Abstract
Dermatomyositis (DM) and polymyositis (PM) are collectively termed autoimmune myopathy. To investigate the difference between muscle- and skin-infiltrating T cells and to address their role for myopathy, we characterized T cells that were directly expanded from the tissues. Enrolled into this study were 25 patients with DM and three patients with PM. Muscle and skin biopsied specimens were immersed in cRPMI medium supplemented with interleukin (IL)-2 and anti-CD3/CD28 antibody-conjugated microbeads. The expanded cells were subjected to flow cytometry to examine their phenotypes. We analysed the cytokine concentration in the culture supernatants from the expanded T cells and the frequencies of cytokine-bearing cells by intracellular staining. There was non-biased in-vitro expansion of tissue-infiltrating CD4+ and CD8+ T cells from the muscle and skin specimens. The majority of expanded T cells were chemokine receptor (CCR) type 7–CD45RO+ effecter memory cells with various T cell receptor (TCR) Vβs. The skin-derived but not muscle-derived T cells expressed cutaneous lymphocyte antigen (CLA) and CCR10 and secreted large amounts of IL-17A, suggesting that T helper type 17 (Th17) cells may have a crucial role in the development of skin lesions. Notably, the frequency of IL-4-producing chemokine (C-X-C motif) receptor (CXCR)4+ Th2 cells was significantly higher in the muscle-derived cells and correlated inversely with the serum creatine phosphokinase (CPK) and lactate dehydrogenase (LDH) levels. stromal-derived factor (SDF)-1/CXCL12, a ligand for CXCR4, was expressed at a high level in the vascular endothelial cells between muscular fasciculi. Our study suggests that T cell populations in the muscle and skin are different, and the Th2 cell infiltrate in the muscle is associated with the low severity of myositis in DM.
Keywords: CXCR4, dermatomyositis, T cells, IL-4
Introduction
Dermatomyositis (DM) and polymyositis (PM) are two clinical phenotypes of autoimmune myopathy, and share clinical features of systemic proximal muscle weakness associated with muscle cell destruction. In addition to the muscle symptoms, DM presents with unique skin manifestations, including violaceous oedematous erythema on the upper eyelids (heliotrope rash) and hyperkeratotic erythemas that emerge symmetrically on dorsal aspects of joints (Gottron's sign) 1. In DM and PM, impaired immunological tolerance for self-antigens causes inflammatory responses against host muscle tissues.
Previous studies have shown that the pathogenesis of DM is different from that of PM in the muscle pathophysiology 2. In the muscle lesions, the perivascular infiltrate is composed mainly of CD4+ T cells as well as B cells and macrophages in DM, whereas CD8+ T cells and macrophages infiltrate predominantly in PM 3. In the skin lesions of DM, the vast majority of infiltrating cells are mature CD4+ T cells producing interleukin (IL)-2, interferon (IFN)-γ and/or IL-4 4. These findings suggest that CD4+ T helper (Th) cells are involved in the muscle and skin lesions of DM, and both Th1 and Th2 cells infiltrate at least into the skin lesions. However, the infiltrating T cells have not been characterized fully, and their subpopulations remain to be clarified. Most of the previous studies on T cells in DM and PM have been performed in peripheral blood mononuclear cells (PBMCs) because of the availability from the patients. Even when the muscle and skin tissues were used as experimental samples, T cell subsets were determined by immunohistochemistry or gene expression, and direct assessment of T cells by isolation and/or cultivation has not been performed.
To further characterize T cells in the muscle and skin lesions of DM and PM, we expanded skin- and muscle-infiltrating T cells using our established procedure. Our method enables us to expand tissue-infiltrating T cells proportionally to the original populations, and the expanded T cells are relevant to the pathogenic cells in the tissue 5. Results suggest that T cell subpopulations in the muscle and skin are different, and the muscle infiltrate of IL-4-producing CXCR4+ T cells is associated with the low severity of myositis in DM.
Materials and methods
Patients
Enrolled into this study were 25 patients with DM and three patients with PM (Table 1). Twenty-six of these patients fulfilled Bohan and Peter's criteria of definite DM or PM 1,6. Two patients (cases 8 and 22) were diagnosed as probable DM, because they were diagnosed clinically as having amyopathic DM, which showed typical skin manifestations and interstitial lung disease without histological myositis. The study was performed according to the Declaration of Helsinki, and the study protocol was approved by the ethical committee of Hamamatsu University School of Medicine. Written informed consent was obtained from all participants.
Table 1.
Patient profile
| No. | Age (years) | Sex | Disease duration (months) | Samples | CPK(U/l) | LDH(U/l) | Diagnosis | Interstitial lung disease | Internal malignancy | ANA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | F | 3 | Skin/muscle | 644 | 559 | Definite DM | + | 1280 | |
| 2 | 75 | F | 2 | Skin/muscle | 200 | 413 | Definite DM | + | Colon | 40 |
| 3 | 75 | M | 2 | Skin/muscle | 830 | 467 | Definite DM | + | 80 | |
| 4 | 58 | M | 2 | Skin/muscle | 2448 | 647 | Definite DM | + | 80 | |
| 5 | 57 | M | 3 | Skin/muscle | 941 | 795 | Definite DM | − | 80 | |
| 6 | 51 | F | 1 | Skin/muscle | 774 | 368 | Definite DM | − | Uterus | 40 |
| 7 | 41 | F | 1 | Skin/muscle | 2063 | 764 | Definite DM | + | 5120 | |
| 8 | 46 | F | 11 | Skin/muscle | 32 | 302 | Probable DM | + | 40 | |
| 9 | 33 | F | 2 | Skin/muscle | 4988 | 553 | Definite DM | − | 40 | |
| 10 | 77 | F | 3 | Skin/muscle | 434 | 416 | Definite DM | − | Lung | 160 |
| 11 | 65 | M | 5 | Skin/muscle | 1415 | 398 | Definite DM | + | − | |
| 12 | 32 | M | 1 | Skin/muscle | 908 | 435 | Definite DM | + | − | |
| 13 | 37 | F | 9 | Muscle | 785 | 318 | Definite PM | + | 80 | |
| 14 | 53 | F | 5 | Muscle | 274 | 233 | Definite DM | + | 40 | |
| 15 | 23 | M | 1 | Muscle | 4387 | 739 | Definite DM | − | − | |
| 16 | 43 | M | 5 | Muscle | 4957 | 916 | Definite DM | + | 40 | |
| 17 | 51 | F | 10 | Muscle | 3923 | 628 | Definite DM | − | 640 | |
| 18 | 81 | M | 1 | Muscle | 1532 | 532 | Definite DM | − | Oesophagus | 160 |
| 19 | 44 | F | 2 | Muscle | 226 | 274 | Definite DM | + | 40 | |
| 20 | 39 | F | 2 | Muscle | 50 | 172 | Definite PM | − | 40 | |
| 21 | 46 | F | > 20 | Muscle | 386 | 331 | Definite PM | + | − | |
| 22 | 63 | F | 3 | Skin | 64 | 246 | Probable DM | + | 40 | |
| 23 | 50 | F | 6 | Skin | 855 | 456 | Definite DM | + | 320 | |
| 24 | 30 | M | 5 | Skin | 82 | 418 | Definite DM | + | − | |
| 25 | 45 | F | 1 | Skin | 206 | 298 | Definite DM | + | − | |
| 26 | 56 | F | 12 | Skin | 384 | 285 | Definite DM | + | − | |
| 27 | 55 | F | 3 | Skin | 4732 | 800 | Definite DM | + | 640 | |
| 28 | 74 | F | 4 | Skin | 1432 | 437 | Definite DM | − | − |
ANA = anti-nuclear antibody; CPK = creatine phosphokinase (normal values <204 U/l); DM = dermatomyositis; PM = polymyositis; F = female; M = male; LDH, lactate dehydrogenase (normal value < 208 U/l).
Reagents, antibodies and culture medium
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or peridinin chlorophyll (PerCP)-conjugated monoclonal antibodies (mAbs) against CD3, CD4, CD8, CD45RO, cutaneous lymphocyte antigen (CLA) and human leucocyte antigen D-related (HLA-DR) were purchased from BD Pharmingen (San Diego, CA, USA). A series of antibodies against 24 T cell receptor β chain variable region (TCR-Vβ) gene products (IOtest Beta Mark, TCR-Vβ repertoire kit) and FITC-conjugated TCR-Vβ2, pan-TCRγδ mAb were purchased from Beckman Coulter (Marseille, France). Chemokine receptor mAbs for CCR1–CCR10 and CXCR1–CXCR6 were obtained from R&D Systems (Minneapolis, MN, USA). FITC- or PE-conjugated mAbs against IL-4, IL-5, IL-13 and IFN-γ (BD Pharmingen), IL-17, forkhead box protein 3 (FoxP3) (eBioscience, San Diego, CA, USA) were purchased. Cells were cultured in RPMI-1640 medium (Life Technologies, Carlsbad, CA, USA) supplemented with L-glutamine, sodium pyruvate, 2-mercaptoethanol, non-essential amino acids (Life Technologies) and 10% heat-inactivated fetal calf serum or pooled human antibody serum (cRPMI), as described previously 7. Polyclonal antibody for stromal-derived factor (SDF)-1/CXCL12 was purchased from Santa Cruz Biotech (Dallas, TX, USA).
Cell preparations
A 50–100 mm3 muscle specimen biopsied from femoral quadriceps and a 4-mm skin specimen from skin lesions of individual patients were cut into two pieces for expansion of infiltrating T cells and histological investigation. No immunosuppressive agents including corticosteroids were administered prior to the biopsy. For expansion of T cells, samples were immersed in cRPMI supplemented with 50 U/ml human recombinant IL-2 (R&D Systems) and anti-CD3/CD28 antibody-conjugated microbeads (T-cell Expander; Dynal, Copenhagen, Denmark), as reported previously 5. We obtained >107 cells/specimen by this method.
Flow cytometric analysis (FCM) and cytokine production assessments
Aliquots of 106 cells were washed once with phosphate-buffered saline (PBS, pH 7·4) containing 1% bovine serum albumin (BSA) and 0·1% NaN3, and incubated with a panel of fluorescence-conjugated mAbs for 30 min at 4°C in the dark. After washing, the harvested cells were resuspended in PBS and subjected to FCM. More than 5 × 104 cells per sample were analysed on a fluorescence activated cell sorter (FACS)caliber flow cytometer or FACSCanto2 (BD Pharmingen) by gating lymphocytes. Results were analysed using a FlowJo software (TreeStar, Ashland, OR, USA).
For the cytokine production assay, the cells (2 × 105/well) were stimulated with immobilized anti-CD3 mAb-coated 96-well plates (BD Biocoat; BD Pharmingen) for 48 h, and the culture supernatants were harvested to measure cytokine levels [IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ and tumour necrosis factor (TNF)-α] with the human Th1/Th2/Th17 Cytokine Beads Array kit (CBA; BD Pharmingen), according to the manufacturer's protocols.
Alternatively, we performed intracellular cytokine staining. The cells were incubated in cRPMI containing 10−8 M phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St Louis, MO, USA), 10−6 M calcium ionophore (Sigma-Aldrich) and 1 μl/ml of BerGolgiStop™ (BD Pharmingen) for 6 h at 37°C. The cells were harvested and stained with fluorescent-tagged mAbs against cytokines using the Cytofix/Cytoperm Plus Kit with GolgiStop (BD Pharmingen), according to the manufacturer's protocols, followed by staining of fluorescence-tagged antibodies against CD4 and CD8.
Histopathological and immunohistochemical studies
Biopsy specimens from the lesions were fixed in 4% formalin and stained routinely with haematoxylin and eosin (H&E) for standard histopathology. Deparaffinized specimens were autoclaved in 10 mM citrate buffer (pH 6·0) for 10 min at 120°C to retrieve the antigenic epitopes and were then processed for CD4, CD8, CD3 and stromal cell-derived factor (SDF)-1 expression analysis by the avidin–biotin complex method. Nuclear staining was performed with haematoxylin. The sections were scanned by a digital image scanner, NanoZoomer (Hamamatsu Photonics, Hamamatsu, Japan), and were analysed.
Statistical analyses
For non-parametric analysis, Wilcoxon's signed-rank test and the Mann–Whitney U-test were used for matched-pairs analysis and non-matched-pairs analysis, respectively. Student's t-test was used for parametric analysis. Non-parametric correlation coefficients (Rs) were calculated using Spearman's procedure. P < 0·05 was considered statistically significant.
Results
Non-biased in-vitro expansion of tissue-infiltrating CD4+ and CD8+ T cells from muscle and skin specimens
To obtain large numbers of the tissue-infiltrating T cells, we expanded cells that migrated from the specimens to the culture medium with anti-CD3/CD28 antibodies and IL-2 for 14 days, as described previously 5. This method constantly allowed us to obtain more than 107 cells without a substantial phenotypical alternation. Using FCM, we found that both CD4+ and CD8+ T cells were expanded from the patients' muscle and skin samples, as shown in representative data (Fig. 1a). There were high variations in the CD4/CD8 ratios of both muscle- and skin-derived cells among the patients (Fig. 1b). To assess whether these cells propagated proportionally and reflected the original populations of the muscle- and skin-infiltrating T cells, we compared the CD4 : CD8 ratio of the expanded T cells (FCM analysis) with that of the tissue-infiltrating T cells (immunohistochemical analysis). Immunostaining exhibited the infiltration of CD3+, CD4+ and CD8+ T cells in the lesional muscle (Fig. 1c) and skin (Fig. 1d) of DM. As a considerable number of CD4+ dendritic cells reside in the muscle and skin lesions, we enumerated CD3+ and CD8+ cells and estimated the CD4+ T cell number by subtracting the numbers of CD8+ cells from those of CD3+ cells. There was a close correlation of the CD4 : CD8 ratio between the expanded T cells and the original infiltrate in both skin (Fig. 1e) and muscle lesions (Fig. 1f). These results suggest that the expanded T cells reflect the original tissue-infiltrating T cells.
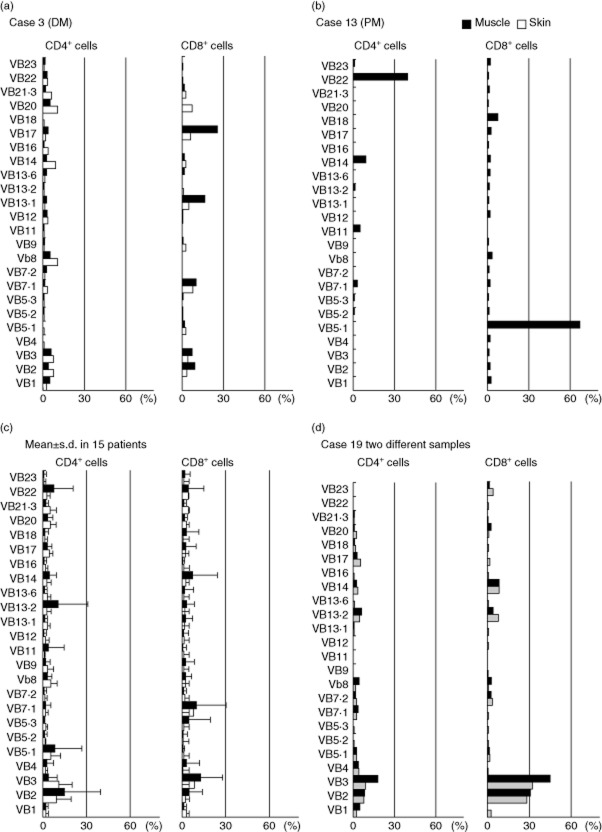
Fig. 1.
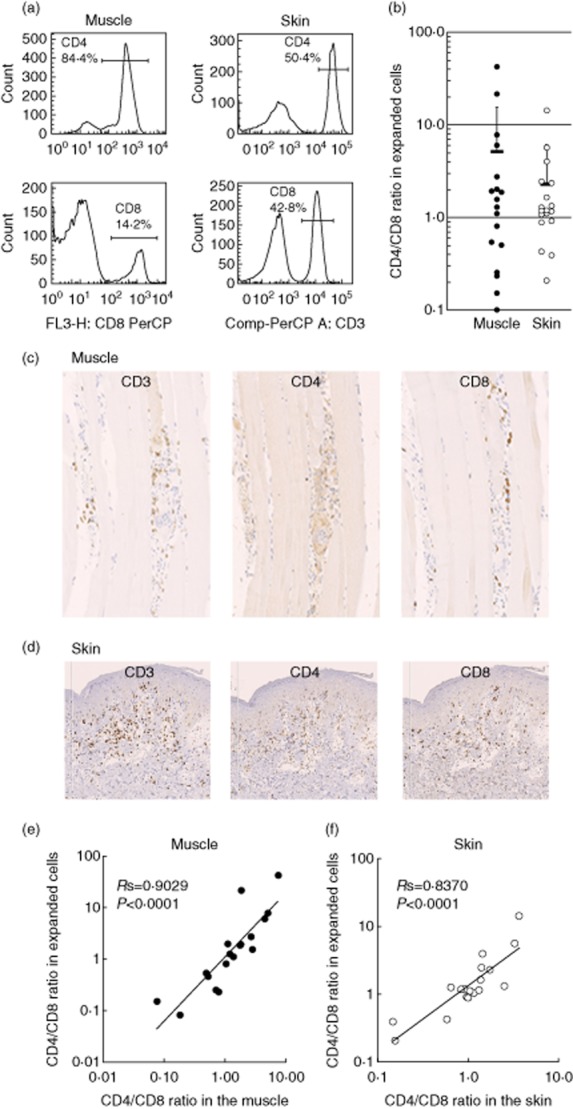
CD4/CD8 ratios in muscle- and skin-derived T cells in dermatomyositis (DM) patients. (a) Flow cytometric analysis (FCM) of expanded T cells. The numbers represent CD4+ or CD8+ cells. (b) The CD4 : CD8 ratio of expanded T cells from muscle lesions (closed circles, n = 18) and skin lesions (open circles, n = 18) of DM patients. Horizontal bars indicate mean ± standard deviation (s.d.). (c,d) Immunohistochemical staining of muscle and skin lesions with monoclonal antibodies against CD3, CD4 and CD8. Original magnification ×200. (e) Relationships between the CD4 : CD8 ratio of the original tissue-infiltrating T cells and that of expanded T cells from muscle lesions (closed circles) and skin lesions (open circles).
TCR Vβ usage of muscle- and skin-derived T cells
The TCR Vβ repertoire of the expanded T cells was examined using a panel of antibodies against various Vβ chains. We found deviated TCR Vβ usage in muscle- and skin-infiltrating T cells in all the patients examined. As represented by case 3 (Fig. 2a), Vβs of the preferentially expanded T cells were different between the muscle- and skin-derived T cells even in the same patients, suggesting that T cells reacted differentially to antigens in each of the tissues. In the three PM patients, as represented by case 13 (Fig. 2b), the skewed CD4+ and CD8+ T cell Vβ usage in the muscle lesions was more marked than that of DM patients. We analysed the mean ± standard deviation (s.d.) of the frequencies of T cells bearing individual Vβs in 15 DM/PM patients and found no common Vβ in the expanded T cells among the patients (Fig. 2c), suggesting a different T cell repertoire in each patient. To verify reflection of the original tissue-infiltrating T cells, we took two muscle biopsy samples from one patient (case 19) and obtained expanded T cells. Virtually the same distribution of Vβ usage was observed in the two samples (Fig. 2d), again validating the non-biased expansion during culture.
Fig. 2.
T cell receptor (TCR) Vβ usage of muscle (closed)- and skin-infiltrating (open) T cells in dermatomyositis/polymyositis (DM/PM) patients. The numbers indicate the percentage of T cells bearing each TCR Vβ in total T cells (CD4+ and CD8+ T cells). (a) Representative data of DM patient (case 3). (b) Representative data of PM patient (case 13). (c) The mean frequencies ± standard deviation (s.d.) of T cells bearing each TCR Vβ in 15 patients. (d) TCR Vβ usage of two muscle samples (black and grey) in case 19.
Chemokine receptor expressions of muscle- and skin-derived T cells
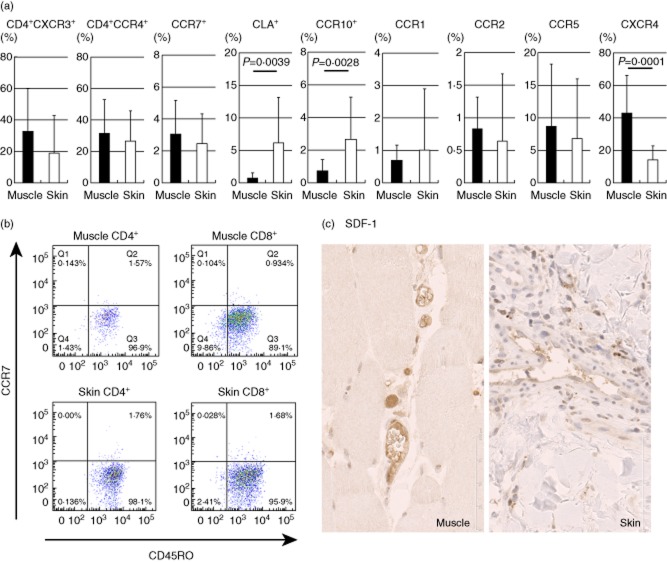
The chemokine receptor expression was investigated in the expanded T cells from muscle and skin samples. No significant differences were observed between the muscle- and skin-derived T cells in the frequency of CD4+CXCR3+ (mostly Th1) or CD4+CCR4+ (mostly Th2) cells (Fig. 3a). The majority of expanded T cells did not express CCR7 (Fig. 3a) but bore CD45RO in both tissues (Fig. 3b), indicating their effector memory phenotype. Notably, the frequencies of the CLA+ and CCR10+ cells were significantly higher in the skin-derived T cells than in the muscle-derived cells, indicating the functional relevance of these skin-homing receptors 8,9. It has been reported that chemokines CCL2, CCL3, CCL4 and CCL5 are produced in vessels of autoimmune myopathy and their receptors, CCR1, CCR2 and CCR5, are expressed in muscle-infiltrating mononuclear cells 10. In our study, however, the frequencies of cells expressing these chemokine receptors were not different between the muscle- and skin-derived cells (Fig. 3a). Of particular interest is the observation that the frequency of CXCR4+ T cells was significantly higher in the muscle-derived cells than in the skin-derived cells (Fig. 3a). It has been shown that SDF-1/CXCL12, a ligand for CXCR4, is expressed in vascular endothelial cells of the patients' muscle of DM and PM 11. In our study of five samples, SDF-1 expression was also observed at a high level in the vascular endothelial cells located between muscular fasciculi and at a moderate level in the dermal vessel epithelial cells (Fig. 3c).
Fig. 3.
Chemokine receptor expressions of T cells expanded from muscle lesions (closed) and skin lesions (open) in dermatomyositis (DM) and polymyositis (PM) patients (n = 24). (a) Numbers indicate mean percentages of total T cells. Mean values ± standard deviation (s.d.) are indicated. (b) Representative fluorescence activated cell sorter (FACS) dot-plots of CD45RO and CCR7. (c) Immunohistochemistry of muscle and skin lesions with antibodies against stromal-derived factor (SDF)-1, original magnification ×400.
Cytokine production patterns of muscle- and skin-derived T cells and their association with laboratory markers
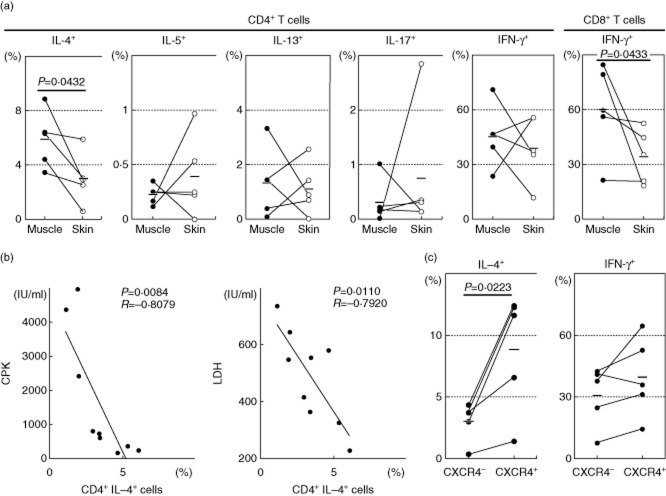
Expanded T cells (2 × 105/well) were stimulated with immobilized anti-CD3 antibody for 48 h, and the concentration values of various cytokines in the culture supernatants were measured. High amounts of IL-4 were found in the supernatants of both skin- and muscle-derived T cells (Fig. 4a). When the IL-4 levels in the muscle and skin T cells were analysed in the individual patients, IL-4 was produced at significantly higher levels by the muscle T cells than by the skin T cells (Fig. 4b). There was no significant difference in IFN-γ (Fig. 4c). Notably, the skin-derived T cells produced significantly higher amounts of IL-17A than did the muscle-derived T cells (Fig. 4a). In three DM patients with severe skin manifestations (cases 22, 23 and 27) the skin-derived T cells secreted large amounts of IL-17A (2751, 21 980 and 5147 pg/ml, respectively), suggesting that IL-17A may have a crucial role in the development of skin lesions of DM.
Fig. 4.
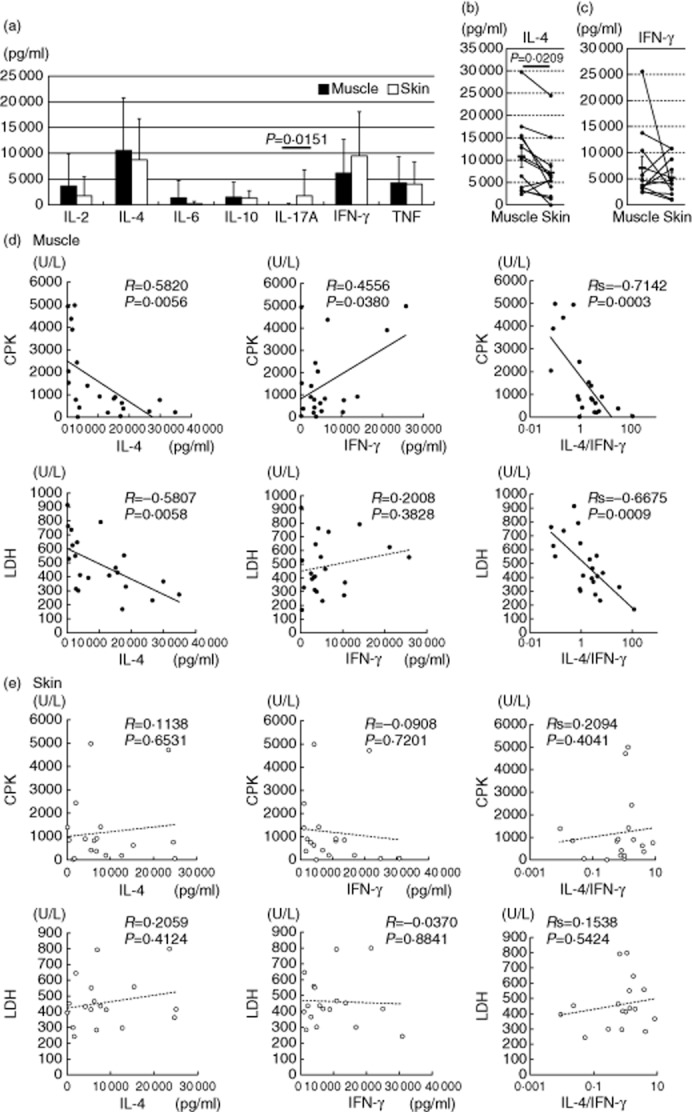
Cytokine production patterns of the tissue-infiltrating T cells. (a) Cytokine concentrations in the culture supernatants from anti-CD3 antibody-stimulated expanded T cells (n = 28). Mean values ± standard deviation (s.d.) are indicated. (b,c) Interleukin (IL)-4 and interferon (IFN)-γ concentrations in the supernatants from the stimulated muscle (closed)- and skin (open)-derived T cells in the same patients. (d,e) Correlation between cytokine levels and clinical parameters in the muscle- and skin-derived T cells.
We analysed the relationship between the levels of produced IL-4 and IFN-γ and serum creatine phosphokinase (CPK) and lactate dehydrogenase (LDH) levels, which are clinical parameters of muscle damage 1. The levels of IL-4 production by muscle-derived T cells correlated inversely with the serum CPK and LDH levels (Fig. 4d). Their IFN-γ production correlated weakly with the CPK levels, but not with the LDH levels. There were highly significant correlations between the IL-4 : IFN-γ ratio and CPK or LDH. The skin-derived T cells did not show such a correlation with CPK or LDH (Fig. 4e). Although the serum LDH level can be influenced by other organ involvements such as interstitial lung disease (ILD), the inverse correlation between the IL-4 : IFN-γ ratio and the LDH level remained even after exclusion of patients with ILD (n = 8, Rs2 = 0·580, P = 0·028). These findings suggest that IL-4 and IFN-γ have opposite roles in the muscle damage, and IL-4 is associated with the low severity of myositis.
Intracellular cytokine staining of muscle- and skin-derived T cells
Intracytoplasmic cytokine staining of the expanded T cells was performed after stimulation with PMA and calcium ionophore, and cells were analysed by FCM. In the individual patients, IL-4-producing T cells were found at significantly higher frequencies in the muscle-derived T cells than in the skin-derived T cells (Fig. 5a), confirming the culture supernatant data. Only low percentages of CD4+ T cells produced IL-5 and IL-13. One patient showed a high frequency of IL-17-producing CD4+ T cells. There was a tendency that the percentage of IFN-γ-producing CD8+ T cells was increased in the muscle T cells compared to the skin T cells. Again, the percentages of IL-4+CD4+ cells correlated inversely with the serum CPK and LDH levels (Fig. 5b), while there was no significant correlation between the percentages of IFN-γ-producing cells and the serum levels of CPK or LDH (data not shown).
Fig. 5.
Intracellular cytokine expressions of muscle- and skin-infiltrating T cells in the patients (n = 5). (a) The percentage of cytokine-producing T cells as assessed by intracellular cytokine staining and flow cytometric analysis (FCM). Horizontal bars indicate mean values. (b) Correlations between the percentage of interleukin (IL)-4-producing CD4+ T cells and clinical parameters (n = 9). (c) IL-4 and interferon (IFN)-γ expression by chemokine (C-X-C motif) receptor (CXCR)4-positive or negative CD4+ T cells (n = 5).
As the muscle-derived T cells expressed CXCR4 at high frequencies, we examined the cytokine profile of CD4+CXCR4+ cells. The frequencies of IL-4-producing CD4+ T cells were higher in CXCR4+ cells than in CXCR4− cells (Fig. 5c), suggesting that CXCR4+ T cells are Th2 cells.
Discussion
To characterize T cells infiltrating into the lesional muscle and skin of DM and PM, we used a T cell expansion method from tissue-infiltrating T cells with immobilized anti-CD3/CD28 antibodies and 14-day cultivation with IL-2 5. In a comparison between the expanded T cells and the original tissue-infiltrating T cells, we observed expansion of CCR7–CD45RO+ effector memory T cells without biased propagation of CD4+ and CD8+ T cells. We focused upon TCR Vβ usage, chemokine receptor expression and cytokine production in a comparison between the muscle- and skin-derived T cells. We found skewed TCR Vβ usage of both the muscle- and skin-derived T cells in all the patients examined. However, the muscle lesions appear to have more marked Vβ preponderance than the skin lesions, as represented by case 13 (see Fig. 2b). No common Vβ was found in the expanded T cells among the patients (Fig. 2c), suggesting a different T cell repertoire in each patient. This suggests that T cells react differentially to antigens specific to each of the tissues, presumably in the context of major histocompatibility complex (MHC).
It is reasonable to assume that the skin-derived T cells expressed skin-homing receptors, CLA and CCR10 8,9, at significantly higher frequencies than the muscle-derived cells. In contrast, the frequency of CXCR4+ T cells was significantly higher in the muscle-derived cells than in the skin-derived cells. As reported previously 11, we observed that SDF-1, a ligand for CXCR4, was expressed at a high level in the vascular endothelial cells located between muscular fasciculi. Preferential infiltration of CXCR4+ T cells may be attributable to SDF-1 produced by the endothelial cells.
By measuring the culture supernatant cytokines and the intracellular cytokine-bearing T cell frequencies, we found that the expanded T cells were comprised predominantly of IFN-γ-producing Th1 and IL-4-producing Th2 cells. In all the patients with DM, the frequencies of IL-4+ T cells were lower than those of IFN-γ+ cells in the lesional muscle. We observed a strong inverse correlation between IL-4 production by muscle-infiltrating T cells and the CPK- and LDH-assessed muscle damage and a weak positive correlation between IFN-γ production and muscle damage. Thus, the compartmental IL-4/IFN-γ cytokine balance was associated with the severity of muscle injury. Conversely, the skin-derived T cells contained a substantial number of IL-17A-producing Th17 cells in some of the DM patients, which are well known as pathogenic T cells for psoriasis 12,13. Clinically, DM shares hyperkeratotic erythema with psoriasis 14. Th17-producing cytokines, IL-17A and IL-22, may also contribute to the skin lesions of DM.
Histopathological studies of the muscle lesions demonstrated that CD4+ T cells, B cells and macrophages predominantly infiltrated around endomysium capillaries with deposition of activated C5b-9 membrane attack complex 15. A surge of circulating B cells and Th2 cells was observed frequently during the active phase of DM 3,16. In contrast, a high number of CD8+ T cells infiltrated into muscle fibres in PM, suggesting a significant contribution of autoreactive cytotoxic CD8+ T cells to the pathogenesis of PM 17,18. Although DM and PM share major clinical features, the type-2 and type-1 responses may be predominant in the development of muscle lesions of DM and PM, respectively. In certain cases of juvenile and adult DM, however, excessive tissue infiltrates of CD8+ T cells were observed 4,19. Meanwhile, recent gene expression analyses demonstrated a strong linkage to an IFN signature in the target tissues of DM 20–22, and type-I IFN-producing plasmacytoid dendritic cells markedly infiltrated the muscle of DM patients 23. These findings indicate that both type 1 and type 2 IFN and Th2 cytokines are expressed in DM. In the muscle lesions of DM, it is possible that the infiltrating Th2 cells serve as regulators against IFN-induced inflammation. Alternatively, as IL-4 is a critical factor in muscle growth by maturating myotubules and a recruitment factor of muscle stem cells 24,25, IL-4-producing Th2 cells contribute to the muscle regeneration of DM.
In the muscle-derived CD4+ T cells, the frequencies of IL-4-producing cells were higher in CXCR4+ cells than in CXCR4− cells. It is therefore considered that the IL-4-producing Th2 cells preferentially express CXCR4 and can be recruited to the muscle by virtue of the tissue-producing SDF-1. The possible ameliorating or protective role of IL-4 and the CXCR4-SDF-1 engagement for T cell chemotaxis may provide a new perspective for therapeutic approaches in this intractable disease.
Acknowledgments
This work was supported by Grants-in Aid for Science Research from the Ministry of Health, Labour, and Welfare of Japan and the Ministry of Education, Science, Sports, and Culture of Japan.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 3.Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- 4.Caproni M, Torchia D, Cardinali C, et al. Infiltrating cells, related cytokines and chemokine receptors in lesional skin of patients with dermatomyositis. Br J Dermatol. 2004;151:784–791. doi: 10.1111/j.1365-2133.2004.06144.x. [DOI] [PubMed] [Google Scholar]
- 5.Hashizume H, Hansen A, Poulsen LK, Thomsen AR, Takigawa M, Thestrup-Pedersen K. In vitro propagation and dynamics of T cells from skin biopsies by methods using interleukins-2 and -4 or anti-CD3/CD28 antibody-coated microbeads. Acta Derm Venereol. 2010;90:468–473. doi: 10.2340/00015555-0927. [DOI] [PubMed] [Google Scholar]
- 6.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 7.Hashizume H, Takigawa M, Tokura Y. Characterization of drug-specific T cells in phenobarbital-induced eruption. J Immunol. 2002;168:5359–5368. doi: 10.4049/jimmunol.168.10.5359. [DOI] [PubMed] [Google Scholar]
- 8.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santamaria-Babi LF. CLA(+) T cells in cutaneous diseases. Eur J Dermatol. 2004;14:13–18. [PubMed] [Google Scholar]
- 10.Civatte M, Bartoli C, Schleinitz N, Chetaille B, Pellissier JF, Figarella-Branger D. Expression of the beta chemokines CCL3, CCL4, CCL5 and their receptors in idiopathic inflammatory myopathies. Neuropathol Appl Neurobiol. 2005;31:70–79. doi: 10.1111/j.1365-2990.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 11.De Paepe B, Schroder JM, Martin JJ, Racz GZ, De Bleecker JL. Localization of the alpha-chemokine SDF-1 and its receptor CXCR4 in idiopathic inflammatory myopathies. Neuromuscul Disord. 2004;14:265–273. doi: 10.1016/j.nmd.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Krueger JG, Fretzin S, Suarez-Farinas M, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130:145–54 e9. doi: 10.1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 14.Haroon M, Devlin J. Gottrons's papule in amyopathic dermatomyositis mimicking psoriasis. Clin Rheumatol. 2009;28:1245–1246. doi: 10.1007/s10067-009-1244-6. [DOI] [PubMed] [Google Scholar]
- 15.Kissel JT, Halterman RK, Rammohan KW, Mendell JR. The relationship of complement-mediated microvasculopathy to the histologic features and clinical duration of disease in dermatomyositis. Arch Neurol. 1991;48:26–30. doi: 10.1001/archneur.1991.00530130034016. [DOI] [PubMed] [Google Scholar]
- 16.Ishii W, Matsuda M, Shimojima Y, Itoh S, Sumida T, Ikeda S. Flow cytometric analysis of lymphocyte subpopulations and TH1/TH2 balance in patients with polymyositis and dermatomyositis. Intern Med. 2008;47:1593–1599. doi: 10.2169/internalmedicine.47.0967. [DOI] [PubMed] [Google Scholar]
- 17.Benveniste O, Herson S, Salomon B, et al. Long-term persistence of clonally expanded T cells in patients with polymyositis. Ann Neurol. 2004;56:867–872. doi: 10.1002/ana.20293. [DOI] [PubMed] [Google Scholar]
- 18.Nishio J, Suzuki M, Miyasaka N, Kohsaka H. Clonal biases of peripheral CD8 T cell repertoire directly reflect local inflammation in polymyositis. J Immunol. 2001;167:4051–4058. doi: 10.4049/jimmunol.167.7.4051. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno K, Yachie A, Nagaoki S, et al. Oligoclonal expansion of circulating and tissue-infiltrating CD8+ T cells with killer/effector phenotypes in juvenile dermatomyositis syndrome. Clin Exp Immunol. 2004;137:187–194. doi: 10.1111/j.1365-2249.2004.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilgic H, Ytterberg SR, Amin S, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60:3436–3446. doi: 10.1002/art.24936. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg SA. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep. 2010;12:198–203. doi: 10.1007/s11926-010-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong D, Kea B, Pesich R, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLOS ONE. 2012;7:e29161. doi: 10.1371/journal.pone.0029161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez de Padilla CM, Vallejo AN, McNallan KT, et al. Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis Rheum. 2007;56:1658–1668. doi: 10.1002/art.22558. [DOI] [PubMed] [Google Scholar]
- 24.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 25.Lafreniere JF, Mills P, Bouchentouf M, Tremblay JP. Interleukin-4 improves the migration of human myogenic precursor cells in vitro and in vivo. Exp Cell Res. 2006;312:1127–1141. doi: 10.1016/j.yexcr.2006.01.002. [DOI] [PubMed] [Google Scholar]