Abstract
The transcription factor Friend leukaemia virus integration 1 (Fli-1) is implicated in the pathogenesis of systemic lupus erythematosus in both human patients and murine models of lupus. Murphy Roths large (MRL)/lpr mice and New Zealand mixed (NZM)2410 mice, murine models of lupus, with decreased expression of Fli-1 had significantly prolonged survival and reduced nephritis. Lupus nephritis is a major cause of mortality and morbidity in patients, and inflammatory cell infiltration plays a key role in the development of the disease. To study how the expression of Fli-1 affects the infiltration of inflammatory cells into the kidneys, we generated congenic enhanced green fluorescent protein (GFP) transgenic MRL/lpr mice. A significantly increased number of GFP-expressing inflammatory cells infiltrated the kidneys of wild-type MRL/lpr mice compared to Fli-1 heterozygous (Fli-1+/−) MRL/lpr mice after injection of GFP+ cells. Expression of inflammatory chemokine mRNA, including chemokine (C-C motif) ligand (CCL)2, CCL3, CCL4 and CCL5, was significantly lower in the kidneys from Fli-1+/− MRL/lpr mice compared to wild-type littermates. Numbers of infiltrated cells into the kidneys correlate with expression levels of CCL2, CCL4 and CCL5, but not the titres of anti-dsDNA autoantibodies in these mice. Significantly increased inflammatory cells from wild-type MRL/lpr mice infiltrated into kidneys compared to the cells from Fli-1+/− MRL/lpr mice. The chemotaxis of inflammatory cells from Fli-1+/− MRL/lpr mice towards each chemokine was decreased significantly compared to inflammatory cells from wild-type MRL/lpr mice in the transwell migration assay in vitro. Our results indicate that Fli-1 affects lupus nephritis development by regulating the expression of chemokines in the kidney and the migration of inflammatory cells.
Keywords: chemokines, chemotaxis, Fli-1, infiltration, lupus nephritis
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that causes inflammation in multiple organs, including the kidney 1–3. Lupus nephritis is a major cause of mortality in patients with SLE, and approximately 60% of lupus patients will develop clinically relevant nephritis at some time during the course of their disease 1,2. Lupus nephritis is characterized by variable degrees of glomerular and tubulointerstitial inflammation with increased infiltrated mononuclear cells (T cells, B cells, monocytes and macrophages 4,5). The infiltration of inflammatory cells into the kidney plays a critical role in lupus nephritis progression 4–7. Many reports have demonstrated that the infiltration of inflammatory cells and the degree of interstitial inflammation correlates with the severity of renal injury 4–7.
Proinflammatory cytokines and chemokines have been shown to play a critical role in the infiltration of inflammatory cells into the kidneys and the development of glomerulonephritis 8. Previous studies have demonstrated that inflammatory chemokines contribute to the initiation or maintenance of immune cell infiltration, persistent inflammation and tissue damage in lupus nephritis 9–11. Chemokine expression and chemotaxis of inflammatory cells are both important in the development of lupus nephritis 8. For example, chemokine (C-C motif) ligand 2 (CCL2)-deficient Murphy Roths large (MRL)/MpJ-Faslpr (MRL/lpr) mice, have dramatically reduced macrophage and T cell infiltration in the kidney, decreased proteinuria, limited renal disease and significantly prolonged survival 12. Previous studies have demonstrated that following immune complex deposition and before inflammatory cell infiltration, proinflammatory cytokine and chemokine production was up-regulated in the kidneys in murine models of SLE 11.
Friend leukaemia virus integration 1 (Fli-1) is a member of the Ets family of transcription factors 13,14. Members of the Ets gene family are found in the genomes of diverse organisms such as Drosophila, Xenopus, sea urchin, chicken, mouse and human 15–17. Ets proteins bind to DNA sequences that contain a consensus GGA(A/T) core motif (Ets-binding site) and function as either transcriptional activators or repressors 16–18. Ets proteins regulate the expression of genes critical for the control of cellular proliferation, differentiation and apoptosis. Fli-1 has a conserved Ets binding domain and is expressed in endothelial cells, fibroblasts and haematopoietic lineages, including T and B cells 13,14,17,18. Fli-1 is also expressed in the glomerulus, small artery and capillaries in the kidney 19. Targeted, constitutive disruption of the Fli-1 gene results in haemorrhage of the neural tube and causes embryotic death due, in part, to thrombocytopenia 20. Expression of Fli-1 is implicated in both human SLE patients and murine models of lupus 21,22. SLE patients with active disease have elevated expression of Fli-1 mRNA in peripheral blood lymphocytes compared to healthy controls, and overall Fli-1 expression parallels disease activity measures 21. Over-expression of the Fli-1 protein in transgenic mice with a normal background resulted in the development of a lupus-like disease 22. We have reported that in murine models of lupus, including both MRL/lpr mice and New Zealand mixed (NZM)2410 mice with decreased expression of Fli-1, the mice demonstrate significantly prolonged survival and reduced lupus nephritis with markedly reduced infiltration of inflammatory cells into the kidneys 23,24. To further define the role of Fli-1 on lupus nephritis development, we generated congenic green fluorescent protein (GFP) transgenic MRL/lpr mice. In this study, we found that significantly increased inflammatory cells infiltrated the kidneys of wild-type MRL/lpr mice compared to Fli-1 heterozygous (Fli-1+/−) littermates; the infiltration correlates with the expression of inflammatory chemokines in kidneys, but not anti-dsDNA autoantibodies. The expression of inflammatory chemokines in kidneys of Fli-1+/− MRL/lpr mice was significantly lower compared to wild-type littermates. Furthermore, we demonstrated that expression of Fli-1 in inflammatory cells affects chemotaxis towards inflammatory chemokines in vitro. We have demonstrated previously that Fli-1 directly regulates the expression of the inflammatory chemokine CCL2 25. Taken together, our results show that Fli-1 affects lupus nephritis disease development both by regulating chemokines and cytokines in the kidney and by promoting inflammatory cell migration.
Materials and methods
Mice
MRL/lpr mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Fli-1+/− MRL/lpr mice were generated by back-crossing with Fli-1+/− C57BL/6 mice for more than 12 generations, as reported previously 23. To generate congenic GFP transgenic MRL/lpr mice, MRL/lpr mice were back-crossed with transgenic enhanced GFP C57BL/6 mice for 12 generations 26. All mice were housed under pathogen-free conditions at the animal facility of the Ralph H. Johnson Veterans Affairs Medical Center, and all animal experiments were approved by the Institutional Animal Care and Use Committee.
Genotyping the mice by polymerase chain reaction (PCR)
To genotype the mice, PCR was used to detect fragments of the wild-type Fli-1 and Fli-1+/− allele, as described previously 23. The PCR primers used were as follows: Fli-1 exon IX/forward primer (positions 1156–1180), GACCAACGGGGAGTTCAAAATGACG; Fli-1 exon IX/reverse primer (positions 1441–1465), GGAGGATGGGTGAGACGGGACAAAG; and Pol II/reverse primer, GGAAGTAGCCGTTATTAGTGGAGAGG. DNA was isolated from tail snips (4-week old mice) using the QIAamp Tissue Kit (Qiagen, Valencia, CA, USA). PCR analyses were performed under the following conditions: one cycle at 95°C for 5 min, followed by 36 repeating cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min followed by 72°C for 7 min. A 309-base pairs (bp) fragment indicates the presence of the wild-type allele, and a 406-bp fragment is amplified from the mutant allele.
Measurement of anti-dsDNA autoantibodies
Anti-dsDNA antibodies were measured by enzyme-linked immunosorbent assay (ELISA), as described previously 23.
Adoptive transfer of GFP+ spleen cells from GFP+ MRL/lpr mice
Twenty-two to 24-month-old GFP+ MRL/lpr mice with active disease were killed and spleens were collected to make single-cell suspensions. Spleen cells collected from these mice were injected intravenously (into the tail vein) to a group of wild-type or Fli-1+/− MRL/lpr littermates at the age of 18–24 weeks with a dose of 1 million cells/per mouse. The mice were killed and sera and kidneys were collected for measurement of anti-dsDNA autoantibody titre and analysis of infiltrated GFP+ cells in kidneys 18 h after injection.
Immunohistochemistry
Sections (5 μM) cut from frozen kidneys were used for counting GFP+ cells and immunofluorescence staining. For immunofluorescence staining, sections were blocked in phosphate-buffered saline (PBS) with 10% normal rat serum and incubated with biotin-labelled antibodies against CD3, CD19 or CD11b for 1 h (BD Biosciences, San Jose, CA, USA). The sections were stained with R-phycoerythrin (PE)-labelled streptavidin (BD Biosciences). GFP+, CD3+, CD19+ or CD11b+ cells in kidneys were counted by randomly selecting 10 glomeruli and tubules per section in a blinded fashion and analysed with a Nikon Eclipse 80i microscope equipped with a digital camera.
Measurement of chemokine expression in the kidneys by real-time PCR
Total RNA was prepared from kidneys taken from wild-type MRL/lpr mice and Fli-1+/− littermates using Trizol reagent, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). Two μg of RNA was used to synthesize cDNA using the SuperScript First-Strand Synthesis System (Invitrogen). Real-time PCR was performed in triplicate using Platinum SYBR Green quantitative PCR (qPCR) SuperMix UDG (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions, with three independent RNA preparations. Primers for CCL2, CCL3, CCL4, CCL5 and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from SABiosciences (Qiagen), and the cycling conditions for all genes followed instructions from the company. Relative expression analysis was conducted using the program provided by SABiosciences.
In-vitro cell migration assays
To investigate whether reduced expression of Fli-1 in inflammatory cells influences their chemotaxis towards each chemokine in immune cells in vitro, we performed cell migration assays with spleen cells from wild-type and Fli-1+/− MRL/lpr mice using 24-well Transwell plates, as described previously 27. In brief, spleens were collected to make single-cell suspensions and resuspended into prewarmed migration medium (RPMI-1640, 20 mM Hepes, 1% bovine serum albumin). One million cells were added to the upper chamber and the lower chamber contained prewarmed migration medium with or without 500 ng/ml of the indicated recombinant mouse chemokine (R&D Systems, Minneapolis, MN, USA). The cells that migrated into the lower chamber were collected 3 h later and the total cell number was counted using a haemocytometer. Fold change was calculated as (immune cells moved to the lower chamber towards each chemokine/immune cells moved without chemokine) and compared between the two groups.
Measurement of chemokine receptor expression
To measure the expression of the chemokine receptors CCR2 and CCR5 on inflammatory cells, single-cell suspensions were prepared from a group of wild-type MRL/lpr mice and Fli-1+/− littermates at the age of 22–24 weeks. The cells were stained with fluorochrome-conjugated anti-CCR2 or CCR5 antibodies (BD Pharmingen, San Diego, CA, USA). The percentage and mean florescence intensity (MFI) of positive cells were measured by flow cytometry and calculated and compared between wild-type and Fli-1+/− MRL/lpr mice.
Statistical analysis
An unpaired Student's t-test was used to determine significant differences between the means of two groups. A statistical power calculation was also performed and the number indicated in the results only when the power was less than 80% in order to detect a significant difference [with significance level (alpha = 0·05)]. Bivariate correlations between variables were determined using Pearson's correlation. A P-value < 0·05 was considered to be statistically significant.
Results
Kidneys from wild-type MRL/lpr mice attract a significantly increased number of inflammatory cells compared to those from Fli-1+/− MRL/lpr mice
To investigate the mechanisms behind how Fli-1 affects the infiltration of inflammatory cells into the kidney during lupus nephritis disease development, we generated congenic transgenic GFP MRL/lpr mice by back-crossing MRL/lpr mice with GFP transgenic B6 mice for 12 generations. GFP transgenic wild-type MRL/lpr mice were killed at the age of 22–24 weeks, and spleen cells were collected as inflammatory cells. Eleven wild-type MRL/lpr mice and six of their Fli-1+/− MRL/lpr littermates were injected intravenously with 1 million spleen cells from wild-type MRL/lpr mice. The mice were killed 18 h later, their kidneys collected, and frozen sections were made from the kidneys. The GFP+ cells were counted from 10 random sections by a fluorescent microscope. As shown in Figs 1 and 2, there were significantly more GFP+ cells in the kidneys from wild-type MRL/lpr mice compared to Fli-1+/− littermates. The immune cell infiltration was observed mainly around the small artery, vein and capillaries in the kidney interstitium (Fig. 2, and data not shown). The frozen sections were stained with specific antibodies to CD3, CD19 or CD11b and PE-labelled avidin. As shown in Fig. 1, all CD3+, CD11b+ and CD19+ cells were significantly higher in kidneys from wild-type MRL/lpr mice compared to Fli-1+/− littermates. To investigate if the expression of Fli-1 in inflammatory cells affects the infiltration of inflammatory cells, we collected GFP+ spleen cells from GFP Fli-1+/− MRL/lpr mice at the age of 22–24 weeks. Five wild-type MRL/lpr mice and five of their Fli-1+/− littermates were injected intravenously with cells from GFP+ Fli-1+/− MRL/lpr mice at a dose of 1 million cells/per mouse. The GFP+, CD3+, CD11b+ and CD19+ inflammatory cells were counted as described above. As shown in Fig. 1, the kidneys from wild-type MRL/lpr mice had increased numbers of GFP+, CD3+, CD11b+ and CD19+ cells compared to Fli-1+/− littermates, although the difference was not statistically significant, due probably to the small number of mice used; a statistical power calculation showed that we only have 52·6% power to detect a significant difference [with significance level (alpha = 0·05)]. When the infiltrated inflammatory cells in kidneys from wild-type MRL/lpr mice were compared between mice that received GFP+ cells from wild-type mice and mice that received GFP+ cells from Fli-1+/− mice, the number of infiltrated cells was increased significantly in mice that received GFP+ cells from wild-type mice (Fig. 1).
Fig. 1.
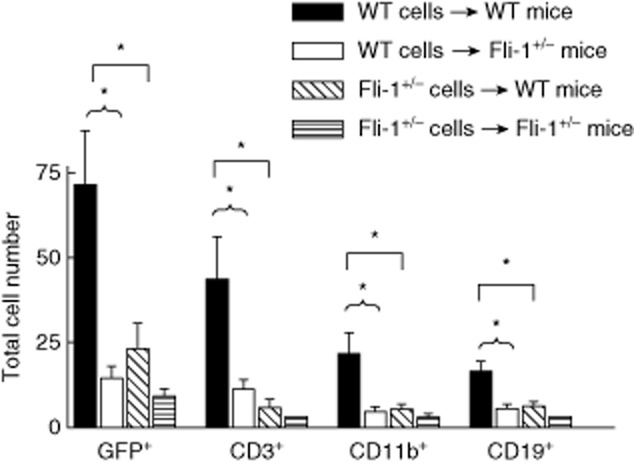
Increased green fluorescent protein (GFP+) inflammatory cells infiltrated into kidneys in wild-type Murphy Roths large (MRL)/lpr mice compared to Friend leukaemia virus integration 1 (Fli-1+/−) littermates; 18–24-week-old wild-type and Fli-1+/− MRL/lpr mice were injected intravenously (i.v.) with 1 million spleen cells collected from GFP+ wild-type MRL/lpr mice (wild-type MRL/lpr mice, black bar, n = 11; Fli-1+/− MRL/lpr mice, white bar, n = 6), or were injected i.v. with 1 million spleen cells from GFP+ Fli-1+/− MRL/lpr mice (wild-type MRL/lpr mice, diagonal-lined bar, n = 5; Fli-1+/− MRL/lpr mice, straight-lined bar, n = 5). The mice were killed and kidneys were collected and analysed for infiltrated GFP+ cells 18 h after injection. CD3+, CD19+ or CD11b+ cells were stained with specific antibodies and counted in 10 randomly selected high-power fields (HPF) and the data presented are the mean number/per mouse/per 10 HPF ± standard error (s.e.). A single asterisk indicates P < 0·05.
Fig. 2.
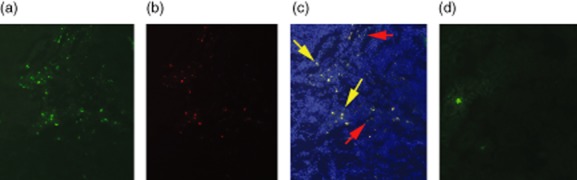
Representative sections of kidney from wild-type and Friend leukaemia virus integration 1 (Fli-1+/−) mice with infiltrating increased green fluorescent protein (GFP+) inflammatory cells. Kidney sections from wild-type Murphy Roths large (MRL)/lpr mice with green fluorescence showing GFP+ cells (a), with red fluorescence showing CD3+ cells after staining with anti-CD3 antibody (b) or merged image (c). Red arrows indicate GFP+ only cells, yellow arrows indicate GFP+ and CD3+ cells.). Kidney sections from Fli-1+/− MRL/lpr mice with green fluorescence showing few GFP+ infiltrating cells (d).
Kidneys from Fli-1+/− MRL/lpr mice had significantly decreased expression of inflammatory chemokines
Because inflammatory chemokines have a critical role in attracting inflammatory cells, we measured the expression of key chemokines in kidneys from both wild-type MRL/lpr mice and Fli-1+/− littermates by reverse transcription–polymerase chain reaction (RT–PCR). Mice were killed and RNA was extracted from kidneys after the infiltration experiments were performed. As shown in Fig. 3, the expression of all inflammatory chemokines measured, including CCL2, CCL3, CCL4 and CCL5, was decreased significantly in the kidneys from Fli-1+/− MRL/lpr mice compared to the wild-type littermates. To eliminate the possibility that a higher number of infiltrated GFP+ cells in kidneys from wild-type MRL/lpr mice would influence the results, we compared the chemokine expression levels in kidneys from wild-type and Fli-1+/− MRL/lpr mice without the injection of GFP+ cells. All four chemokines are expressed significantly higher in kidneys from wild-type MRL/lpr mice (data not shown).
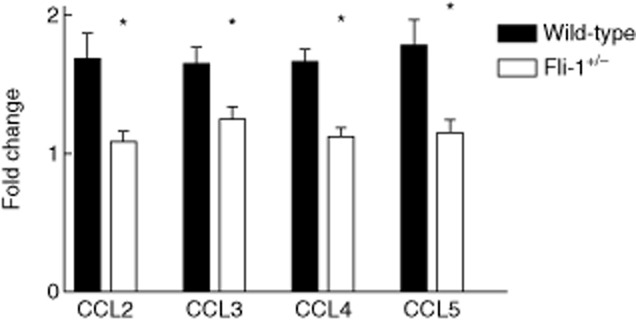
Fig. 3.

Increased expression of inflammatory chemokines in kidneys of wild-type mice compared to Friend leukaemia virus integration 1 (Fli-1+/−) Murphy Roths large (MRL)/lpr mice. Total RNA was prepared from kidneys at the ages of 22–24 weeks (wild-type MRL/lpr, n = 11, Fli-1+/− MRL/lpr mice, n = 6). Total RNA was converted to cDNA with the SuperScript First-Strand Synthesis System. Real-time polymerase chain reaction was performed in triplicate with the appropriate primers. A single asterisk indicates P < 0·05.
The number of infiltrating inflammatory cells is associated with expression levels of chemokines in the kidneys, but not anti-dsDNA autoantibody titres of the mice
The immune complex formed with autoantibodies is known to play a role in the development of lupus nephritis. Serum anti-dsDNA autoantibody titres were measured when the inflammatory infiltration in-vivo experiments were performed. As shown in Fig. 4, anti-dsDNA autoantibodies titres were lower in sera from Fli-1+/− MRL/lpr mice compared to wild-type controls at dilutions of 1:100–1:800, although the difference was not statistically significant. When the association of the number of infiltrated cells with the expression levels of the chemokines tested was determined, we found that the number of inflammatory cells that infiltrated the kidney was correlated statistically with the expression level of CCL2, CCL4 or CCL5 (Fig. 5). The association between the number of inflammatory cells infiltrating the kidney and the expression of CCL3 was close, although not statistically significant. However, the numbers of inflammatory cells in these mice that infiltrated into the kidney were not correlated with anti-dsDNA antibody titres.
Fig. 4.
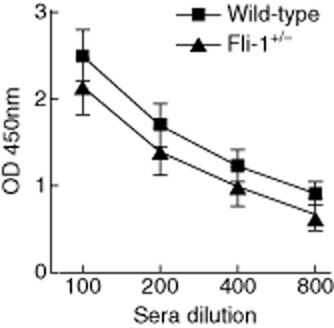
Comparison of anti-dsDNA titres between wild-type and Friend leukaemia virus integration 1 Fli-1+/−) Murphy Roths large (MRL)/lpr mice. Sera were collected from wild-type and Fli-1+/− MRL/lpr mice at the ages of 22–24 weeks. Anti-dsDNA autoantibody titres were determined by enzyme-linked immunosorbent assay (ELISA). Data presented are the mean optical density (OD) 450 ± standard error (s.e.) at 1:100–1:800 dilutions in each group (wild-type MRL/lpr mice, n = 9; Fli-1+/− MRL/lpr mice, n = 6). A single asterisk indicates P < 0·05.
Fig. 5.
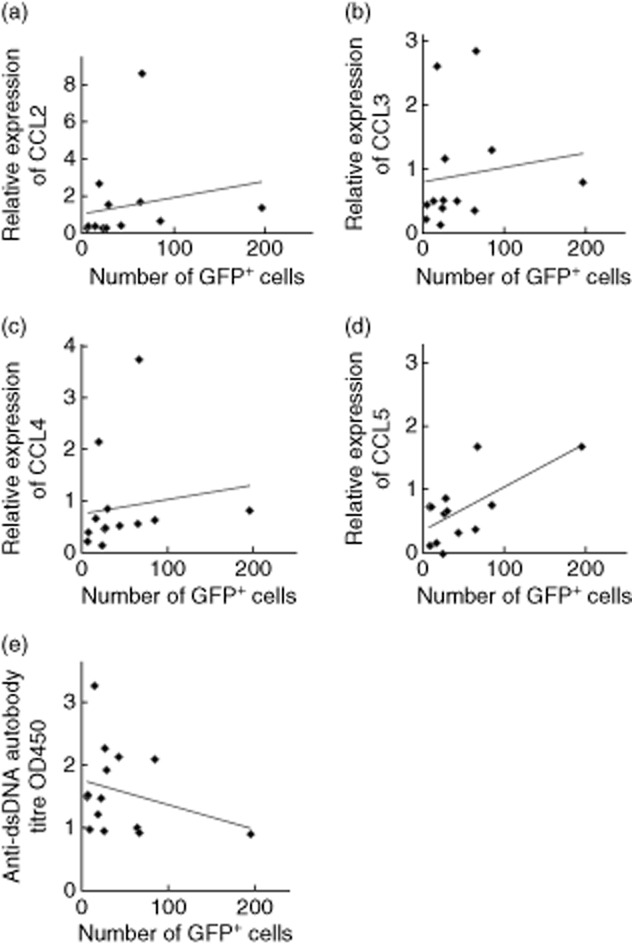
Correlation between the numbers of infiltrated inflammatory cells in kidneys and the expression levels of chemokines and anti-dsDNA autoantibody titres in MRL/lpr mice.(a) Correlation between the number of infiltrated inflammatory cells in kidneys and the expression level of chemokine (C-C motif) ligand (CCL)2 (r = 0·62, P = 0·015). (b) Correlation between the number of infiltrated inflammatory cells in kidneys and the expression level of CCL3 (r = 0·46, P = 0·052). (c) Correlation between the number of infiltrated inflammatory cells in kidneys and the expression level of CCL4 (r = 0·5, P = 0·041). (d) Correlation between the number of infiltrated inflammatory cells in kidneys and the expression level of CCL5 (r = 0·51, P = 0·037). (e) Correlation between the number of infiltrated inflammatory cells in kidneys and anti-dsDNA autoantibody titres at a 1:200 dilution (r = −0·22, P = 0·211).
Chemotaxis of inflammatory cells from Fli-1+/− MRL/lpr mice was impaired significantly in vitro
Compared to inflammatory cells from wild-type MRL/lpr mice, significantly reduced numbers of inflammatory cells from Fli-1+/− MRL/lpr mice infiltrated the kidneys (Fig. 1). Next, we measured the migration rates of the inflammatory cells from wild-type and Fli-1+/− MRL/lpr mice to chemokines in vitro using a Transwell migration assay. Fold change in migration for each chemokine was calculated as the total migrated cells in the lower chamber that contained the chemokine/total cells in the lower chamber without chemokine. As shown in Fig. 6, the migration of inflammatory cells from Fli-1+/− MRL/lpr mice towards each chemokine (CCL2, 3, 4 and 5) was decreased significantly compared to the migration in wild-type mice.
Fig. 6.
Chemotaxis of inflammatory cells from Friend leukaemia virus integration 1 (Fli-1+/− Murphy Roths large (MRL)/lpr mice was decreased significantly in vitro. One million spleen cells from wild-type or Fli-1+/−) MRL/lpr mice were placed into the upper chamber of 24-well Transwell plates. The lower chamber contained prewarmed medium with or without 500 ng/ml of the indicated recombinant mouse chemokine (wild-type MRL/lpr mice, n = 6; Fli-1+/− MRL/lpr mice, n = 4). The cells that migrated into the lower chamber were collected after 3 h and the total cell number was counted. The fold change was calculated as the number of cells that moved to lower chamber towards each chemokine/cells moved without chemokine. A single asterisk indicates P < 0·05.
Expression of CCR2 and CCR5 on inflammatory cells from Fli-1+/− MRL/lpr mice was similar compared to wild-type mice
To determine if the reduced chemotaxis of inflammatory cells from Fli-1+/− MRL/lpr mice is duo to the altered expression of chemokine receptors, we measured the expression of CCR2 and CCR5 on spleen cells from Fli-1+/− and wild-type controls by flow cytometry. The percentages of CCR2+ cells were higher in spleen cells from Fli-1+/− MRL/lpr mice, although the differences were not statistically significant except for CD3+ cells (Table 1). Conversely, percentages of CCR5+ cells were higher in spleen cells from wild-type MRL/lpr mice, although the differences were not statistically significant (Table 1). The MFI of CCR2 and CCR5 between spleen cells from wild-type and Fli-1+/− MRL/lpr mice were not statistically different.
Table 1.
Comparison of expression of chemokine receptor (CCR) type 2 and CCR5 on spleen cells between wild-type and Friend leukaemia virus integration 1 (Fli-1+/−) Murphy Roths large (MRL)/lpr mice†
| CCR2 | CCR5 | ||||||
|---|---|---|---|---|---|---|---|
| CD3+* | CD19+ | CD11b+ | CD3+ | CD19+ | CD11b+ | ||
| Percentage positive cells | Wild-type | 3·98 ± 0·96‡ | 42·82 ± 11·54 | 31·41 ± 7·65 | 3·39 ± 0·97 | 0·70 ± 0·18 | 2·83 ± 0·56 |
| Fli-1+/− | 7·63 ± 0·83 | 59·91 ± 10·93 | 43·50 ± 6·84 | 2·01 ± 1·40 | 0·41 ± 0·16 | 1·70 ± 0·16 | |
| Mean fluorescence intensity | Wild-type | 118·6 ± 17·3§ | 117·0 ± 17·5 | 163·4 ± 14·8 | 103·5 ± 19·9 | 41·1 ± 4·1 | 154·8 ± 26·4 |
| Fli-1+/− | 114·2 ± 5·3 | 90·7 ± 4·8 | 140·5 ± 9·4 | 71·4 ± 25·0 | 40·1 ± 3·7 | 115·0 ± 11·6 | |
Indicates P < 0·05.
Mice were killed at the age of 24 weeks (n = 8 in each group). The cells were stained with fluorochrome-conjugated anti-CCR2 or CCR5 antibodies as well as CD3, CD19 or CD11b antibody and measured by flow cytometry.
Percentage positive cells ± standard error (s.e.).
Mean florescence intensity ± s.e.
Discussion
Infiltration of inflammatory cells into the kidneys plays a critical role in lupus nephritis disease development. The expression of the transcription factor Fli-1 has been implicated in SLE development in both human patients and murine models of lupus 21–24. MRL/lpr and NZM2410 mice with reduced expression of Fli-1 exhibited significantly reduced nephritis and prolonged survival 23,24. In this report, using inflammatory cells from congenic GFP transgenic MRL/lpr mice, we have demonstrated significantly decreased infiltration of inflammatory cells into kidneys in Fli-1+/− MRL/lpr mice compared to wild-type littermates and that inflammatory cell infiltration is correlated closely with the expression of inflammatory chemokines located in the kidneys, but is not associated with anti-dsDNA autoantibody titres.
The effects of chemokines in inflammatory cell infiltration have been demonstrated in CCL2-deficient MRL/lpr mice 12. These mice had significantly reduced inflammatory cell infiltration into the kidneys, decreased proteinuria and prolonged survival compared to wild-type controls 12. Perez De Lema et al. have reported that proinflammatory chemokines are up-regulated prior to inflammatory cell infiltration during the initiation of lupus nephritis in a lupus mouse model 11. By generating enhanced GFP transgenic MRL/lpr mice, we were able to easily track the infiltrated inflammatory cells into kidneys during the development of lupus nephritis disease. We have found that the expression of all four inflammatory chemokines tested, including CCL2, CCL3, CCL4 and CCL5, in kidneys from Fli-1+/− MRL/lpr mice was significantly lower compared to wild-type littermate controls when GFP+ inflammatory cells were injected. The number of infiltrated cells was correlated statistically with the expression of these inflammatory chemokines in kidneys. We have reported that Fli-1 binds directly to the promoter of CCL2, and endothelial cells isolated from kidneys of Fli-1+/− NZM2410 mice had significantly lower production of CCL2 compared to wild-type NZM2410 mice 25. We have further demonstrated that CCL2 promoter activity was increased significantly by Fli-1 in a dose-dependent manner when transfected into NIH3T3 cells (Lennard Richard et al., unpublished data). CCL5 is an important chemokine in recruiting leucocytes to inflammatory sites 10,11,28. We found endothelial cells transfected with specific Fli-1 siRNA produced significantly less CCL5 in the supernatants compared to cells transfected with non-specific siRNA after stimulation with lipopolysaccharides (LPS), Fli-1 directly binds to the promoter of CCL5 by chromatin immunoprecipitation (ChIP) assay and CCL5 promoter activity was increased by Fli-1 in a dose-dependent manner (Lennard Richard et al., unpublished data). Thus, the lower expression of CCL2 and CCL5 in kidneys from Fli-1+/− MRL/lpr mice is probably due, in part, to decreased Fli-1 expression. We do not know if Fli-1 regulates the expression of CCL3 and CCL4 at this time.
In human lupus patients, elevated expressions of chemokines including CCL2, CCL3 and CCL5 have been reported, and this increase is associated with disease activity and clinical manifestations of SLE 29,30. The expressions of CCL3 in kidneys from lupus patients were correlated with the cellular crescents and the number of CD68-positive (macrophage marker) infiltration cells 30. Liu et al. also reported that expressions of CCL2, CCL3 and CCL4 in kidneys from lupus patients were correlated strongly with infiltrating macrophages 31.
In this study,we found that the number of infiltrated inflammatory cells does not correlate with anti-dsDNA autoantibody titres in the MRL/lpr mice. Fli-1+/− MRL/lpr mice had significantly reduced inflammatory cells in kidneys with measurable autoantibody titres (Figs 1 and 4). It is well known that end-stage organ damage in SLE is caused by aberrant autoantibodies that are locally deposited in the form of immune complexes leading to progressive inflammation and tissue destruction 1–3. In our previous studies, we have demonstrated that Fli-1+/− MRL/lpr and NZM2410 mice had significantly reduced renal disease despite the fact that autoantibodies were still generated and the deposit of immune complexes occurred in the kidneys of these mice 23,24. Another study showed that NZM2410 mice with oestrogen receptor disruption had significantly reduced renal pathology, in spite of increased autoantibody titres compared to wild-type littermates 32. Several reports have demonstrated that some lupus patients did not develop renal injury even with persistent, high serum titres of immunoglobulin (Ig)G anti-dsDNA antibodies, and in most lupus patients the autoantibodies can be detected several years before clinical illness 33,34. Thus, these results indicate that the local response in the kidney to stimulation also plays an important role in the development of lupus nephritis.
Compared to the number of inflammatory cells isolated from wild-type MRL/lpr mice, significantly fewer inflammatory cells isolated from Fli-1+/− MRL/lpr mice infiltrated the kidneys (Fig. 1). Inflammatory cells from Fli-1+/− MRL/lpr mice also had significantly decreased chemotaxis towards CCL2, CCL3, CCL4 and CCL5 in vitro compared to cells from wild-type MRL/lpr mice (Fig. 6). It has been reported that the expression of chemokine receptors affects chemotaxis and lupus nephritis development. Perez-De Lema et al. reported that CCR2-deficient MRL/lpr had decreased renal lesions, proteinuria and prolonged survival with decreased T cell and macrophage infiltration into the kidney 35. On the contrary, CCR5-deficient MRL/lpr mice showed aggressive renal disease with increased mononuclear cell infiltration into the kidney due to decreased consumption of their ligands 36. We found that the expression levels of CCR2 and CCR5 between inflammatory cells from wild-type and Fli-1+/− MRL/lpr mice were not altered significantly. A recent report demonstrated that Fli-1 directly regulates expression of neuraminidase 1 in T cells; expression of neuraminidase 1 was lower in T cells from Fli-1+/− MRL/lpr mice compared to those from wild-type MRL/lpr mice 37. Glycosphingolipids have been shown to be involved in cellular signalling, metabolism and migration 38. Thus, Fli-1 may affect chemotaxis partly by regulating the expression of neuraminidase 1. The mechanisms behind how the expression level of Fli-1 in inflammatory cells can alter their chemotaxis towards chemokines is still under active investigation.
In summary, reduced Fli-1 expression affects inflammatory cell infiltration into the kidney by moderating inflammatory chemokine expression in local kidneys and the chemotaxis of inflammatory cells. Our studies provide new understanding and insights into how the transcription factor Fli-1 impacts lupus nephritis disease development, which will help to develop novel treatments for lupus nephritis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR056670 to X. Z.) and the Medical Research Service, Department of Veterans Affairs (to X. Z.). We would like to thank Ms Eva Karam and Ms Sarah Williams for their excellent technical support and Dr Mara Lennard Richard for critical review of the manuscript. We thank Dr M. Okabe (Osaka University, Japan) for providing enhanced GFP C57BL/6 mice.
Disclosure
The authors have no financial or commercial conflicts of interest.
References
- 1.D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 2.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME. Lupus nephritis: a critical review. Autoimmun Rev. 2012;12:174–194. doi: 10.1016/j.autrev.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10:413–424. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 4.Boucher A, Droz D, Adafer E, Noël LH. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. Kidney Int. 1986;29:1043–1049. doi: 10.1038/ki.1986.105. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37:100–109. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- 6.de Zubiria Salgado A, Herrera-Diaz C. Lupus nephritis: an overview of recent findings. Autoimmune Dis. 2012;2012:21. doi: 10.1155/2012/849684. Article ID 849684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castiglione A, Bucci A, Fellin G, d'Amico G, Atkins RC. The relationship of infiltrating renal leucocytes to disease activity in lupus and cryoglobulinaemic glomerulonephritis. Nephron. 1988;50:14–23. doi: 10.1159/000185110. [DOI] [PubMed] [Google Scholar]
- 8.Nowling TK, Gilkeson GS. Mechanisms of tissue injury in lupus nephritis. Arthritis Res Ther. 2011;13:250–259. doi: 10.1186/ar3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoja C, Liu XH, Donadelli R, et al. Renal expression of monocyte chemoattractant protein-1 in lupus autoimmune mice. J Am Soc Nephrol. 1997;8:720–729. doi: 10.1681/ASN.V85720. [DOI] [PubMed] [Google Scholar]
- 10.Moore KJ, Wada T, Barbee SD, Kelley VR. Gene transfer of RANTES elicits autoimmune renal injury in MRL-Fas(1pr) mice. Kidney Int. 1998;53:1631–1641. doi: 10.1046/j.1523-1755.1998.00911.x. [DOI] [PubMed] [Google Scholar]
- 11.Pérez de Lema G, Maier H, Nieto E, et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12:1369–1382. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 12.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med. 1999;190:1813–1824. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-David Y, Giddens EB, Letwin K, et al. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Rev. 1991;5:908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- 14.Watson DK, Smyth FE, Thompson DM, et al. The ERGB/Fli-1 gene: isolation and characterization of a new member of the family of human ETS transcription factors. Cell Growth Differ. 1992;3:705–713. [PubMed] [Google Scholar]
- 15.Seth A, Ascione R, Fisher RJ, Mavrothalassitis GJ, Bhat NK, Papas TS. The ets gene family. Cell Growth Differ. 1992;3:327–334. [PubMed] [Google Scholar]
- 16.Karim FD, Urness LD, Thummel CS, et al. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990;4:1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- 17.Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91:896–903. doi: 10.1002/jcb.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papas TS, Bhat NK, Spyropoulos DD, et al. Functional relationships among ETS gene family members. Leukemia. 1997;11(Suppl 3):557–566. [PubMed] [Google Scholar]
- 19.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 20.Spyropoulos DD, Pharr PN, Lavenburg KR, et al. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgiou P, Maroulakou IG, Green JE, et al. Expression of ets family of genes in systemic lupus erythematosus and Sjogren's syndrome. Int J Oncol. 1996;9:9–18. [PubMed] [Google Scholar]
- 22.Zhang L, Eddy A, Teng TT, et al. An immunological renal disease in transgenic mice that overexpress Fli-1, a member of the ets family of transcription factor genes. Mol Cell Biol. 1995;15:6961–6970. doi: 10.1128/mcb.15.12.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XK, Gallant S, Molano I, et al. Decreased expression of the ets family transcription factor Fli-1 markedly prolongs survival and significantly reduces renal disease in MRL/lpr mice. J Immunol. 2004;173:6481–6489. doi: 10.4049/jimmunol.173.10.6481. [DOI] [PubMed] [Google Scholar]
- 24.Mathenia J, Reyes-Cortes E, Williams S, et al. Impact of Fli-1 transcription factor on autoantibody and lupus nephritis in NZM2410 mice. Clin Exp Immunol. 2012;362:362–371. doi: 10.1111/j.1365-2249.2010.04245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki E, Karam E, Williams S, Watson DK, Gilkeson G, Zhang XK. Fli-1 transcription factor affects glomerulonephritis development by regulating expression of monocyte chemoattractant protein-1 in endothelial cells in the kidney. Clin Immunol. 2012;145:201–208. doi: 10.1016/j.clim.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 27.Adalid-Peralta L, Mathian A, Tran T, et al. Leukocytes and the kidney contribute to interstitial inflammation in lupus nephritis. Kidney Int. 2008;73:172–180. doi: 10.1038/sj.ki.5002625. [DOI] [PubMed] [Google Scholar]
- 28.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 29.Fu Q, Chen X, Cui H, et al. Association of elevated transcript levels of interferon-inducible chemokines with disease activity and organ damage in systemic lupus erythematosus patients. Arthritis Res Ther. 2008;10:R112. doi: 10.1186/ar2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wada T, Furuichi K, Segawa-Takaeda C, et al. MIP-1alpha and MCP-1 contribute to crescents and interstitial lesions in human crescentic glomerulonephritis. Kidney Int. 1999;56:995–1003. doi: 10.1046/j.1523-1755.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu ZH, Chen SF, Zhou H, Chen HP, Li LS. Glomerular expression of C-C chemokines in different types of human crescentic glomerulonephritis. Nephrol Dial Transplant. 2003;18:1526–1534. doi: 10.1093/ndt/gfg172. [DOI] [PubMed] [Google Scholar]
- 32.Svenson JL, EuDaly J, Ruiz P, Korach KS, Gilkeson GS. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clin Immunol. 2008;128:259–268. doi: 10.1016/j.clim.2008.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gladman DD, Urowitz MB, Keystone EC. Serologically active clinically quiescent systemic lupus erythematosus: a discordance between clinical and serologic features. Am J Med. 1979;66:210–215. doi: 10.1016/0002-9343(79)90529-1. [DOI] [PubMed] [Google Scholar]
- 34.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 35.Pérez de Lema G, Maier H, Franz TJ, et al. Chemokine receptor Ccr2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J Am Soc Nephrol. 2005;16:3592–3601. doi: 10.1681/ASN.2005040426. [DOI] [PubMed] [Google Scholar]
- 36.Turner JE, Paust HJ, Bennstein SB, et al. Protective role for CCR5 in murine lupus nephritis. Am J Physiol Renal Physiol. 2012;302:F1503–1515. doi: 10.1152/ajprenal.00382.2011. [DOI] [PubMed] [Google Scholar]
- 37.Richard EM, Thiyagarajan T, Bunni MA, et al. Reducing FLI1 levels in the MRL/lpr lupus mouse model impacts T cell function by modulating glycosphingolipid metabolism. PLOS ONE. 2013;8:e75175. doi: 10.1371/journal.pone.0075175. doi: 10.1371/journal.pone.0075175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–896. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]


