Abstract
SEW2871, a selective sphingosine-1-phosphate type 1 receptor (S1P1) agonist, has been shown to be effective in protecting kidneys against ischaemia–reperfusion injury by reducing CD4+ T cell infiltration in mice. However, the effects of SEW2871 on colitis remain unclear. The aim of this study was to investigate the effects of SEW2871 on established colitis in interleukin (IL)-10 gene-deficient (IL-10–/–) mice, a murine model of Crohn's disease (CD). SEW2871 was administered by gavage at a dose of 20 mg/kg/day for 2 weeks to IL-10–/– mice. Severity of colitis, serum amyloid A, tissue myeloperoxidase (MPO), T cells in blood and colon lamina propria (LP) and proinflammatory cytokine productions were evaluated. Furthermore, the phospho-signal transducer and activator of transcription (STAT)-3 (p-STAT-3) expression in lymphocytes isolated from colon LP was also assessed. The 2-week administration of SEW2871 ameliorated established colitis in IL-10–/– mice, associated with a reduction of serum amyloid A concentration, a decreased colon MPO concentration, a depletion of the peripheral CD4+CD45+ T cells and a reduction of the homing of T cells into colon LP. Moreover, typical cytokines of T helper type 1 (Th1) and Th17 cells and p-STAT-3 expression were also suppressed by SEW2871 treatment. SEW2871 treatment ameliorates established experimental colitis in IL-10–/– mice, which may provide a new therapeutic approach for human CD therapy.
Keywords: Crohn's disease, IL-10 deficient mice, inflammation, SEW2871, treatment
Introduction
Crohn's disease (CD), one of the major forms of inflammatory bowel disease (IBD), is generally considered to be a chronic inflammatory disorder associated with a dysregulated mucosal immune response 1. Although tremendous progress has been made in our understanding of the pathogenesis of CD, its precise aetiology still remains unclear 2. Despite this, increasing evidence suggests that an imbalanced mucosal T cell response may play an important role in the pathogenesis of CD 3. For many years it has been assumed that Crohn's disease is mediated mainly by T helper type 1 (Th1) cells, which has been supported by increased levels of Th1 cytokines such as interferon (IFN)-γ and interleukin (IL)-12 4. Recently, a novel subset of IL-17-producing CD4+ Th cells termed Th17 cells have been implicated in the pathogenesis of CD 5. Th17 cells make IL-21, which in turn activates signal transducer and activator of transcription (STAT)-3 in Th17 cells, with the downstream effect of enhancing retinoic acid-related orphan receptor (ROR)-γt expression and expanding Th17 cell responses, which produce IL-17, also termed IL-17A 6–9. It was also reported that high levels of tyrosine phosphorylated STAT-3 (p-STAT-3) was observed in rested intestinal T cell lines obtained from CD patients 10.
IL-10 gene-deficient (IL-10–/–) mice develop Crohn's-like chronic colitis spontaneously when bred and maintained under specific pathogen-free (SPF) conditions, with massive infiltration of activated lymphocytes and macrophages in the lamina propria (LP) of the colon, accompanied by a CD4+ Th1 and Th17 response with secreting tumour necrosis factor (TNF)-α, IL-1β, IL-12, IFN-γ and IL-17A 11–13. Moreover, high levels of p-STAT-3 in colon tissues of IL-10–/– mice were also observed 14.
Sphingosine-1-phosphate (S1P), regarded primarily as an intermediate of sphingolipid metabolism, is now recognized as a powerful mediator of many vital cellular processes 15. S1P is recognized by cells expressing S1P receptors. Of the five types of S1P receptor, type 1 S1P receptors (S1P1) are expressed preferentially by lymphocytes, and determine lymphocyte emigration from and retention in the lymphoid tissues 16,17. Accumulating evidence has revealed the pivotal role of S1P in the development of inflammatory diseases such as autoimmune type 1 diabetes, rheumatoid arthritis and multiple sclerosis 16,18. It was reported recently that FTY720, a non-selective S1P receptor agonist, ameliorated intestinal inflammation in several murine colitis models [IL-10–/– mice 19, trinitrobenzene sulphonic acid (TNBS)-induced colitis 20, dextran sulphate sodium (DSS)-induced and CD4+CD62L+ T cell-transfer colitis 21]. Moreover, both KRP-203 and W-061 (another two S1P receptor agonists) were significantly effective in the treatment of chronic ongoing colitis in IL-10–/– mice and DSS-induced acute colitis, respectively 22,23. Compared with non-selective S1P receptor agonists, selective S1P1 agonists had the advantage of not inducing bradycardia, as type 3 S1P receptor (S1P3) was implicated directly in this complication 24. Thus, targeting S1P receptors appears to be a promising therapeutic approach for the treatment of IBD.
SEW2871, another selective S1P1 agonist, was identified originally by high-throughput screening of commercial chemical libraries with a fluorescent imaging plate reader (FLIPR) calcium flux assay, and was found to induce lymphopenia in mice via a S1P1-dependent mechanism 24,25. Although it is a S1P1-selective agonist, it is structurally unrelated to S1P and its phosphorylation is not required for binding to the receptor compared with FTY720 25. SEW2871 has already been proved to be efficient in protecting kidneys against ischaemia–reperfusion injury and preserving renal function by reducing CD4+ T cell infiltration in mice 26,27. Hence, we performed the present study to evaluate the therapeutic effects of SEW2871 on established experimental colitis in IL-10–/– mice.
Materials and methods
Animals
Both IL-10–/– and wild-type (WT) mice on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were housed and maintained under SPF conditions at the Model Animal Research Center of Nanjing University (Nanjing, China). As the C57BL/6 mutants were relatively resistant to colitis 28, 20 to 24-week-old IL-10–/– mice were used in our study. All mice received humane care in accordance with the law concerning the protection and control of animals in China.
Drug treatment of mice
SEW2871 (Cayman Chemical, Ann Arbor, MI, USA) was dissolved in 100% dimethyl sulphoxide and diluted with 50% Tween 20 for use. We chose a dose of SEW2871 (20 mg/kg/day for 2 weeks) by gavage for the treatment, which was well tolerated, as reported previously by Lien et al. 26. In brief, the mice were divided into three groups: (i) treatment group: IL-10–/– mice received SEW2871 by gavage (20 mg/kg/day); (ii) control group: IL-10–/– mice that received equal volumes of distilled water alone; and (iii) WT group: WT mice also received equal volumes of distilled water. After administration, the mice were killed by excessive anaesthesia with pentobarbital sodium and their blood and colons were collected for experiments.
Histological evaluation of the colon
After the mice were killed, tissue samples were obtained from proximal colon. The samples were fixed in 10% formalin for 24 h and paraffin-embedded. Fixed tissues were then sectioned at 6 μm thickness and stained with haematoxylin and eosin (H&E). The histological score of H&E-stained samples of the proximal colon was determined by two independent pathologists in a blinded manner according to the method described by Singh et al. 29. Briefly, a score (0–4) was given, based on the following criteria: grade 0, no change from normal tissue; grade 1, one or a few multi focal mononuclear cell infiltrates in the LP, minimal hyperplasia and no mucus depletion; grade 2, intestinal lesions involved with several multi focal, mild, inflammatory cell infiltrates in the LP composed of mononuclear cells with no inflammation in the submucosa; grade 3, lesions involved moderate inflammation and epithelial hyperplasia; and grade 4, inflammation involved most of the intestinal sections. Each segment of the proximal colon was given a score based on the criteria described above.
Serum amyloid A (SAA) enzyme-linked immunosorbent assay (ELISA)
Blood samples were collected by cardiac puncture under aseptic conditions using a 1-ml syringe and spun at 1500 g for 10 min at 4°C. The supernatant serum was frozen at −80°C for later assay of SAA protein with the SAA ELISA kit (BioSource, Welland, ON, Canada), according to the manufacturer's protocol.
Tissue myeloperoxidase (MPO) determination
MPO was assessed as a marker for neutrophil leucocyte infiltration and accumulation into the inflamed colon tissue 20. MPO concentration in colon tissues was evaluated using an ELISA kit (Cell Sciences, Canton, MA, USA) by a method described previously 14, and data were presented as pg/mg tissue.
Flow cytometric analysis
Blood samples were processed on the day of collection for flow cytometric analysis using the method that we have described previously 30. In brief, erythrocytes were lysed with lysis buffer (Sigma, St Louis, MO, USA) for 10 min at room temperature. Cell suspensions were then washed twice in Roswell Park Memorial Institute (RPMI)-1640 (Sigma), and isolated cells were thoroughly suspended in each tube with 500 μl RPMI-1640.
For isolation of LP lymphocytes, we used the method reported previously by Brown et al. 31. Briefly, the removed colons were placed into ice-cold Hanks's balanced salt solution (HBSS) and opened longitudinally, washed to remove faecal contents, and dissected into 1-cm pieces. The pieces were incubated in HBSS containing 2·5% fetal bovine serum (FBS) (BioSource) and 1 mM dithiothreitol (DTT) (Sigma) to remove mucus and were digested twice in HBSS containing 1 mM ethylenediamine tetraacetic acid (EDTA) (Sigma) for 20 min at 37 C. They then were washed three times with HBSS and incubated in HBSS with 1 mM collagenase type IV (Sigma) for 2 h at 37 C. The digested tissues were filtered and washed twice with HBSS. After that, cells were resuspended in 40% Percoll (Pharmacia Biotech, Little Chalfont, UK) and layered on 75% Percoll before centrifugation at 600 g for 20 min. The LP lymphocytes were collected at the interface of the Percoll gradient and washed with RPMI-1640. The cells were used immediately for experiments.
For immunofluorescent staining, cells isolated from blood and colon tissues were counted and approximately 1 million cells transferred to each flow test tube. These cells were stained with fluorescein isothiocyanate-conjugated anti-CD4 (RM4-5; BD Biosciences, San Jose, CA, USA), phycoerythrin-conjugated anti-CD45 (30-F11; BD Biosciences) or an appropriate negative control. Then, the stained cells were incubated at room temperature for 30 min in the dark. The cells were washed twice with 2 ml RPMI-1640 at room temperature and suspended in 500 μl RPMI-1640. Data were acquired on a fluorescence activated cell sorter (FACS)Calibur (BD Biosciences), followed by analysis using FlowJo software (version 7·6·1; TreeStar, Ashland, OR, USA).
Quantitative real-time–polymerase chain reaction (qRT–PCR) analysis
Total RNA was extracted from frozen colon tissues using Trizol RNA isolation reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The RNA concentration was determined by absorbance at 260 nm in relation to absorbance at 280 nm with a spectrophotometer (Bio-Rad, Hercules, CA, USA). qRT–PCR analysis for proinflammatory cytokine expression was performed using the method described previously 32. The primer sets used for qRT–PCR are described in Table 1.
Table 1.
Primer sets for quantitative real-time PCR.
| TNF-α | Forward: | 5′- TGGGAGTAGACAAGGTACAACCC-3′ |
| Reverse: | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ | |
| IFN-γ | Forward: | 5′- AACGCTACACACTGCATCTTGG-3′ |
| Reverse: | 5′- GCCGTGGCAGTAACAGCC-3′ | |
| IL-1β | Forward: | 5′-CAACCAACAAGTGATATTCTCCATG-3′ |
| Reverse: | 5′-GATCCACACTCTCCAGCTGCA-3′ | |
| IL-12p40 | Forward: | 5′-ACAGCACCAGCTTCTTCATCAG-3′ |
| Reverse: | 5′-TCTTCAAAGGCTTCATCTGCAA-3′ | |
| IL-17A | Forward: | 5′-GCTCCAGAAGGCCCTCAGA -3′ |
| Reverse: | 5′-AGCTTTCCCTCCGCATTGA-3′ | |
| β-actin | Forward: | 5′-GACGGCCAGGTCATCACTATTG-3′ |
| Reverse: | 5′-AGGAAGGCTGGAAAAGAGCC-3′ |
TNF = tumour necrosis factor; IFN = interferon; IL = interleukin.
Western blot analysis
For analysis of the effects of SEW2871 on STAT3 signalling, lymphocytes were isolated from mouse colon LP homogenized in 2–3 ml of lysis buffer with a protease inhibitor cocktail (Sigma). Debris was eliminated by centrifugation at 10 000 g at 4°C for 15 min. Immunoblotting was performed as described previously 14. The primary antibodies against STAT-3 and p-STAT-3 were purchased from Cell Signaling Technology (Danvers, MA, USA). Detection was performed by incubating the membranes with ECL Plus (Amresco, Solon, OH, USA) and exposed to X-ray film. Relative changes in protein expression were estimated from the pixel density using UN-SCAN-IT version 6·1, normalized to β-actin, and calculated as target protein expression : β-actin expression ratios.
Statistical analysis
Statistical analyses were performed using spss version 17·0 software (SPSS, Inc., Chicago, IL, USA). Data were expressed as means ± standard error of the mean (s.e.m.). The measurements were subjected to one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test. Differences were considered significant when P < 0·05.
Results
SEW2871 treatment significantly ameliorated established colitis in IL-10–/– mice
After the mice were killed, we first removed colons and measured their lengths, as reduced colon length is an indication of inflammation and fibrosis. The length of colon in IL-10–/– mice was significantly shorter compared with WT mice (Fig. 1). After treatment with SEW2871, the length of the colon was significantly longer than that in the water-treated mice (Fig. 1), suggesting a reduction in the severity of disease. To confirm this, microscopic study was then conducted with H&E-stained colonic specimens from different groups and the degree of inflammation was scored. Mucosa from IL-10–/– mice receiving water showed obvious signs of inflammation, with clear inflammatory infiltration and disruption in crypt architecture compared with WT mice. In contrast, in the mucosa of mice that were treated with SEW2871, inflammation signs were reduced significantly compared with water-treated controls (Fig. 2a–c), accompanied by a lower mean histological score (Fig. 2d).
Fig. 1.
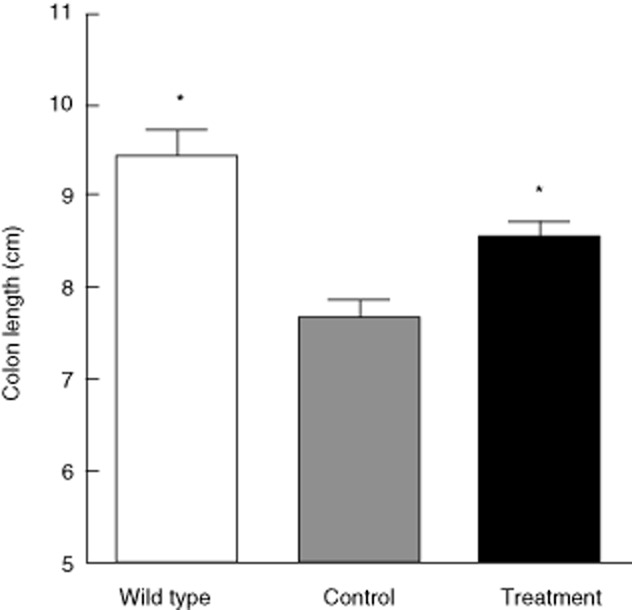
Change of colon lengths in mice treated with water or SEW2871. Data are presented as means ± standard error of the mean (s.e.m.) (n = 6 for each group; *P < 0·05 versus water-treated interleukin (IL)-10–/– controls).
Fig. 2.
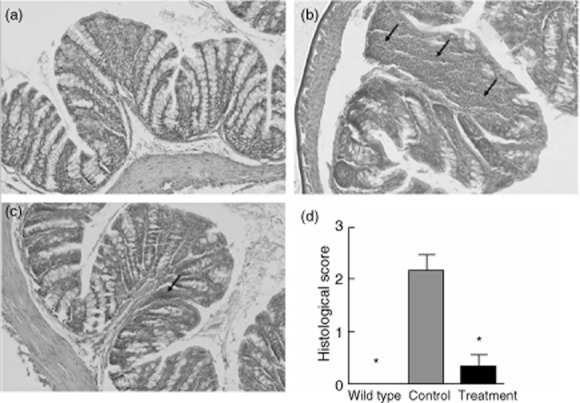
Histological features and inflammation scores of the proximal colons from mice in three groups. Representative haematoxylin and eosin (H&E)-stained sections from three groups (×200 magnification) are shown. Arrow indicates infiltration of inflammatory cells. (a) Normal wild-type (WT) group. (b) Water-treated interleukin (IL)-10–/– control group, which showed significant lymphocyte infiltration in colon lamina propria (LP). (c) SEW2871 treatment group, which showed markedly decreased lymphocyte infiltrates after SEW2871 treatment. (d) The histological inflammation scores of all three groups. Data are presented as means ± standard error of the mean (s.e.m.) (n = 6–8 for each group; *P < 0·05 versus water-treated IL-10–/– controls).
We subsequently examined the MPO concentration in colon tissues, which is widely accepted as a marker to quantify the degree of the accumulation of inflammatory cells, especially neutrophils. As shown in Fig. 3a, MPO concentration was reduced significantly by treatment with SEW2871 compared with IL-10–/– controls. Furthermore, development of colitis in IL-10–/– mice was associated with a significant increase of plasma SAA levels, and treatment with SEW2871 reduced SAA protein concentration significantly in plasma (Fig. 3b).
Fig. 3.
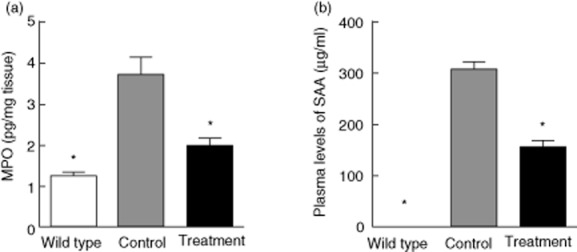
SEW2871 treatment ameliorated spontaneous colitis in IL-10–/– mice. (a) Myeloperoxidase (MPO) concentration in colon tissues of each group was determined using an enzyme-linked immunosorbent assay (ELISA) kit. (b) The serum amyloid A (SAA) concentration was determined by ELISA. Data are all presented as means ± standard error of the mean (s.e.m.) (n = 6 for each group; *P < 0·05 versus water-treated IL-10–/– controls).
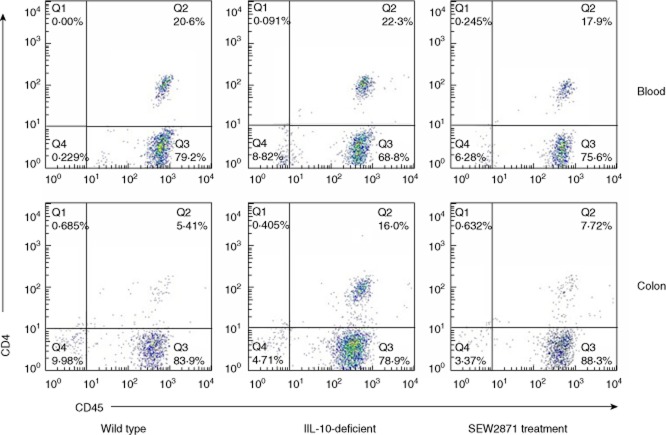
SEW2871 treatment reduced CD4+CD45+ T lymphocytes in blood and colon LP
It has been reported that S1P receptor agonists exert their immunomodulatory effectiveness through the blocking of T cell egress to peripheral tissues 18. Flow cytometric analysis was next performed to observe the change of CD4+CD45+ T lymphocytes in blood and colon LP. As shown in Fig. 4, the percentage of CD4+CD45+ T cells in blood in water-treated IL-10–/– controls was higher than WT mice, while this difference was not statistically significant (P =0.27). When IL-10–/– mice were treated with SEW2871, the percentage of CD4+CD45+ T cells in blood was decreased significantly (P <0.05). In LP of colon tissues, we also observed a significant increase in the percentage of CD4+CD45+ T cells in IL-10–/– mice compared with WT mice (P <0.05). Similar to the findings in blood, a significant decrease was also observed after treatment with SEW2871 in IL-10–/– mice (P < 0·05).
Fig. 4.
SEW2871 treatment reduced CD4+CD45+ T lymphocytes in blood and colon lamina propria (LP) (n = 6–8 for each group). Representative results are shown.
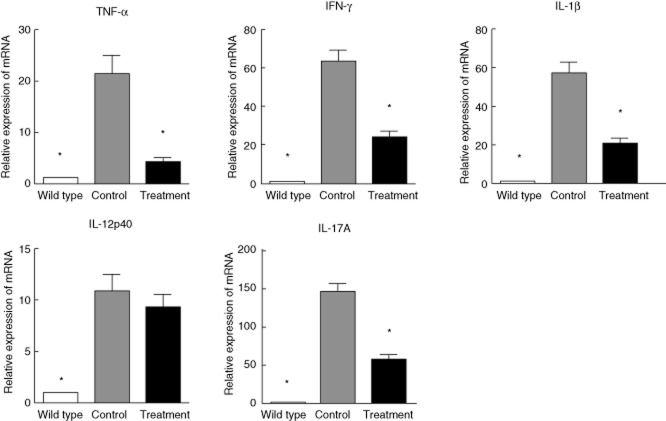
SEW2871 suppressed colonic proinflammatory cytokine mRNA expression in IL-10–/– mice
In the next series of our study, we analysed whether or not ameliorated disease in SEW2871-treated mice was associated with changes in the production of cytokines and gene expression for inflammatory cytokine was quantified in colon tissues. As shown in Fig. 5, all the markers of Th1 and Th17 cells were increased significantly in IL-10–/– mice when compared with WT mice. TNF-α, IFN-γ, IL-1β and IL-17A mRNA levels were significantly lower in mice treated with SEW2871 than IL-10–/– controls. Although IL-12p40 was also suppressed, there was no significant difference between the water and SEW2871 treatment groups.
Fig. 5.
Effect of SEW2871 on colonic proinflammatory cytokine mRNA expression using quantitative real-time–polymerase chain reaction (qRT–PCR) analysis. Data are presented as means ± standard error of the mean (s.e.m.) (n = 6 for each group; *P < 0·05 versus water-treated IL-10–/– controls).
SEW2871 treatment reduced the protein expression of p-STAT-3
To further determine the therapeutic effects of SEW2871, Western blotting of STAT-3 and p-STAT-3 expression in lymphocytes isolated from mouse colon LP was performed among the three groups. As shown in Fig. 6, treatment with SEW2871 reduced p-STAT-3 expression significantly, but the levels of total STAT-3 proteins were not affected significantly.
Fig. 6.
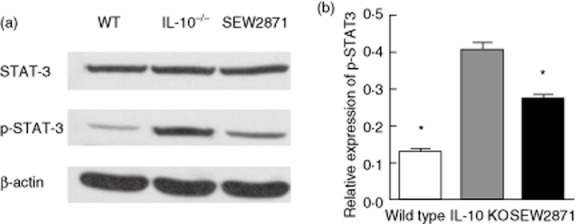
Western blot analysis of signal transducer and transcription (STAT)-3 and p-STAT-3 expressions in lymphocytes isolated from mouse colon lamina propria (LP). (a) The expressions of STAT-3 and p-STAT-3 were statistically analysed relative to β-actin expression by densitometry. (b) SEW2871 treatment suppresses p-STAT-3 expression. Data are presented as means ± standard error of the mean (s.e.m.) (n = 4–6 for each group; *P < 0·05 versus water-treated IL-10–/– controls).
Discussion
Tremendous progress has been made in our understanding of the treatment of CD in the past several decades 33. Recently, the relationship between S1P and inflammation has been clarified extensively, and diverse pharmacological agents that target the functions of S1P and its receptors show therapeutic potential for treating a wide range of inflammatory and autoimmune disorders 18,34,35.
IL-10–/– mice display similar characteristics to those of humans with an aberrant immunological response to enteric antigens, which are used widely for our understanding of the molecular mechanisms involved in CD 11,28,36. In this study, we utilized the IL-10 deficiency-induced mouse model of spontaneous experimental colitis to investigate the therapeutic effects of SEW2871 on chronic inflammation. Our results demonstrated that SEW2871 was effective for established colitis in IL-10–/– mice.
Treatment with SEW2871 resulted in a remarkable reduction of all aspects of the severity of colitis that we examined. Previous studies have shown that IL-10–/– mice develop chronic enterocolitis spontaneously in both Th1 and Th17 with typical proinflammatory cytokines released, which is consistent with the findings in human CD 37. We next measured the transcriptional levels of TNF-α, IFN-γ, IL-1β, IL-12p40 and IL-17A in colon tissues; the results were in line with the findings of histological evaluation. Moreover, as inhibiting only various proinflammatory cytokines such as IFN-γ and IL-12 in IL-10–/– mice has not shown satisfactory effects, perhaps the direct regulation of activated lymphocytes may be necessary for the control of colitis 19,22. We next performed experiments to observe the change of CD4+CD45+ T lymphocytes in blood and colon LP. Considering the effect of SEW2871 on inhibiting lymphocyte recirculation 24, the reduction of LP CD4+CD45+ T lymphocytes in both blood and LP might result from the decreased homing of circulating lymphocytes, and suppression of the proinflammatory cytokine production thereby could be a result of the reduced LP-activated T lymphocytes.
Finally, we performed experiments to examine the effect of SEW2871 on STAT-3 activation, phosphorylation of the transcription factors. Recent studies have revealed that STAT-3 activation plays an important role in the adaptive immune responses of colitis 38. STAT-3 activation by IL-6 and IL-23, in combination with factors such as IL-1β and RoRγt, is associated with Th17 formation 39. In contrast, STAT-3 activation by IL-10 is important for the function of regulatory T cells (Tregs), especially their ability to suppress the pathogenic Th17 response 39,40. Although the exact role of STAT-3 activation in the pathogenesis of IBD is still not clear, the expression of activated STAT-3 in the mucosa of human IBD patients as well as in animal colitis models has been well studied. Musso et al. reported that in IBD patients STAT-3 activation was confined to actively inflamed areas using immunofluorescence, and activated STAT-3 was also detected in isolated LP mononuclear cells from inflamed IBD tissues using immunoblotting 41. Lovato et al. has demonstrated that aberrant activation was observed of STAT-3 in intestinal T cells from patients with CD 10. Furthermore, using model mice, Suzuki et al. found that STAT-3 is activated in many types of colitis irrespective of the cause, suggesting that, rather than initiation, STAT-3 activation is related to the progression or development of the disease 42. More importantly, Bai et al. found that STAT-3 activation played an important role in the inflammatory process of TNBS-induced colitis, and administration of STAT-3 anti-sense oligonucleotide showed a beneficial effect on colitis through down-regulating the production of proinflammatory cytokines and inducing apoptosis of activated LP mononuclear cells 43, which suggested that STAT-3 could be a considerable target for the treatment of IBD. In this study, as shown in Fig. 6, our results indicated that an important beneficial effect of SEW2871 was associated with a reduction of p-STAT-3 expression in mouse colon LP.
In conclusion, our study demonstrates that the administration of SEW2871 effectively ameliorates the established colitis in IL-10–/– mice. The therapeutic effects were associated with a depletion of the peripheral lymphocytes and a reduction of the homing of lymphocytes into the LP. Furthermore, typical cytokines of Th1 and Th17 cells and p-STAT-3 expression were also suppressed by treatment. These data suggest that SEW2871 might be a candidate for the treatment of patients with CD.
Acknowledgments
The present study was supported in part by funding from the National Ministry of Health for the Digestive Disease (grant 201002020) and the National Natural Science Foundation of China (grants 81200263, 30972881 and 81170365). This study was also partly supported by the Model Animal Research Center, Nanjing University (Nanjing, China). The authors also thank Jianhui Liu (Department of General Surgery, the Second Affiliated Hospital of Nanjing Medical University) for his excellent technical assistance.
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombel JF, Watson AJ, Neurath MF. The 10 remaining mysteries of inflammatory bowel disease. Gut. 2008;57:429–433. doi: 10.1136/gut.2007.122192. [DOI] [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 4.Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 5.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 6.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 9.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 10.Lovato P, Brender C, Agnholt J, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem. 2003;278:16777–16781. doi: 10.1074/jbc.M207999200. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 12.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Lytle C, Tod TJ, Vo KT, Lee JW, Atkinson RD, Straus DS. The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis. 2005;11:231–243. doi: 10.1097/01.mib.0000160805.46235.eb. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Yu C, Zhu WM, et al. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol Immunol. 2010;47:2467–2474. doi: 10.1016/j.molimm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Edmonds Y, Milstien S, Spiegel S. Development of small-molecule inhibitors of sphingosine-1-phosphate signaling. Pharmacol Ther. 2011;132:352–360. doi: 10.1016/j.pharmthera.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunisawa J, Kiyono H. Immunological function of sphingosine 1-phosphate in the intestine. Nutrients. 2012;4:154–166. doi: 10.3390/nu4030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 18.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima T, Ito T, Kishi D, et al. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis. 2004;10:182–192. doi: 10.1097/00054725-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 21.Deguchi Y, Andoh A, Yagi Y, et al. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol Rep. 2006;16:699–703. [PubMed] [Google Scholar]
- 22.Song J, Matsuda C, Kai Y, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 23.Sanada Y, Mizushima T, Kai Y, et al. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLOS ONE. 2011;6:e23933. doi: 10.1371/journal.pone.0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanna MG, Liao J, Jo E, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 25.Takabe K, Paugh SW, Milstien S, Spiegel S. ‘Inside-out’ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 27.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia–reperfusion injury. Kidney Int. 2007;71:1223–1231. doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 28.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh UP, Singh S, Taub DD, Lillard JW., Jr Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10–/– mice. J Immunol. 2003;171:1401–1406. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Dong J, Zuo L, et al. Anti-mouse CD52 monoclonal antibody ameliorates iron-deficient anaemia in IL-10 knockout mice. Br J Nutr. 2014;111:987–995. doi: 10.1017/S0007114513003413. [DOI] [PubMed] [Google Scholar]
- 31.Brown JB, Lee G, Grimm GR, Barrett TA. Therapeutic benefit of pentostatin in severe IL-10–/– colitis. Inflamm Bowel Dis. 2008;14:880–887. doi: 10.1002/ibd.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Tian Y, Zhu W, et al. Triptolide induces suppressor of cytokine signaling-3 expression and promotes lamina propria mononuclear cells apoptosis in Crohn's colitis. Int Immunopharmacol. 2013;16:268–274. doi: 10.1016/j.intimp.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology. 2011;140:1838–1846. doi: 10.1053/j.gastro.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugimoto K. Role of STAT3 in inflammatory bowel disease. World J Gastroenterol. 2008;14:5110–5114. doi: 10.3748/wjg.14.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, de Haar C, Peppelenbosch MP, van der Woude CJ. New insights into the role of STAT3 in IBD. Inflamm Bowel Dis. 2012;18:1177–1183. doi: 10.1002/ibd.21884. [DOI] [PubMed] [Google Scholar]
- 40.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musso A, Dentelli P, Carlino A, et al. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis. 2005;11:91–98. doi: 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki A, Hanada T, Mitsuyama K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai A, Hu P, Chen J, et al. Blockade of STAT3 by antisense oligonucleotide in TNBS-induced murine colitis. Int J Colorect Dis. 2007;22:625–635. doi: 10.1007/s00384-006-0229-z. [DOI] [PubMed] [Google Scholar]