Abstract
Type 1 diabetes (T1DM) is a T cell-mediated autoimmune disease that selectively destroys pancreatic β cells. The only possible cure for T1DM is to control autoimmunity against β cell-specific antigens. We explored whether the natural compound curcumin, with anti-oxidant and anti-inflammatory activities, might down-regulate the T cell response that destroys pancreatic β cells to improve disease outcome in autoimmune diabetes. We employed two accelerated autoimmune diabetes models: (i) cyclophosphamide (CYP) administration to non-obese diabetic (NOD) mice and (ii) adoptive transfer of diabetogenic splenocytes into NODscid mice. Curcumin treatment led to significant delay of disease onset, and in some instances prevented autoimmune diabetes by inhibiting pancreatic leucocyte infiltration and preserving insulin-expressing cells. To investigate the mechanisms of protection we studied the effect of curcumin on key immune cell populations involved in the pathogenesis of the disease. Curcumin modulates the T lymphocyte response impairing proliferation and interferon (IFN)-γ production through modulation of T-box expressed in T cells (T-bet), a key transcription factor for proinflammatory T helper type 1 (Th1) lymphocyte differentiation, both at the transcriptional and translational levels. Also, curcumin reduces nuclear factor (NF)-κB activation in T cell receptor (TCR)-stimulated NOD lymphocytes. In addition, curcumin impairs the T cell stimulatory function of dendritic cells with reduced secretion of proinflammatory cytokines and nitric oxide (NO) and low surface expression of co-stimulatory molecules, leading to an overall diminished antigen-presenting cell activity. These in-vitro effects correlated with ex-vivo analysis of cells obtained from curcumin-treated mice during the course of autoimmune diabetes. These findings reveal an effective therapeutic effect of curcumin in autoimmune diabetes by its actions on key immune cells responsible for β cell death.
Keywords: dendritic cells, inflammation, NOD mouse, T-bet, T lymphocytes
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease that arises from the selective and progressive loss of insulin-producing β cells by means of self-reactive T lymphocytes 1,2. Treatment with insulin remains the most suitable therapy for T1DM patients. However, in many patients tight glycaemic control is difficult to achieve, leading to long-term vascular damage associated with kidney failure, heart disease, retinopathy and neuropathy 3. Clinical manifestations of T1DM are evident only when more than 80% of the β cell mass has been destroyed 4. It is possible to predict, with a certain degree of accuracy, those candidates who will progress to T1DM long before the appearance of clinical manifestations, and prediction can be determined by measuring serum levels of autoantibodies in the relatives of T1DM patients 5. Thus, early therapeutic interventions would be beneficial to prevent T1DM. Considering the inflammatory nature of T1DM, it is plausible to speculate that treatment with anti-inflammatory agents/drugs would be beneficial. Curcumin, a polyphenolic compound extracted from the rhizome of the spice plant Curcuma longa, has been used extensively for treatment of a wide spectrum of health problems. Curcumin possesses anti-depressant, anti-oxidative, anti-inflammatory and neuroprotective actions and acts through several intracellular mechanisms affecting multiple targets. Curcumin has been proved to be effective for the treatment of different forms of cancer, allergic reactions, asthma, Alzheimer's disease and pathological disorders in which aberrant self-reactivity takes place, such as inflammatory bowel disease, rheumatoid arthritis, experimental autoimmune encephalomyelitis and psoriasis 6–10. The biosafety of curcumin has been proved exhaustively because of its use as a spice, colouring food agent and at higher doses in Indian Ayurvedic medicine 11. Also, curcumin inhibits the growth of tumour cells in vivo in athymic mice 12. The best-known mechanism is through its ability to modulate transcription factors such as nuclear factor (NF)-κB, activator protein 1 (AP-1), signal transducer and activator of transcription (STAT) and their downstream signalling pathways 13,14.
The hypothesis that administration of curcumin would ameliorate diabetes has been tested successfully in a murine model of insulin resistance 15 and in a clinical trial on T2DM prediabetic patients 16. Its efficacy and safety has been tested previously in clinical trials 17,18.
Curcumin has also been tested on a streptozotocin (STZ)-induced T1DM mouse model, resulting in prevention of islet damage along with an in-vitro protective effect on β cells when cultured in the presence of inflammatory cytokines 19,20. Despite the importance of these studies using STZ to induce β cell death chemically, it is still unknown whether curcumin might be effective to prevent and/or ameliorate autoimmunity in an animal model that resembles human disease more closely, particularly from an immunological standpoint. In this study, we report the therapeutic effect of curcumin and its putative mechanisms of action employing acute variants of the non-obese diabetic (NOD) mouse model 21.
Materials and methods
Animals
The NOD, NODscid and NOD.BDC2·5 transgenic T cell receptor (tgTCR) (BDC2·5) mice (Jackson Laboratory, Bar Harbor, ME, USA) were bred in a pathogen-free environment. BALB/c mice were purchased from the Facultad de Ciencias Exactas y Naturales (FCEyN), University of Buenos Aires, Animal Facility. Studies were approved by the Animal Research and Care Committee (CICUAL no. 0001) FCEyN.
Accelerated models of T1DM and curcumin treatment
Cyclophosphamide (CYP)-induced diabetes was performed by injecting twice 200 mg/kg body weight intraperitoneally (i.p.) 14 days apart in female NOD mice. Adoptive transfer of diabetes was performed in female NODscid mice, as described previously 22. Curcumin (95% spectrum) was injected daily i.p. for 7 days and then every other day until the end of the experiment. Diabetes was diagnosed when glycaemia reached ≥300 mg/dl in two consecutive readings (Optium Xceed®; Abbott Laboratories, North Chicago, IL, USA).
Histological examination
The pancreata were fixed in 10% formaldehyde and embedded in paraffin. Insulin immunolabelling was performed on 7 μm tissue sections with anti-human insulin monoclonal antibody (mAb) (BioGenex, Fremont, CA, USA). Sections were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin (Ig)G (Jackson ImmunoResearch, West Grove, PA, USA), peroxidase activity developed with 3,3-diaminobenzidine (Dako, Glostrup, Denmark), and then counterstained with haematoxylin. At least 10 islets from each animal were scored for insulitis according to the percentage of infiltration using the following criteria: 0, no insulitis; 1: <25%; 2: 25–50%; 3: 50–75%; and 4: >75%.
T lymphocyte proliferation and enzyme-linked immunosorbent assays (ELISAs)
Splenocytes were stained with carboxyfluorescein-diacetate succinimidyl ester (CFSE; Fluka-Sigma Aldrich, St Louis, MO, USA), cultured in RPMI 10% fetal bovine serum (FBS) and stimulated with phorbol myristate acetate (PMA)/ionomicyn (Sigma Aldrich) or the mimotope (M) (Ac-MVLPLWVRME-NH2), respectively. CD-4 T lymphocytes were stained for fluorescence activated cell sorter (FACS) analysis with biotinylated anti-CD4 (clone GK1·5) followed by streptavidin–allophycocyanin (eBiosciences, San Jose, CA, USA). Non-viable cells were excluded from analysis by 7-aminoactinomycin D (7-AAD) staining. ELISA kits (BD Pharmingen, San Jose, CA, USA) were used to quantify interferon (IFN)-γ and interleukin (IL)-4 in supernatants.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis
Splenocytes, 5 × 106 per well, were cultured in six-well plates, stimulated as described, and curcumin was added prior to cell lysis. For phospho-NF-κB detection, 2·5 × 105 splenocytes were cultured in 96-well plates, stimulated with anti-CD3ε (10 μg/ml; eBiosciences) and curcumin (10 μM) or vehicle for 15, 30, 120 and 240 min. Lysis was performed with 50 mM sodium phosphate/1% v/v SDS/40 mM 2-mercaptoethanol (2-ME)/2 mM ethylenediamine tetraacetic acid (EDTA) and loaded in 10% SDS-PAGE. Incubation with anti-T-bet antibody (Santa Cruz, Dallas, TX, USA), anti-phospho-NF-κB p65 (Ser536) antibody (Cell Signalling Technology Inc., Danvers, MA, USA) and HRP-conjugated anti-mouse or anti-rabbit (Bio-Rad, Hercules, CA, USA) were followed by enhanced chemiluminescence (ECL) (Pierce Biotechnology, Rockford, IL, USA) detection.
Quantitative RT–qPCR
Total RNA was isolated using TriReagent (Sigma Aldrich). Reverse transcription was performed using murine leukaemia virus reverse transcriptase (MMLTV-RT) (Promega, Madison, WI, USA) in the presence of RNAsin RNAse inhibitor (Promega) for 1 h at 37°C followed by inactivation at 95°C. The following primers were used: mouse forkhead box protein 3 (FoxP3), forward: 5′-CCCAGGAAAGACAGCAACCTT-3′ and reverse: 5′-TTCTCACAACCAGGCCACTTG-3′; mouse GATA-binding protein 3 (GATA-3): forward: 5′-CTACCGGGTTCGGATGTAAGTC-3′ and reverse: 5′-GTTCACACACTCCCTGCCTTCT-3′; mouse T-bet: forward: 5′-GCCAGGGAACCGCTTATATG-3′ and reverse: 5′-GACGATCATCTGGGTCACATTGT-3′.
Transient transfections and luciferase activity
EL4 T cells (murine T cell lymphoma) were transfected by electroporation 23,24. Luciferase activity was measured using the luciferase measure kit (Promega). Cells were co-transfected with respiratory syncytial virus (RSV)-β-galactosidase expression vector for normalization. The murine IFN-γ promoter coupled to the luciferase reporter vector (IFN-γ-Luc) was obtained as described by Liberman et al. 23. T-bet binding sites coupled to the luciferase reporter vector is described in Liberman et al. 24. The murine T-bet expression vector (pcDNA3-T-bet) is described in Liberman et al. 23,25.
In-vitro generation of dendritic cells (DC)
DC were generated from progenitor cells as described 26 and cultured in complete medium [RPMI-1640 from Invitrogen Life Technologies (Grand Island, NY, USA), 10% FCS, glutamine, non-essential amino acids, sodium pyruvate, HEPES, 2-ME and antibiotics] supplemented with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 (1000 U/ml of each). DC were pretreated for 2 h with either 20 μM curcumin or vehicle [dimethylsulphoxide (DMSO)] and then stimulated with 1 μg/ml lipopolysaccharide (LPS) (Sigma) and 50 ng/ml IFN-γ (R&D Systems, Minneapolis, MN, USA). After treatment, DC were analysed by FACS. IL-12p70, IL-6 and TNF-α were evaluated using ELISA kits (BD Pharmingen). Nitrite was measured as an indicator of nitric oxide (NO) production using Griess reagent (1% sulphanilamide and 0·1% naphthyl ethylene diamine dihydrochloride in 2·5% phosphoric acid) at 570 nm.
Flow cytometry and endocytosis assay
Cells were stained as described previously 26. DC were stained with biotinylated anti-CD11c (clone HL3), phycoerythrin-conjugated-anti-CD80 (clone 16-10A1), -anti-CD86 (clone GL-1), -anti-CD40 (clone 1C10) and -anti-major histocompatibility complex II (MHC-II) (clone M5/114·15·2) and streptavidin–allophycocyanin (eBiosciences). Isotype-matched mAb were used as negative controls. For endocytosis assay, DC (1 × 106 cells) were incubated at 37°C for 1 h with 10 mg/ml fluorescein isothiocynate (FITC)–dextran. Endocytosis was analysed by FACS.
Mixed lymphocyte reaction
Splenocyte (BALB/c) were enriched in T lymphocytes by nylon wool column. Cells were eluted with 10 ml of warm RPMI 10% FCS and further diluted at a concentration of 2 × 106 cells/ml in complete medium. NOD DC were co-cultured with the T lymphocytes at a ratio of 1:10, 1:20 and 1:40 and cultured for 72 h in complete RPMI and 1 μCi/well [3H]-TdR was added for the last 18 h. Cells were harvested and [3H]-TdR uptake was measured.
Crossed antigen presentation
BDC2·5 mice were treated daily either with 25 mg/kg curcumin or vehicle (DMSO) i.p. for 7 days. Splenocyte suspensions were enriched in T cell or antigen-presenting cells (APC) after elution from nylon wool columns. The APC-enriched fraction (referred to as APC) was incubated with 1 mg/ml M and co-cultured with the T lymphocyte fraction (referred to as T cells, 2 × 105) at a ratio of 1:10; 1:20 and 1:40. APC from curcumin-treated animals were cultured with T cells from the vehicle-treated group and vice versa 72 h and 1 μCi/well [3H]-TdR was added the last 18 h.
Statistical analysis
Results are presented as mean ± standard error of the mean (s.e.m.). Comparison between all means was performed using analysis of variance (anova) followed by Bonferroni's multiple comparison test. Comparison between two means was performed by Student's t-test (one- or two-tailed). Incidence of diabetes between groups was compared by Kaplan–Meyer analysis and the log-rank test. A P < 0·05 was considered to indicate a statistically significant difference.
Results
Administration of curcumin prevents autoimmune diabetes in the cyclophosphamide-accelerated model of disease
Natural development of diabetes occurs in 60–80% of 12–30-week-old female NOD mice 21. In order to overcome this asynchronous process we employed CYP, which induces rapid and synchronized diabetes in NOD mice 27. Mice received curcumin injections i.p. from the day before the first CYP injection (at day 0) daily for 7 days and then every other day until the end of the experiment (day 60) (Fig. 1a). Vehicle-treated mice developed hyperglycaemia between days 20 and 24. Curcumin administration of 50, 25 and 5 mg/kg led to 66·7% (n = 6), 57·1% (n = 8) and 41·2% (n = 12) of diabetes-free mice at day 60, respectively (P < 0·05 versus control, log-rank test), suggesting that curcumin had a dose–dependent effect on the reduction of diabetes incidence. No statistically significant differences were detected between the highest doses of curcumin (25 and 50 mg/kg of body weight). Therefore, we used curcumin 25 mg/kg dose hereafter.
Fig. 1.
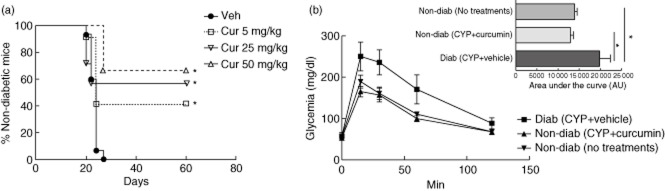
Curcumin prevented diabetes in cyclophosphamide (CYP)-injected non-obese diabetic (NOD) mice. (a) Kaplan–Meier plot of cumulative diabetes incidence in mice treated with curcumin (Cur) 5 mg/kg (n = 12), 25 mg/kg (n = 8) and 50 mg/kg (n = 6) or vehicle (Veh, n = 15). *P < 0·05 versus vehicle, by log-rank (Mantel–Cox) test. (b) Glucose tolerance of diabetic vehicle-treated (Diab, CYP + vehicle) (n = 4), non-diabetic curcumin-treated (Non-diab, CYP + curcumin) (n = 4) and non-diabetic untreated control mice (Non-diab, No treatments) (n = 4). Inset bar-chart shows quantification of the area under the curve (AUC). Data are shown as mean ± standard error of the mean (s.e.m.). *P < 0·05 versus diabetic mice, by analysis of variance (anova) followed by Bonferroni's multiple comparison test.
A glucose tolerance test was performed to assess the physiological response of β cells. Curcumin-treated animals had a similar glucose clearance compared to non-diabetic, untreated age- and sex-matched mice [area under the curve (AUC): 12 926 ± 698 (n = 4) versus 13 896 ± 599 (n = 4), respectively]. As expected, overtly diabetic mice had an elevated AUC: 19 821 ± 2553 (n = 4) in comparison to normoglycaemic groups: the untreated and curcumin-treated mice (P < 0·05). Administration of curcumin did not cause apparent toxicity in NOD mice.
Administration of curcumin delays disease onset employing the adoptive transfer model of autoimmune diabetes
We challenged the beneficial effects of curcumin using the adoptive transfer of disease into NODscid mice as a model of T1DM 26,28. In this model, diabetes onset is more rapid (days 20–30) and aggressive than the natural progression of the disease in NOD mice. Curcumin treatment significantly delayed the onset of T1DM (median = 49 days) in comparison to the control group (median = 29 days; P < 0·001 versus control, by log-rank test) (Fig. 2).
Fig. 2.
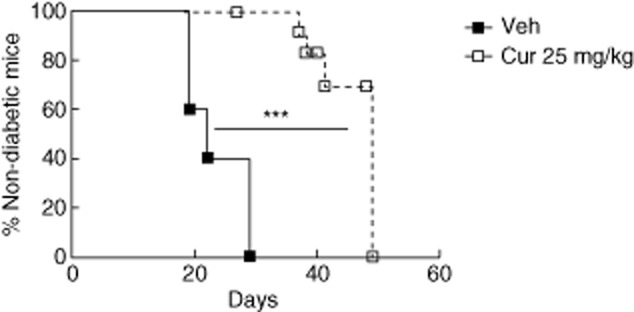
Curcumin delayed the adoptive transfer of autoimmune diabetes. Kaplan–Meier plot of cumulative diabetes incidence in non-obese diabetic (NODscid) mice adoptively transferred with diabetogenic splenocytes and treated with curcumin 25 mg/kg (n = 11) or vehicle (n = 5). ***P < 0·001 versus vehicle, by log-rank (Mantel–Cox) test.
Curcumin inhibits pancreatic leucocyte infiltration
Islet infiltration (insulitis) initiates the destruction of β cells and, eventually, diabetes 29. To investigate whether curcumin administration in NODscid mice reconstituted with diabetogenic splenocytes had an effect on islet infiltration, we harvested pancreata for histological analysis after 20 or 35 days after adoptive disease transfer. Islets from vehicle-injected NODscid mice showed insulitis at day 20, and this infiltration was aggravated at day 35 (Fig. 3a). Insulitis augmentation correlated with a reduction of insulin staining, indicative of specific β cell loss. By contrast, the administration of curcumin prevented insulitis maintaining an intact pancreatic architecture and intense immunostaining of insulin (Fig. 3a). Quantification of islet infiltration is shown in Fig. 3c.
Fig. 3.
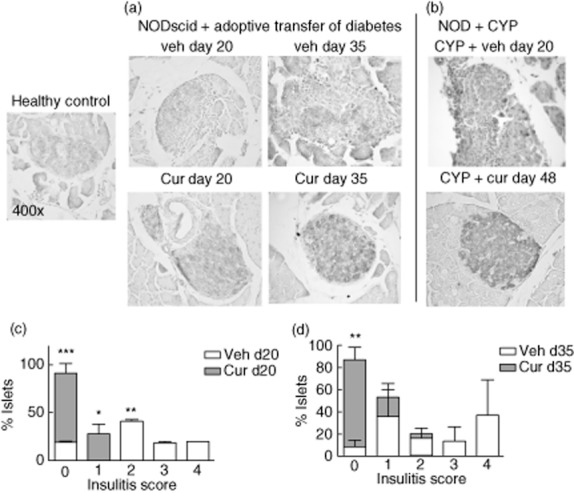
Curcumin prevented insulitis. Immunostaining of β cells (light brown) from (a) curcumin- and vehicle-treated non-obese diabetic (NODscid) mice at 20 and 35 days after adoptive transfer of diabetes. (b) Cyclophosphamide (CYP)-challenged curcumin- and vehicle-treated NOD mice. Representative islets are shown, ×400 magnification. Insulitis quantification was performed 20 (c) and 35 days (d) after adoptive transfer. *P < 0·05; **P < 0·01; ***P < 0·001 versus vehicle by analysis of variance (anova), followed by Bonferroni's multiple comparison test.
Also, we harvested pancreata from animals challenged with CYP and treated with curcumin. Histological analysis showed that curcumin reduced insulitis and preserved insulin expression in β cells (Fig. 3b).
Curcumin impairs polyclonal and antigen-specific T lymphocyte proinflammatory responses
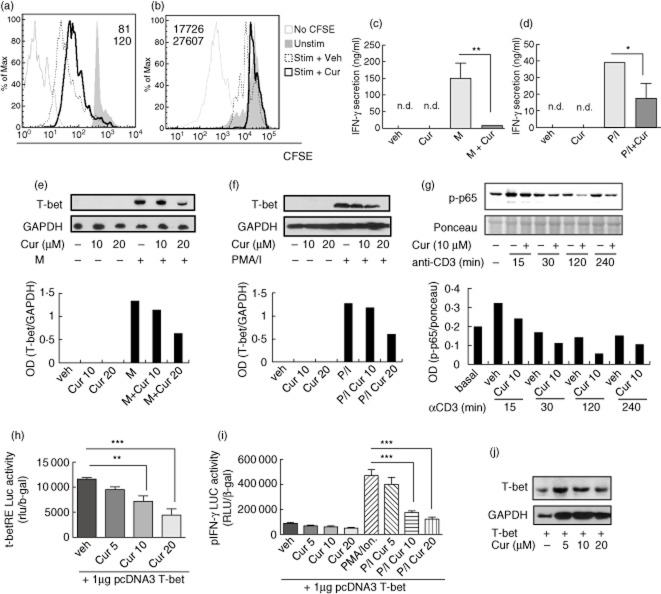
To define the mechanisms by which curcumin is involved in the prevention/delay of autoimmune diabetes, we examined its effect on antigen-specific T cell responses in vitro. M-stimulated BDC2·5-splenocytes 28 had a strong proliferative response in vitro and 10 μM curcumin treatment resulted in a decrease in the proliferation of CD4+ T lymphocytes, as assessed by CFSE dilution (Fig. 4a). Similar effects were observed when stimulating with PMA/I (Fig. 4b). T helper type 1 (Th1) lymphocytes and their hallmark cytokine IFN-γ are central in T1DM pathogenesis 29. Curcumin reduced M-stimulated IFN-γ release, resulting in 18-fold less cytokine production (P < 0·01 versus M-stimulated splenocytes, Fig. 4c).
Fig. 4.
Curcumin impaired antigen-specific and polyclonal T lymphocyte responses in vitro. CD4+ T lymphocyte proliferation was reduced by 10 μM curcumin (thick line) when (a) BDC2·5-splenocytes were stimulated with mimotope (M) (dashed line) or (b) non-obese diabetic (NOD) splenocytes were stimulated with P/I (dashed line). Flow cytometric data show carboxyfluorescein-diacetate succinimidyl ester/mean fluorescence intensity (CFSE/MFI) of stimulated (normal type) and stimulated + curcumin (bold type) conditions. Shaded area in the histogram represents CFSE incorporation by non-stimulated T lymphocytes and continuous thin line represents background staining. Interferon (IFN)-γ secretion of stimulated splenocytes with (c) M or (d) phorbol myristate acetate/ionomycin (PMA/I) was diminished by 10 μM curcumin. *P < 0·05; **P < 0·01 versus M or P/I alone, analysis of variance (anova) followed by Bonferroni's multiple comparison test. Western blot of T-bet expression is shown for (e) M- or (f) PMA/I-stimulated splenocytes incubated or not with 10–20 μM curcumin [normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH)]. Western blot of (g) phosphonuclear factor (NF)-κB p65 (Ser536) (p-p65) is shown for plate-bound anti-CD3ε stimulated NOD splenocytes treated or not with 10 μM curcumin (Cur 10 or Veh), normalized to loaded protein (ponceau). Representative of at least two independent experiments for each stimulation condition. T-bet transcriptional activity on (h) its response elements and (i) on IFN-γ promoter performed with T-bet over-expression in EL-4 T-cells. Mean ± standard error of the mean (s.e.m.) from two to three independent experiments are represented. **P < 0·01; ***P < 0·001; anova followed by Bonferroni's multiple comparison test. (j) Western blot of T-bet is representative of two independent experiments.
Curcumin also reduced IFN-γ release from PMA/I-stimulated splenocytes (Fig. 4d). Ovalbumin (OVA) stimulation did not affect cell proliferation or IFN-γ release (data not shown).
TBX21 (T-box transcription factor, also known as T-bet) is a key transcription factor that governs Th1 differentiation and controls the expression of IFN-γ 25. Curcumin reduced antigen-specific expression of T-bet by splenocytes (Fig. 4e). The same effect was observed when NOD splenocytes were stimulated with PMA/I (Fig. 4f).
Antigen stimulation of TCR signalling to NF-κB is required for T cell proliferation and differentiation of effector cells. To gain insight into curcumin-induced signalling we determined whether it might inhibit NF-κB activation in NOD T lymphocytes. Plate-bound anti-CD3ε stimulation led to NF-κB activation in culture splenocytes and curcumin treatment impaired induction of phospho-NF-κB p65 (Ser536), as shown by Western blot analysis (Fig. 4g).
Then, we asked whether curcumin might be able to modulate GATA-3, a master transcription factor involved in Th2 development. We did not detect splenocyte GATA-3 expression by Western blot due possibly to the strong Th1 bias of NOD splenocytes. GATA-3 expression levels were not modified in the EL-4 T-cell line by curcumin (data not shown).
The transcriptional activity regulation of T-bet by curcumin was studied transfecting EL-4 T cells with a reporter plasmid containing T-bet response elements and a −3447 base pairs (bp) IFN-γ promoter cloned upstream of the luciferase gene (T-bet-RE-Luc and pIFN-γ-Luc) together with high expression levels of T-bet 23. Curcumin inhibited T-bet transcriptional activity on its response elements (10 and 20 μM versus control, P < 0·01 and P < 0·001, respectively) and on the activity of IFN-γ promoter after PMA/I stimulation (10–20 μM, P < 0·001, Fig. 4h–i). Over-expressed T-bet levels were not changed by curcumin, confirming its effects exclusively at the transcriptional level (Fig. 4j).
Effect of long-term curcumin administration on immune splenocyte response ex vivo
We assayed whether the observed in vitro inhibitory effects of curcumin on T lymphocytes also take place in vivo. The spontaneous IFN-γ secretion of splenocytes from curcumin-treated adoptively transferred mice was significantly lower than the control group (95·5% P < 0·05, Fig. 5a). In line with this, we observed lower proliferation in splenocytes from curcumin-treated mice compared to those from the control group (56·9%, P < 0·05, Fig. 5a). IL-4 and IL-10 secretion were undetectable (not shown). T-bet mRNA levels were significantly lower in curcumin-treated mice (52·8%, P < 0·01) relative to controls (Fig. 5b). However, there were non-significant changes of FoxP3 [P = 0·1, 95% confidence interval (CI)] and GATA-3 (P = 0·073, 95% CI) spleen mRNA levels in curcumin-treated mice compared with the control group (Fig. 5b).
Fig. 5.
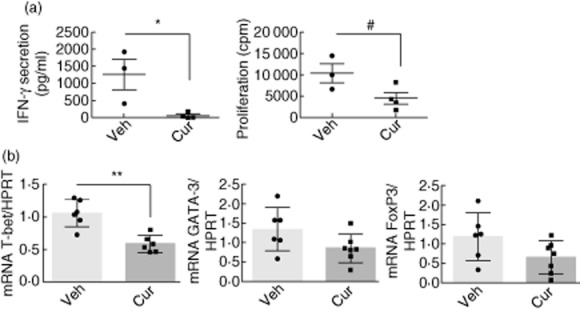
Splenocyte response from adoptively transferred non-obese diabetic (NODscid) mice treated with curcumin in vivo. (a) Interferon (IFN)-γ secretion and proliferation of splenocytes from vehicle- (n = 3) or curcumin-treated (n = 4) NODscid mice 35 days post-transfer. *P < 0·05 by unpaired t-test; #P < 0·05 by unpaired (one-tailed) t-test. (b) Spleen mRNA levels of T-bet, GATA-binding protein 3 (GATA-3) and forkhead box protein 3 (FoxP3) of vehicle- (n = 6) or curcumin-treated (n = 7) mice. **P < 0·01 by unpaired t-test (95% confidence interval). Mean ± standard error of the mean (s.e.m.) is shown.
Curcumin down-regulates LPS/IFN-γ-induced maturation and function of DC
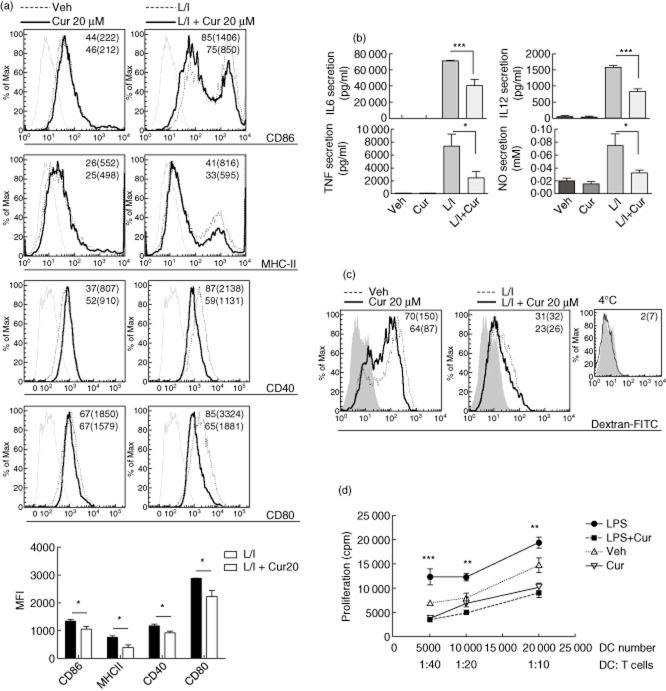
The importance of DC in the pathogenesis of autoimmune diabetes is well documented 30,31. DC were pretreated with 20 μM curcumin, or alternatively with vehicle, for 2 h and stimulated or not with LPS/IFN-γ for 24 h. Curcumin pretreatment of DC (CD11c+) followed by LPS/IFN-γ stimulation exhibited a reduction in the percentage of cells expressing surface CD40, CD80, CD86 and MHC-II when compared with those stimulated with LPS/IFN-γ (Fig. 6a). The mean fluorescence intensity (MFI) of these molecules was also reduced by curcumin pretreatment.
Fig. 6.
Inhibition of lipopolysaccharide/interferon (LPS/IFN)-γ-induced dendritic cell (DC) maturation and function by curcumin. (a) CD11c + DC surface expression of co-stimulatory molecules and major histocompatibility complex (MHC)-II with the corresponding percentage and mean fluorescence intensity (MFI) (brackets) are shown for 20 μM curcumin- (bold type, thick line) and vehicle-treated or LPS/IFN-γ-stimulated conditions (L/I, normal type, dashed line). Representative flow cytometric data from at least three independent experiments and MFI quantification from stimulated (L/I) versus curcumin-treated stimulated DC (L/I + Cur20) is shown; *P < 0·05 by unpaired one-tailed Student's t-test, n = 3. (b) Proinflammatory cytokines and nitric oxide (NO) secretion *P < 0·05; ***P < 0·001 versus LPS/IFN-γ-stimulated DC, n = 3 by analysis of variance (anova) followed by Bonferroni's multiple comparison test. (c) Dextran-fluorescein isothiocyanate (FITC) uptake by CD11c + DC expressed by percentage and MFI (brackets) is shown for 20 mM curcumin- (bold type, thick line) and vehicle-treated or LPS/IFN-γ-stimulated conditions (normal type, dashed line); shaded area represents DC autofluorescence. Representative fluorescence activated cell sorter (FACS) data of three independent experiments. (d) Mixed leucocyte reaction (MLR) shows a reduction in T lymphocyte proliferation when co-cultured with LPS-stimulated curcumin-treated DC versus LPS-stimulated, **P < 0·01; ***P < 0·001; n = 3, by anova followed by Bonferroni's multiple comparison test for each T cell : antigen-presenting cell (APC) ratio.
Curcumin strongly inhibited IL-12p70, IL-6 and TNF-α secretion two- (P < 0·001), 1·7- (P < 0·05) and three-fold (P < 0·001) compared with LPS/IFN-γ stimulation. IL-10 secretion was not detectable (not shown). Curcumin inhibited inducible NO synthase in activated macrophages 32. Consistent with its anti-oxidant capacity, curcumin significantly inhibited NO release in LPS/IFN-γ-stimulated DC (P < 0·05, Fig. 6b).
Endocytosis is critical to mount an efficient immune response by DC. Analysis of curcumin-treated immature DC revealed a reduced ability of mannose receptor-mediated endocytosis (Fig. 6c).
The modulatory effects of curcumin on DC maturation suggest that this agent might alter their function. Thus, we assayed a mixed leucocyte reaction using responder T lymphocytes (BALB/c, H2d) and DC (NOD, H2g7). As expected, LPS-stimulated DC showed the strongest proliferative allogeneic T cell response, whereas curcumin treatment of LPS-stimulated DC led to a significantly impaired proliferation of responder T cells (Fig. 6d).
Antigen-specific T lymphocyte proliferation is diminished by curcumin action on both T cells and APC ex vivo
We performed T cell proliferation experiments to determine whether or not the in-vitro immunomodulatory effects of curcumin on both DC and T lymphocytes also take place in vivo. This was addressed by means of a cross-linked antigen presentation assay in which BDC2·5 mice were treated either with curcumin or vehicle during 1 week. APC (from curcumin- or vehicle-treated BDC2·5 mice) were pulsed with M and co-cultured with BDC2·5 T lymphocytes (from curcumin- or vehicle-treated mice). Figure 7 shows reduced T cell proliferation in co-cultures where either APC or T cells isolated from curcumin-treated animals were present. The proliferation was maximal when both cell populations came from the vehicle-injected mice. These data demonstrate that curcumin alters the antigen-specific T cells response ex vivo.
Fig. 7.
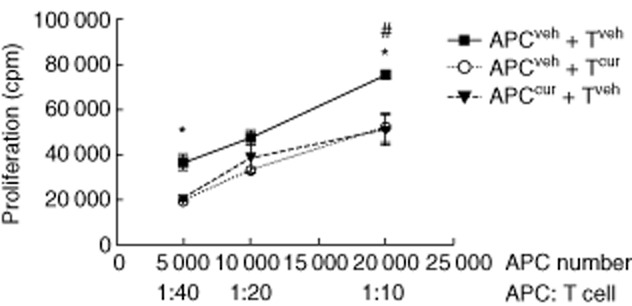
Curcumin administration modulated splenic antigen-presenting cell (APC) and T cell responses. APC and T cells were obtained from curcumin- and vehicle-treated BDC2·5 mice. APC were mimotope (M)-pulsed and co-cultured with T cells. Impairment of T cell proliferation was observed when APC from curcumin-treated mice were co-cultured with T cells from vehicle-treated mice and when APC from the control group were co-cultured with T cells from the curcumin-group. *P < 0·05 and #P < 0·05, respectively, versus co-culture of APC and T cells from vehicle-treated mice (by unpaired t-test for each APC : T cell ratio).
Discussion
In the present work we challenged the well-known anti-oxidant and anti-inflammatory activities of curcumin in autoimmune diabetes employing the NOD mouse in which the immune system plays an essential role in the pathogenesis of disease, as occurs in T1DM 21. The inhibition of proinflammatory cytokine-induced β cell death by curcumin and its beneficial effect on experimental models of diabetes by STZ administration has been reported previously 19. Although the administration of STZ in mice has long been employed as a model of T1DM, this agent induces β cell death by chemical toxicity. In fact, necrotic β cells in STZ-injected mice have been detected as soon as 2–4 h after administration, while leucocyte infiltration is evident only after 3–4 days 33. We show that curcumin protected against the development of CYP-accelerated autoimmune diabetes in NOD mice, a model in which autoimmunity arises by reducing regulatory T cell (Treg) lymphocyte numbers 21. Moreover, in order to resemble the immunological activation state of an individual at the moment of T1DM diagnosis, we employed the transfer of diabetogenic splenocytes to NODscid mice 22. In this study, we report for the first time that in the context of these two accelerated models of disease the administration of curcumin controlled islet-specific autoimmunity, delaying and in some instances completely stopping diabetes progression. Histological pancreata analysis showed that curcumin significantly diminished the presence of inflammatory cells characteristic of insulitis. In accordance, several studies have shown that curcumin inhibits inflammation and autoimmunity in animal models of atherosclerosis, multiple sclerosis, rheumatoid arthritis, sepsis, psoriasis, Alzheimer's disease and experimental colitis 34–38.
The pathogenesis of T1DM is complex, and involves the activation of APC such as DC and macrophages, and the activation of antigen-specific Th1 cells. Proinflammatory cytokines play a determinant role in autoimmune diabetes 39,40. We found that curcumin treatment resulted in a decrease of both T cell proliferation and IFN-γ secretion induced by non-specific stimuli and, importantly, in response to a diabetogenic peptide. These findings are in line with those described by others regarding curcumin inhibition of both T cell differentiation and proliferation 7,41.
T-bet is central in Th1 development 25. Along with STAT-4 and IL-12R signalling, T-bet directs histone post-translational modifications and remodels ifng chromatin, allowing efficient gene transcription 42. Curcumin acts on IL-12-stimulated STAT-4 signalling with an impact upon T cell differentiation and proliferation 7,41. We found that curcumin inhibits T-bet expression levels in both non-specific and antigen-specific-stimulated T lymphocytes. In addition, curcumin inhibited T-bet transcriptional activity on its response gene elements, affecting T-bet-induced IFN-γ promoter activity. These results may account for the observed curcumin-mediated IFN-γ secretion in splenocyte cultures, and suggest that curcumin could play an immunomodulatory role in Th differentiation, strongly inhibiting the Th1 inflammatory profile. In accordance, we found reduced mRNA T-bet levels in the spleens of curcumin-treated mice compared with controls. Meanwhile, GATA-3 and FoxP3 mRNA levels remained unchanged. Thus, curcumin did not exert major effects on Th2 and Treg subpopulations, suggesting that the attenuation of diabetes might be due to regulation of effector T cells at the initiation of autoimmunity. The significance of Th17 in T1DM is uncertain. Th17 cell transfer to NOD mice was found to induce diabetes only after in-vivo conversion to Th1 43. Blockade of IL-17 did not prevent autoimmune diabetes 44. Moreover, we did not observe abundant IL-17-producing splenocytes in NOD mice 40.
NOD mice possess several abnormalities regarding their immune system. Their APC have impaired ability to mediate tolerance induction (reviewed in 21). NOD DC have an abnormally high antigen-presenting stimulatory capacity governed by hyperactivation of NF-κB 45. DC activation is a critical step for the induction of a strong immune response, and within the signalling pathways involved in this process the nuclear translocation of NF-κB p65 subunit plays an important role. In this respect, inhibition of NF-κB activation blocked maturation of DC 46. It has been reported that treatment of immature DC with curcumin decreased stimulation-induced activation of NF-κB and repressed LPS-induced NF-κB promoter activity. Also, curcumin inhibited LPS-induced up-regulation of phosphorylation in mitogen-induced protein kinases (MAPKs), suggesting that curcumin inhibited NF-κB activation by suppressing the MAPK intracellular signal 47. It has also been found that curcumin modulates inducible NO synthase and IL-12 production through reduction of the NF-κB pathway in other immune cells, such as monocytes and macrophages 32,48. Interestingly, this is consistent with our findings that curcumin inhibited NO, TNF-α and IL-6 secretion in LPS-stimulated DCs, also reported in earlier studies 7,49. IL-12p70 released by DC and macrophages drives differentiation of Th1 cells 50,51. Delayed onset and reduced incidence of autoimmune diabetes in recipient mice of DC with impaired IL-12p70 production has been reported 52. Importantly, we show that curcumin-treated NOD DC reduced IL-12p70 release when stimulated in vitro. Taken together, the underlying impairment of DC maturation may account, in part, for the curcumin effect on the activation of NF-kB, although we cannot rule out the action of curcumin in other intracellular signal pathways 53. The effect of curcumin in the MAPK pathway remains to be determined in NOD-derived dendritic cells. Curcumin also reduced endocytosis and stimulatory capacity in an allogeneic T lymphocyte response. These results are in accordance to what has been reported using DC from C57BL/6 mice 47 and human monocyte-derived DC 54.
APC from curcumin-treated mice exhibited a reduced ability to support a specific diabetogenic-peptide T cell proliferation. The therapeutic effect achieved with curcumin may be due to the inability of APC to induce an optimal priming signal and/or to impair Th1 lymphocyte response, as demonstrated in vitro. Notably, when control-injected mice APC were employed as stimulators and T cells from curcumin-treated animals as responders, a similar reduction in T cell proliferation was observed, suggesting that curcumin in vivo acts on both APC and T lymphocytes.
Activation of naive T lymphocytes requires both antigen-specific signal through TCR–peptide–MHC-II interaction and co-stimulatory signal by B7 ligand over-expressed on APC which bind to CD28 on their surface. NF-κB signalling is pivotal in controlling the proliferation of naive T cells and the survival of T cells during antigen presentation 55. There are several important molecules involved within the NF-kB signalling pathway, and phospho-NF-κB p65 (Ser536) plays a key role in the activation of proliferation and gene transcription in T lymphocytes. Our results showed that curcumin exerted effective reduction of NF-κB activation in TCR-stimulated T lymphocytes of NOD mice.
Apart from the two signals consisting of TCR and CD28 on the T cell surface interacting with MHC-II and B7 on APC, respectively, naive Th activation requires a third signal 56. This third signal consists of proinflammatory mediators produced by the innate immune response to boost the adaptive immune response and it is important for inducing, enhancing and prolonging the antigen-specific T cell response 57. Interestingly, Tse et al. reported that modulation of a redox balance with the use of an anti-oxidant inhibited the generation of the third signal from the innate immune response which leads to antigen-specific hyporesponsiveness 58. In view of this evidence, we speculate that inhibition of the third signal is another mechanism that might take place with curcumin treatment contributing to antigen-specific hyporesponsiveness in NOD-accelerated models of diabetes. Indeed, the anti-oxidant activity of curcumin led to a decrease NO and TNF-α secretion by stimulated DC.
Taken together, this evidence suggests that curcumin impairing the NF-kB signalling pathway at both T lymphocytes and APC, and inhibiting the third signal, contributes to attenuation of immunity and subsequently ameliorates diabetes in accelerated murine models.
Recently, administration of curcumin was found to improve the management of peripheral complications associated with diabetes in experimental models 59 and in T2DM patients 16 with encouraging results regarding its anti-diabetic properties. Despite its poor bioavailability, the safety, tolerability and non-toxicity of curcumin, with doses up to 12 g/day, are well established 16. The present study highlights the ability of curcumin to modulate key immune cells involved in the attack against β cells, and demonstrates that this agent: (i) attenuates immunity, (ii) delays diabetes onset and, in some instances, (iii) blocks disease progression using aggressive NOD models. The potential therapeutic benefit of curcumin in naturally occurring diabetes in NOD mice remains to be established. We propose that curcumin, in combination with immunomodulatory agents, deserves to be investigated as therapy for T1DM.
Acknowledgments
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica, Argentina and FOCEM-Mercosur (COF 03/11).
Disclosures
The authors declare that there are no conflicts of interest associated with this manuscript.
Author contributions
C. N. C. and M. J. P. contributed to the conception, design and drafting of the article. All authors contributed to the experimental work, analysis, interpretation of data and revised the manuscript critically.
References
- 1.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 2.Roep BO, Kallan AA, De Vries RR. Beta-cell antigen-specific lysis of macrophages by CD4 T-cell clones from newly diagnosed IDDM patient. A putative mechanism of T-cell-mediated autoimmune islet cell destruction. Diabetes. 1992;41:1380–1384. doi: 10.2337/diab.41.11.1380. [DOI] [PubMed] [Google Scholar]
- 3.Davidson MH. Cardiovascular risk factors in a patient with diabetes mellitus and coronary artery disease: therapeutic approaches to improve outcomes: perspectives of a preventive cardiologist. Am J Cardiol. 2012;110:43B–49. doi: 10.1016/j.amjcard.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Reimann M, Bonifacio E, Solimena M, et al. An update on preventive and regenerative therapies in diabetes mellitus. Pharmacol Ther. 2009;121:317–331. doi: 10.1016/j.pharmthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Sosenko JM, Skyler JS, Palmer JP, et al. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care. 2013;36:2615–2620. doi: 10.2337/dc13-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol. 2002;168:6506–6513. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- 8.Motawi TK, Rizk SM, Shehata AH. Effects of curcumin and Ginkgo biloba on matrix metalloproteinases gene expression and other biomarkers of inflammatory bowel disease. J Physiol Biochem. 2012;68:529–539. doi: 10.1007/s13105-012-0168-9. [DOI] [PubMed] [Google Scholar]
- 9.Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–1725. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLOS ONE. 2013;8:e67078. doi: 10.1371/journal.pone.0067078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curic S, Wu Y, Shan B, et al. Curcumin acts anti-proliferative and pro-apoptotic in human meningiomas. J Neurooncol. 2013;113:385–396. doi: 10.1007/s11060-013-1148-9. [DOI] [PubMed] [Google Scholar]
- 13.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann NY Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 14.Soetikno V, Sari FR, Veeraveedu PT, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-kappaB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond) 2011;8:35. doi: 10.1186/1743-7075-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35:2121–2127. doi: 10.2337/dc12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanai M, Yoshimura K, Asada M, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 18.Kanai M, Otsuka Y, Otsuka K, et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol. 2013;71:1521–1530. doi: 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- 19.Kanitkar M, Gokhale K, Galande S, Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol. 2008;155:702–713. doi: 10.1038/bjp.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanpoo M, Petchpiboonthai H, Panyarachun B, Anupunpisit V. Effect of curcumin in the amelioration of pancreatic islets in streptozotocin-induced diabetic mice. J Med Assoc Thai. 2010;93(Suppl. 6):S152–159. [PubMed] [Google Scholar]
- 21.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33:67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 22.Perone MJ, Bertera S, Tawadrous ZS, et al. Dendritic cells expressing transgenic galectin-1 delay onset of autoimmune diabetes in mice. J Immunol. 2006;177:5278–5289. doi: 10.4049/jimmunol.177.8.5278. [DOI] [PubMed] [Google Scholar]
- 23.Liberman AC, Antunica-Noguerol M, Ferraz-de-Paula V, et al. Compound A, a dissociated glucocorticoid receptor modulator, inhibits T-bet (Th1) and induces GATA-3 (Th2) activity in immune cells. PLOS ONE. 2012;7:e35155. doi: 10.1371/journal.pone.0035155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberman AC, Refojo D, Druker J, et al. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein–protein interaction. FASEB J. 2007;21:1177–1188. doi: 10.1096/fj.06-7452com. [DOI] [PubMed] [Google Scholar]
- 25.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 26.Perone MJ, Larregina AT, Shufesky WJ, et al. Transgenic galectin-1 induces maturation of dendritic cells that elicit contrasting responses in naive and activated T cells. J Immunol. 2006;176:7207–7220. doi: 10.4049/jimmunol.176.12.7207. [DOI] [PubMed] [Google Scholar]
- 27.Augstein P, Elefanty AG, Allison J, Harrison LC. Apoptosis and beta-cell destruction in pancreatic islets of NOD mice with spontaneous and cyclophosphamide-accelerated diabetes. Diabetologia. 1998;41:1381–1388. doi: 10.1007/s001250051080. [DOI] [PubMed] [Google Scholar]
- 28.Piganelli JD, Flores SC, Cruz C, et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 29.Roep BO, De Vries RR. T-lymphocytes and the pathogenesis of type 1 (insulin-dependent) diabetes mellitus. Eur J Clin Invest. 1992;22:697–711. doi: 10.1111/j.1365-2362.1992.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 30.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barcala Tabarrozzi AE, Castro CN, Dewey RA, Sogayar MC, Labriola L, Perone MJ. Cell-based interventions to halt autoimmunity in type 1 diabetes mellitus. Clin Exp Immunol. 2013;171:135–146. doi: 10.1111/cei.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995;206:533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- 33.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Liu YL, Liu GX, et al. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int Immunopharmacol. 2013;17:314–320. doi: 10.1016/j.intimp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez-Tortosa MC, Mesa MD, Aguilera MC, et al. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/s0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 36.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joe B, Rao UJ, Lokesh BR. Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol Cell Biochem. 1997;169:125–134. doi: 10.1023/a:1006877928703. [DOI] [PubMed] [Google Scholar]
- 38.Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–634. [PubMed] [Google Scholar]
- 39.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 40.Perone MJ, Bertera S, Shufesky WJ, et al. Suppression of autoimmune diabetes by soluble galectin-1. J Immunol. 2009;182:2641–2653. doi: 10.4049/jimmunol.0800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fahey AJ, Adrian Robins R, Constantinescu CS. Curcumin modulation of IFN-beta and IL-12 signalling and cytokine induction in human T cells. J Cell Mol Med. 2007;11:1129–1137. doi: 10.1111/j.1582-4934.2007.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams CL, Schilling MM, Cho SH, et al. STAT4 and T-bet are required for the plasticity of IFN-gamma expression across Th2 ontogeny and influence changes in ifng promoter DNA methylation. J Immunol. 2013;191:678–687. doi: 10.4049/jimmunol.1203360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bending D, De la Pena H, Veldhoen M, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poligone B, Weaver DJ, Jr, Sen P, Baldwin AS, Jr, Tisch R. Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J Immunol. 2002;168:188–196. doi: 10.4049/jimmunol.168.1.188. [DOI] [PubMed] [Google Scholar]
- 46.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 48.Kang BY, Chung SW, Chung W, Im S, Hwang SY, Kim TS. Inhibition of interleukin-12 production in lipopolysaccharide-activated macrophages by curcumin. Eur J Pharmacol. 1999;384:191–195. doi: 10.1016/s0014-2999(99)00690-1. [DOI] [PubMed] [Google Scholar]
- 49.Kang BY, Song YJ, Kim KM, Choe YK, Hwang SY, Kim TS. Curcumin inhibits Th1 cytokine profile in CD4+ T cells by suppressing interleukin-12 production in macrophages. Br J Pharmacol. 1999;128:380–384. doi: 10.1038/sj.bjp.0702803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alleva DG, Pavlovich RP, Grant C, Kaser SB, Beller DI. Aberrant macrophage cytokine production is a conserved feature among autoimmune-prone mouse strains: elevated interleukin (IL)-12 and an imbalance in tumor necrosis factor-alpha and IL-10 define a unique cytokine profile in macrophages from young nonobese diabetic mice. Diabetes. 2000;49:1106–1115. doi: 10.2337/diabetes.49.7.1106. [DOI] [PubMed] [Google Scholar]
- 51.Marleau AM, Singh B. Myeloid dendritic cells in non-obese diabetic mice have elevated costimulatory and T helper-1-inducing abilities. J Autoimmun. 2002;19:23–35. doi: 10.1006/jaut.2002.0597. [DOI] [PubMed] [Google Scholar]
- 52.Morin J, Faideau B, Gagnerault MC, Lepault F, Boitard C, Boudaly S. Passive transfer of flt-3L-derived dendritic cells delays diabetes development in NOD mice and associates with early production of interleukin (IL)-4 and IL-10 in the spleen of recipient mice. Clin Exp Immunol. 2003;134:388–395. doi: 10.1111/j.1365-2249.2003.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. Biofactors. 2013;39:27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 54.Rogers NM, Kireta S, Coates PT. Curcumin induces maturation-arrested dendritic cells that expand regulatory T cells in vitro and in vivo. Clin Exp Immunol. 2010;162:460–473. doi: 10.1111/j.1365-2249.2010.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harbor Perspect Biol. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtsinger JM, Schmidt CS, Mondino A, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 57.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- 58.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 59.Xavier S, Sadanandan J, George N, Paulose CS. Beta(2)-adrenoceptor and insulin receptor expression in the skeletal muscle of streptozotocin induced diabetic rats: antagonism by vitamin D(3) and curcumin. Eur J Pharmacol. 2012;687:14–20. doi: 10.1016/j.ejphar.2012.02.050. [DOI] [PubMed] [Google Scholar]