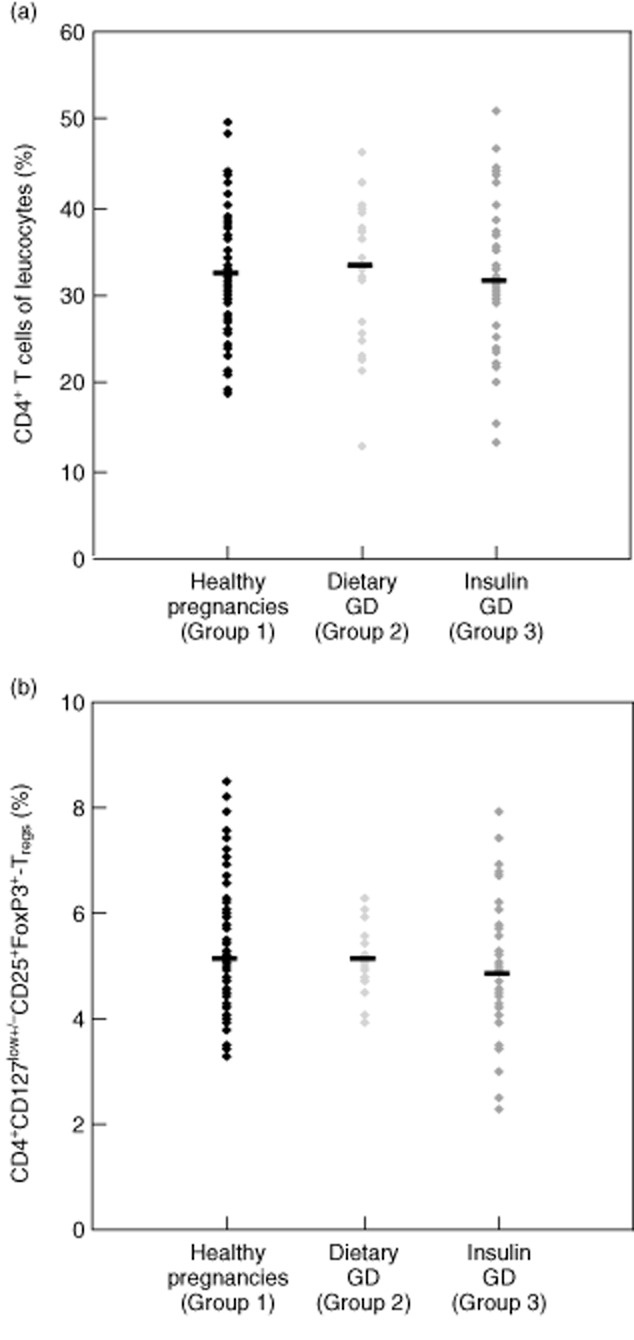
Detection of the percentage of CD4+ T cells of total leucocytes and the percentage of CD4+CD127low+/−CD25+ forkhead box protein 3 (FoxP3+) regulatory T cells (Tregs) within total CD4+ T cells for healthy pregnancies and patients with dietary-adjusted or insulin-dependent gestational diabetes mellitus (GDM). The figure shows the individual and median data obtained for healthy pregnant women (group 1, ♦), pregnancies affected by dietary-adjusted GDM (group 2 ) and pregnancies with insulin-dependent GDM (group 3
) and pregnancies with insulin-dependent GDM (group 3 ).
).
In comparison to healthy pregnancies, the suppressive activity of the total CD4+CD127low+/−CD25+ Treg cell pool is moderately decreased in pregnancies affected by GDM
In order to compare the suppressive capacity of the total CD4+CD127low+/−CD25+ Treg pool between healthy pregnancies (group 1) and patients with GDM (groups 2 and 3), we performed co-culture suppression assays, as described recently 20. For that, CD4+CD127low+/−CD25+ Treg cells were isolated from 33 healthy pregnant women between 24 and 41 weeks of gestation (group 1) and 23 pregnant women affected by GDM (groups 2 and 3) by magnetic affinity cell sorting (MACS) technology. To evaluate the suppressive capacity of the isolated CD4+CD127low+/−CD25+ Tregs, we determined the maximum suppressive activity (Fig. 3a: ratio of Treg cells to Tresp cells 1:1) and calculated the ratio of Treg cells to Tresp cells with which a minimum suppressive activity of at least 15% could be achieved (Fig. 3b: ratio Treg/Tresp). Table 2 and Fig. 3 show the results for these measurements. There were no differences concerning the maximum suppressive activity of the total CD4+CD127low+/−CD25+ Treg pool (Fig. 3a) between healthy pregnancies (group 1) and pregnancies affected by GDM (groups 2 and 3). However, the ratio of Treg/Tresp (Fig. 3b) was decreased significantly (P < 0·01) in patients with GDM (median 1:4) (groups 2 and 3) compared to healthy pregnancies (median 1:16).
Fig. 3.
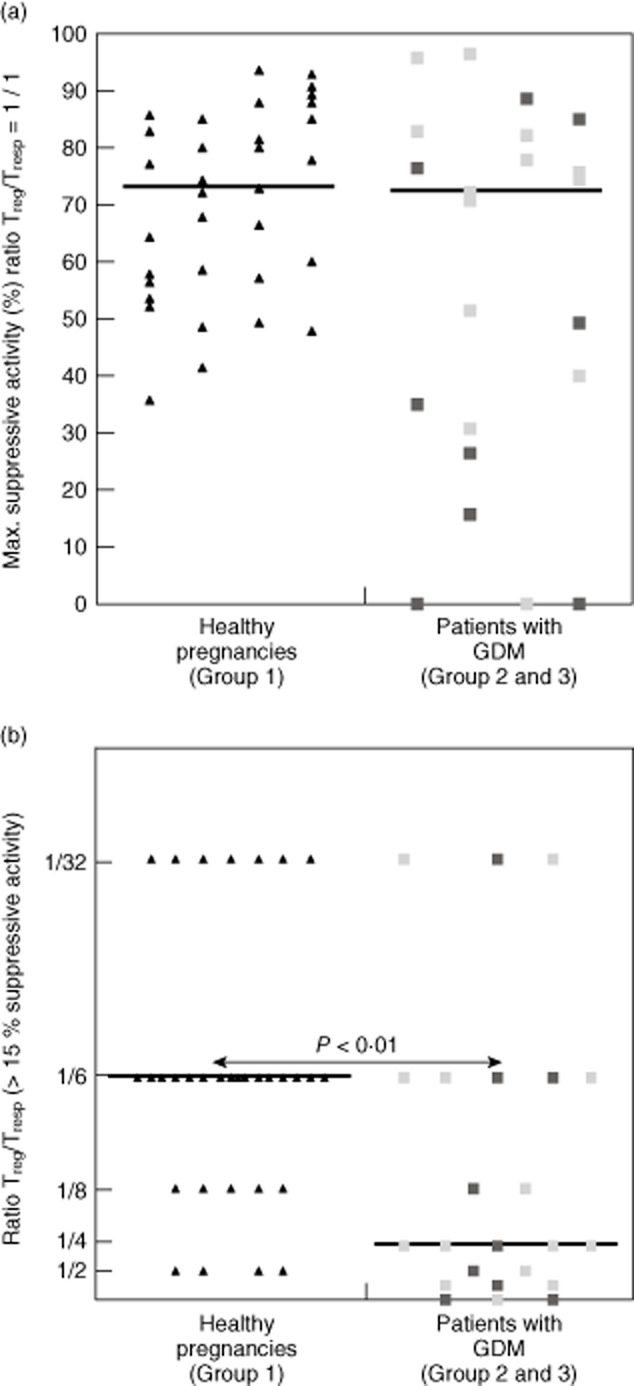
Evaluation of the suppressive activity of the total CD4+CD127low+/−CD25+ regulatory T cell (Treg) pool in healthy pregnancies and pregnant women affected by gestational diabetes mellitus (GDM). The total CD4+CD127low+/−CD25+ Treg pool was isolated using the magnetic-activated cell sorting (MACS) technique and its suppressive activity was examined using suppression assays (see Methods). The figure shows the maximum suppressive activity (ratio Treg/T = 1/1), (a) and the ratio of Treg/responder T cells (Tresp) up to which the purified Tregs could be diluted to achieve a minimum suppressive activity of at least 15% (b). The diagrams represent the individual data obtained for healthy pregnancies (group 1, ▲), for pregnant women with dietary-adjusted GDM (group 2,  ) and for pregnant women with insulin-dependent GDM (group 3,
) and for pregnant women with insulin-dependent GDM (group 3,  ).
).
Table 2.
Percentage of the CD4+CD127low+/−CD25+ forkhead box protein 3(FoxP3+) regulatory T cell (Treg) cell pool within CD4+ T cells, its suppressive activity and its composition with distinct Treg subsets in healthy pregnancies and women with gestational diabetes mellitus (GDM).
| Group 1: healthy pregnancies median (range) | Group 2: dietary-adjusted GDM median (range) | Group 3: insulin-dependent GDM median (range) | |
|---|---|---|---|
| CD4+ T cells | 32·0 (18·8–49·5) | 32·9 (12·7–46·3) | 31·2 (13·3–51·2) |
| Treg cells (%) | 5·1 (3·3–8·5) | 5·1 (3·9–6·3) | 4·8 (2·3–7·9) |
| Suppressive activity | |||
| Max. suppr. activity (%) (Treg/Tresp 1/1) | 73·2 (35·8–93·8) | 74·0 (0–96·4) | |
| Ratio of Treg (> 15% suppr. activity) | 1:16 (1:2–1:32) | 1:4 ↓ (0–1:32) | |
| Subsets of the Treg pool (%): | |||
| DR+ Tregs | 22·8 (12·5–34·5) | 25·6 (16·5–41·4) | 28·8 ↑ (17·2–56·7) |
| DRlow+ Tregs | 20·3 (11·1–30·7) | 22·0 (15·1–35·1) | 24·3 ↑ (16·1–46·4) |
| DRhigh+ Tregs | 2·5 (0·8–5·2) | 2·9 (1·3–6·7) | 3·7 ↑ (1·4–15·1) |
| DR− Tregs | 29·9 (13·7–44·8) | 34·3 ↑ (24·1–44·9) | 31·1 (21·8–44·7) |
| CD45RA+ Tregs | 44·4 (25·6–68·9) | 35·8 ↓ (20·9–49·0) | 36·8 ↓ (8·7–54·5) |
| MFI | |||
| DR+ Tregs | 5047·5 (3033–9053) | 4786 (3576–7228) | 6424·5 ↑ (3786–11207) |
MFI = mean fluorescence intensity; ↑, ↓ = significant differences (P < 0·05) were obtained compared to healthy pregnancies. Tresp = responder T cells.
The composition of the total CD4+CD127low+/−CD25+FoxP3+ Treg cell pool of patients with GDM is different compared to healthy pregnancies
To examine whether the diminished suppressive activity in patients with GDM is associated with changes in the composition of the total CD4+CD127low+/−CD25+FoxP3+ Treg pool with different Treg subsets, we estimated its percentage of DRhigh+ (P4), DRlow+ (P5), DR− memory Tregs (P6) and naive CD45RA+ Tregs (P7) (Fig. 1d) for healthy pregnancies (group 1), for patients with dietary-adjusted GDM (group 2) and patients with insulin-dependent GDM (groups 3). Additionally, we determined the HLA-DR mean fluorescence intensity (MFI) of the DR+ memory Treg subset (P8) for each participant. Table 2 and Fig. 4a–d depict the results for these measurements. In comparison to healthy pregnancies (group 1), we found a significantly decreased percentage of naive CD45RA+ Tregs (Fig. 4d) for both patients with dietary-adjusted GDM (group 2) and patients with insulin-dependent GDM (group 3). Surprisingly, in patients with dietary-adjusted GDM (group 2), the percentage of DR− memory Tregs (Fig. 4c) was increased significantly, while in patients with insulin-dependent GDM (group 3) the percentages of activated DRlow+ (Fig. 4a) and DRhigh+ memory Tregs (Fig. 4b) were increased strongly compared to healthy pregnancies (group 1). As the HLA-DR MFI of total DR+ memory Tregs was increased significantly in patients with insulin-dependent GDM (group 3), but not dietary-adjusted GDM patients, it seems that there is a stronger increase in the percentage of DRhigh+ memory Tregs compared to DRlow+ memory Tregs. The direct statistical comparison between dietary- and insulin-treated pregnancies revealed a significantly decreased percentage of DR− memory Tregs and a significantly increased percentage of DRhigh+ memory Tregs in insulin-dependent GDM patients. In summary, these results demonstrate clearly that both dietary-adjusted and insulin-dependent GDM show a reduced percentage of naive CD45RA+ Treg cells and an increased percentage of CD45RA− memory Treg cells. Obviously, there is an augmented conversion into DR− memory Tregs in patients with less pronounced disease, while patients with severe disease produce predominantly DRlow+ and DRhigh+ memory Treg cells. Similar results were obtained when dietary-treated and insulin-treated groups were pooled (groups 2 and 3, n = 61) and compared with the healthy control group (group 1, n = 64). The percentage of naive CD45RA+ Tregs (median 35·8%, range 8·7–54·5%) was reduced significantly (P < 0·001), while the percentages of DR− memory Tregs (median 32·7%, range 21·8–44·9%, P < 0·05), DRlow+ memory Tregs (median 24·0%, range 15·1–46·4%, P < 0·001) and DRhigh+ memory Tregs (median 3·3%, range 1·3–15·1%, P < 0·001) were increased significantly in pooled GDM patients compared to healthy pregnancies.
Fig. 4.
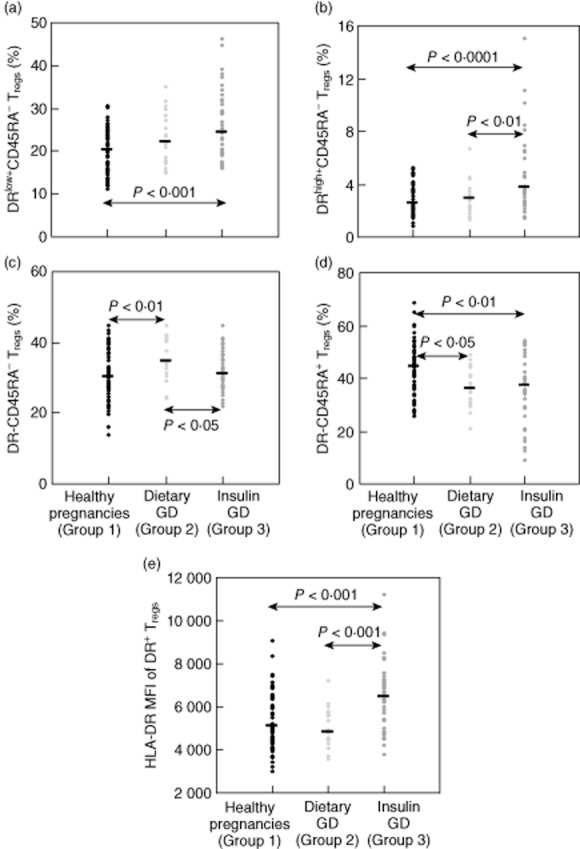
Detection of the percentages of the DRlow+, DRhigh+, DR− and CD45RA+ regulatory T cell (Treg) subsets within total CD4+CD127low+/−CD25+ forkhead box protein 3 (FoxP3+) Tregs and the human leucocyte antigen D-related (HLA-DR) mean fluorescence intensity (MFI) of the DR+ Treg subset in healthy pregnancies and patients with gestational diabetes mellitus (GDM). (a–d) Quantitative changes of the DRlow+ (a), DRhigh+ (b), DR− (c) and CD45RA+ (d) Treg subsets in pregnancies affected by dietary-adjusted GDM (group 2,  ) and insulin-dependent GDM (group 3,
) and insulin-dependent GDM (group 3,  ) compared to healthy pregnancies (group 1, ♦). (e) The HLA-DR MFI of the DR+ Treg subset for healthy pregnancies (group 1, ♦), patients with dietary-adjusted GDM (group 2,
) compared to healthy pregnancies (group 1, ♦). (e) The HLA-DR MFI of the DR+ Treg subset for healthy pregnancies (group 1, ♦), patients with dietary-adjusted GDM (group 2,  ) and patients with insulin-dependent GDM (group 3,
) and patients with insulin-dependent GDM (group 3,  ).
).
Discussion
In this exploratory study, we ascertained Treg frequencies, their suppressive activity and their composition with distinct Treg subsets (DRhigh+, DRlow+, DR− memory Tregs and naive CD45RA+ Tregs) in pregnancies affected by GDM compared to healthy pregnancies. Similar to other gestation-associated diseases (pre-eclampsia, preterm labour) 19,20, GDM was associated with decreased percentages of naive CD45RA+ Tregs, but increased percentages of DR−, DRlow+ and DRhigh+ memory Tregs. Also notable is the fact that dietary-adjustable GDM was characterized by an increased percentage of DR− memory Tregs, while the more severe form of insulin-dependent GDM was associated with an increased percentage of the much more activated DRlow+ and DRhigh+ memory Tregs. Such findings may suggest that according to the severity of the disease, an increased conversion of naive CD45RA+ Tregs into less activated DR− memory Tregs occurs in the presence of dietary GDM, while an increased conversion into much more activated DRlow+ and DRhigh+ memory Tregs occurs in the presence of insulin-dependent GDM.
Interestingly, similar findings are documented for non-regulatory peripheral T lymphocytes, such as CD4+ T helper and CD8+ cytotoxic T cell subsets. Compared to healthy pregnancies, a significantly reduced percentage of naive CD4+CD45RA+ T helper cells was found in patients with dietary-adjustable GDM and in patients with insulin-dependent GDM. In contrast, the percentages of CD4+CD45RO+ memory T helper cells, the activated CD4+CD25+ and CD4+HLA-DR+ T helper cells were in fact increased significantly. In addition, significantly increased percentages of activated CD8+CD25+ and HLA-DR+ cytotoxic T cells were also detected in these patient cohorts 22, indicating that both Tresp cells as well as Treg cells are activated strongly in the presence of GDM.
Especially for Treg cells, it was shown that the highly activated DRlow+ and DRhigh+ memory Tregs have the highest suppressive activity within the total Treg pool in normal healthy control subjects 23. In particular, the DRhigh+ memory Tregs were found to be of potential importance for the suppressive activity of the total Treg pool, as these cells were shown to be reduced significantly in kidney transplant patients with acute rejection, compared to non-rejecting patients 23,24. Surprisingly, we found this relation to be reversed in pregnancy. In contrast to non-pregnant women, whose naive CD45RA+ Tregs showed little suppressive activity, the CD45RA+ Tregs showed the highest suppressive activity in pregnant women, while the DR+ memory Tregs were less suppressive 25. Therefore, changes in the composition of the total Treg pool in the manner that the naive CD45RA+ Tregs decrease, while the DRlow+ DRhigh+ memory Tregs increase, may cause a significant loss of the suppressive activity of the total Treg pool in pregnant women. Meanwhile, such changes are demonstrated for many gestation-associated diseases, such as pre-eclampsia and preterm labour, and may explain the loss of the suppressive activity of the Treg pool in these patients. At present, it is not known which immunological mechanisms cause such differences in the suppressive activity of the individual Treg subsets between pregnant and non-pregnant women.
Our findings may suggest that the normal maternal immune suppression is less effective in GDM patients, due presumably to the fact that the existing Treg pool in GDM patients is strongly activated, but not capable of fulfilling its suppressive activity. However, the exact mechanisms through which such immune activation and inflammation lead to glucose intolerance in pregnancy are still unclear. There are some data which demonstrate that inflammasome activation in adipose tissue interferes with the insulin signalling pathway, leading to insulin resistance [14]. Meanwhile, it is also known that there is a strong relationship between inflammatory signals and rising serum pentraxin 3 (PTX3) levels 26. PTX 3 is known as a marker of vascular inflammation and atherosclerotic plaque formation, and therefore it is considered as a strong prognostic factor of mortality after myocardial infarction 27. A significantly increased rise of PTX3 was also found in GDM patients during oral glucose tolerance test (OGTT). In addition, there was a negative correlation of PTX3 and insulin sensitivity in these patients 28. These data suggest a link between this inflammatory cardiovascular risk marker and markers for glucose intolerance and insulin resistance in pregnant women. Also, recent data showed that PTX3 is elevated significantly in patients affected by pre-eclampsia 29,30. Such findings propose that inflammatory events, due presumably to aberrant immune responses, may be involved in the pathogenesis of both GDM and pre-eclampsia 31.
However, our current data do not yet allow the conclusion that the observed changes in the composition of the total Treg pool and the resulting decrease of its suppressive activity is responsible for the amplification of inflammatory processes observed in GDM patients. Such mechanisms may not be the cause of the disease, but may simply reflect the consequences of the disease rather than a pathway leading to GDM. In addition, it should be noted that our study is based on exploratory analyses and thus the results have to be interpreted with care. As a validation cohort is missing, because it is ethically not acceptable to treat healthy pregnant women with insulin, the data are somewhat suggestive and require further validation. Notably, in a murine obesity model for GDM, it has been demonstrated that GDM leads to chronic hypoxia stress and an excessive inflammatory response in the murine placenta, thus suggesting a severe dysregulation of the immune tolerance network under these conditions 32. Hence, additional investigations concerning the role of immune activation and inflammation in the pathogenesis of GDM are necessary to ultimately clarify whether an aberrant Treg cell compartment is involved in the pathogenesis of GDM, and also presumably in the development of related complications, such as type 2 diabetes, metabolic syndrome and cardiovascular diseases.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft grant STE 885/3-2 (to A. S.). We would like to thank M. Hoerner, C. Schneider and the nursing staff of the delivery room and the ultrasound section of the Department of Obstetrics and Gynecology, University of Heidelberg, for arranging the collection of blood samples.
Disclosure
None of the authors have any conflicts of interest related to this manuscript.
Author contributions
A. S., L. S., D. R. and E. S. designed the study, D. R., A. K. and L. S. performed the study, K. M., L. U., H. F. and C. S. contributed important methods and patients, L. S., D. R., J. S. and A. S. collected and analysed the data, L. S. and A. S. wrote the paper. All authors contributed to the final version of the paper and approved it.
References
- 1.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffreis WS, Robinson JS for the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 2.The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 3.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systemic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 4.Tam WH, Ma RC, Yang X, et al. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: a 15 year follow-up study. Diabetes Care. 2010;33:1382–1384. doi: 10.2337/dc09-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Leeuwen M, Louwerse MD, Opmeer BC, et al. Glucose challenge test for detecting gestational diabetes mellitus: a systematic review. Br J Obstet Gynaecol. 2012;119:393–401. doi: 10.1111/j.1471-0528.2011.03254.x. [DOI] [PubMed] [Google Scholar]
- 6.Harlev A, Wiznitzer A. New insights on glucose pathophysiology in gestational diabetes and insulin resistance. Curr Diab Rep. 2010;10:242–247. doi: 10.1007/s11892-010-0113-7. [DOI] [PubMed] [Google Scholar]
- 7.Kautzky-Willer A, Prager R, Waldhausl W, et al. Pronounced insulin resistance and inadequate beta-cell insulin secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care. 1997;20:1717–1723. doi: 10.2337/diacare.20.11.1717. [DOI] [PubMed] [Google Scholar]
- 8.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type two diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 9.Mao H, Li Q, Gao S. Meta-analysis of the relationship between common type 2 diabetes risk gene variants with gestational diabetes mellitus. PLOS ONE. 2012;7:e45882. doi: 10.1371/journal.pone.0045882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enquobahrie DA, Williams MA, Qiu C, Meller M, Sorensen TK. Global placental gene expression in gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200:206.e1–206.e13. doi: 10.1016/j.ajog.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Atègbo JM, Grissa O, Yessoufou A, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91:4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 12.Richardson AC, Carpenter MW. Inflammatory mediators in gestational diabetes mellitus. Obstet Gynecol Clin North Am. 2007;34:213–224. doi: 10.1016/j.ogc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Steinborn A, Saran G, Schneider A, Fersis N, Sohn C, Schmitt E. The presence of gestational diabetes is associated with increased detection of anti-HLA-class II antibodies in the maternal circulation. Am J Reprod Immunol. 2006;56:124–134. doi: 10.1111/j.1600-0897.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 14.Lappas M. Activation of inflammasomes in adipose tissue of women with gestational diabetes. Mol Cell Endocrinol. 2014;382:74–83. doi: 10.1016/j.mce.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Miyara M, Costantino C, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 16.Aluvihare VR, Kallikourdis M Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 17.Booth NJ, McQuaid AJ, Sobande T, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 18.Baecher-Allan C, Wolf E, Hafler D. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 19.Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90:935–944. doi: 10.1038/icb.2012.33. [DOI] [PubMed] [Google Scholar]
- 20.Steinborn A, Schmitt E, Kisielewicz A, et al. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol. 2012;167:84–98. doi: 10.1111/j.1365-2249.2011.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes – a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoud F, Abul H, Omu A, Haines D. Lymphocyte sub-populations in gestational diabetes. Am J Reprod Immunol. 2005;53:21–29. doi: 10.1111/j.1600-0897.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 23.Schaier M, Seissler N, Schmitt E, et al. DR(high+)CD45RA(–)-Tregs potentially affect the suppressive activity of the total Treg pool in renal transplant patients. PLOS ONE. 2012;7:e34208. doi: 10.1371/journal.pone.0034208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaier M, Seissler N, Becker LE, et al. The extent of HLA-DR expression on HLA-DR(+) Tregs allows the identification of patients with clinically relevant borderline rejection. Transpl Int. 2013;26:290–299. doi: 10.1111/tri.12032. [DOI] [PubMed] [Google Scholar]
- 25.Schlossberger V, Schober L, Rehnitz J, et al. The success of assisted reproduction technologies in relation to composition of the total regulatory T cell (Treg) pool and different Treg subsets. Hum Reprod. 2013;28:3062–3073. doi: 10.1093/humrep/det316. [DOI] [PubMed] [Google Scholar]
- 26.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition and female fertility. Ann Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 27.Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 28.Todoric J, Handisurya A, Knapp B, Tura A, Pacini G, Kautzky-Willer A. Relationship of pentraxin 3 with insulin sensitivity in gestational diabetes. Eur J Clin Invest. 2013;43:341–349. doi: 10.1111/eci.12051. [DOI] [PubMed] [Google Scholar]
- 29.Castiglioni MT, Scavini M, Cavallin R, et al. Elevation of plasma levels of the long pentraxin 3 proceeds preeclampsia in pregnant patients with type 1 diabetes. Autoimmunity. 2009;42:296–298. doi: 10.1080/08916930902831464. [DOI] [PubMed] [Google Scholar]
- 30.Zhou P, Luo X, Qi HB, et al. The expression of pentraxin 3 and tumor necrosis factor-alpha is increased in preeclamptic placental tissue and maternal serum. Inflamm Res. 2012;61:1005–1012. doi: 10.1007/s00011-012-0507-x. [DOI] [PubMed] [Google Scholar]
- 31.Laresgoiti-Servitje E, Gómez-Lopéz N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010;16:510–524. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 32.Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013;6:650–659. [PMC free article] [PubMed] [Google Scholar]


